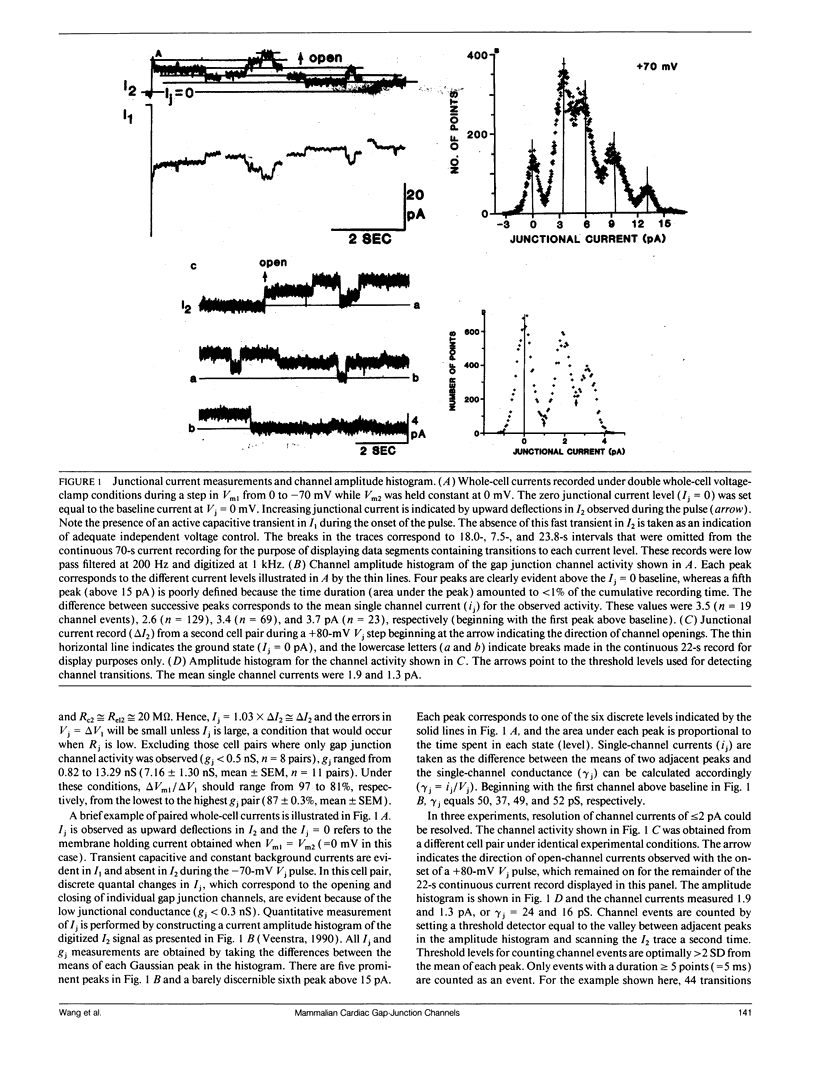
Abstract
Numerous two-cell voltage-clamp studies have concluded that the electrical conductance of mammalian cardiac gap junctions is not modulated by the transjunctional voltage (Vj) profile, although gap junction channels between low conductance pairs of neonatal rat ventricular myocytes are reported to exhibit Vj-dependent behavior. In this study, the dependence of macroscopic gap junctional conductance (gj) on transjunctional voltage was quantitatively examined in paired 3-d neonatal hamster ventricular myocytes using the double whole-cell patch-clamp technique. Immunolocalization with a site-specific antiserum directed against amino acids 252-271 of rat connexin43, a 43-kD gap junction protein as predicted from its cDNA sequence, specifically stained zones of contact between cultured myocytes. Instantaneous current-voltage (Ij-Vj) relationships of neonatal hamster myocyte pairs were linear over the entire voltage range examined (0 less than or equal to Vj less than or equal to +/- 100 mV). However, the steady-state Ij-Vj relationship was nonlinear for Vj greater than +/- 50 mV. Both inactivation and recovery processes followed single exponential time courses (tau inactivation = 100-1,000 ms, tau recovery approximately equal to 300 ms). However, Ij recovered rapidly upon polarity reversal. The normalized steady-state junctional conductance-voltage relationship (Gss-Vj) was a bell-shaped curve that could be adequately described by a two-state Boltzmann equation with a minimum Gj of 0.32-0.34, a half-inactivation voltage of -69 and +61 mV and an effective valence of 2.4-2.8. Recordings of gap junction channel currents (ij) yielded linear ij-Vj relationships with slope conductances of approximately 20-30 and 45-50 pS. A kinetic model, based on the Boltzmann relationship and the polarity reversal data, suggests that the opening (alpha) and closing (beta) rate constants have nearly identical voltage sensitivities with a Vo of +/- 62 mV. The data presented in this study are not consistent with the contingent gating scheme (for two identical gates in series) proposed for other more Vj-dependent gap junctions and alternatively suggest that each gate responds to the applied Vj independently of the state (open or closed) of the other gate.
Full text
PDF


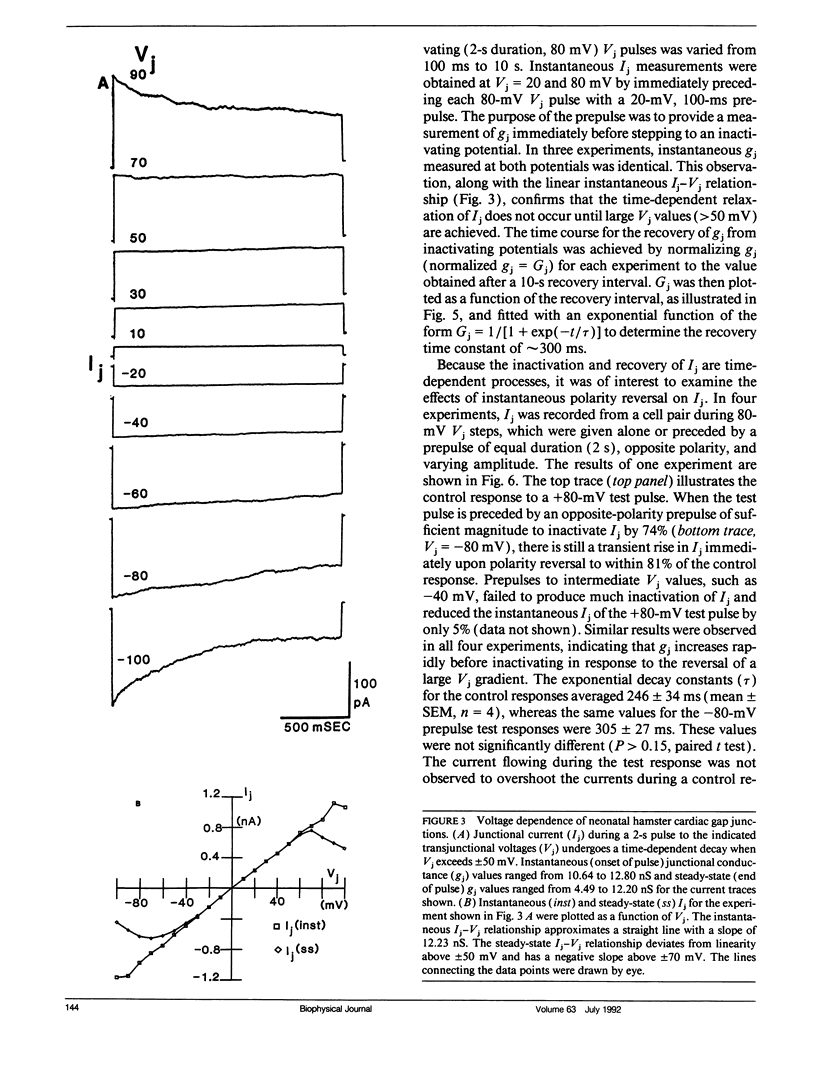


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer E. C., Kistler J., Paul D. L., Goodenough D. A. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989 Feb;108(2):595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin family of gap junction proteins. J Membr Biol. 1990 Jul;116(3):187–194. doi: 10.1007/BF01868459. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987 Dec;105(6 Pt 1):2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt J. M., Spray D. C. Single-channel events and gating behavior of the cardiac gap junction channel. Proc Natl Acad Sci U S A. 1988 May;85(10):3431–3434. doi: 10.1073/pnas.85.10.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara L., Beyer E. C., Swenson K. I., Paul D. L., Goodenough D. A. Cloning and expression of a Xenopus embryonic gap junction protein. Science. 1989 Mar 3;243(4895):1194–1195. doi: 10.1126/science.2466337. [DOI] [PubMed] [Google Scholar]
- Eghbali B., Kessler J. A., Spray D. C. Expression of gap junction channels in communication-incompetent cells after stable transfection with cDNA encoding connexin 32. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1328–1331. doi: 10.1073/pnas.87.4.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman G. I., Moreno A. P., Spray D. C., Leinwand L. A. Functional analysis of human cardiac gap junction channel mutants. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3525–3529. doi: 10.1073/pnas.88.9.3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman G. I., Spray D. C., Leinwand L. A. Molecular characterization and functional expression of the human cardiac gap junction channel. J Cell Biol. 1990 Aug;111(2):589–598. doi: 10.1083/jcb.111.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J. A. Ion channel subconductance states. J Membr Biol. 1987;97(1):1–8. doi: 10.1007/BF01869609. [DOI] [PubMed] [Google Scholar]
- Harris A. L., Spray D. C., Bennett M. V. Kinetic properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981 Jan;77(1):95–117. doi: 10.1085/jgp.77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M. Electrical coupling between ventricular paired cells isolated from guinea-pig heart. J Physiol. 1983 Mar;336:345–357. doi: 10.1113/jphysiol.1983.sp014585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanter H. L., Saffitz J. E., Beyer E. C. Cardiac myocytes express multiple gap junction proteins. Circ Res. 1992 Feb;70(2):438–444. doi: 10.1161/01.res.70.2.438. [DOI] [PubMed] [Google Scholar]
- Laird D. W., Revel J. P. Biochemical and immunochemical analysis of the arrangement of connexin43 in rat heart gap junction membranes. J Cell Sci. 1990 Sep;97(Pt 1):109–117. doi: 10.1242/jcs.97.1.109. [DOI] [PubMed] [Google Scholar]
- Lemanski L. F., Tu Z. H. Immunofluorescent studies for myosin, actin, tropomyosin and alpha-actinin in cultured cardiomyopathic hamster heart cells. Dev Biol. 1983 Jun;97(2):338–348. doi: 10.1016/0012-1606(83)90091-x. [DOI] [PubMed] [Google Scholar]
- Li J. A., Lemanski L. F. Immunofluorescent studies for alpha-actinin in cultured cardiomyopathic hamster heart cells. Anat Rec. 1990 Sep;228(1):46–52. doi: 10.1002/ar.1092280108. [DOI] [PubMed] [Google Scholar]
- Makowski L., Caspar D. L., Phillips W. C., Goodenough D. A. Gap junction structures. II. Analysis of the x-ray diffraction data. J Cell Biol. 1977 Aug;74(2):629–645. doi: 10.1083/jcb.74.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger P., Weingart R. Electric current flow in cell pairs isolated from adult rat hearts. J Physiol. 1985 Sep;366:177–195. doi: 10.1113/jphysiol.1985.sp015791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma A., Tsuboi N. Dependence of junctional conductance on proton, calcium and magnesium ions in cardiac paired cells of guinea-pig. J Physiol. 1987 Jan;382:193–211. doi: 10.1113/jphysiol.1987.sp016363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook M. B., Jongsma H. J., de Jonge B. Single channel currents of homo- and heterologous gap junctions between cardiac fibroblasts and myocytes. Pflugers Arch. 1989 May;414(1):95–98. doi: 10.1007/BF00585633. [DOI] [PubMed] [Google Scholar]
- Rook M. B., Jongsma H. J., van Ginneken A. C. Properties of single gap junctional channels between isolated neonatal rat heart cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H770–H782. doi: 10.1152/ajpheart.1988.255.4.H770. [DOI] [PubMed] [Google Scholar]
- Rook M. B., de Jonge B., Jongsma H. J., Masson-Pévet M. A. Gap junction formation and functional interaction between neonatal rat cardiocytes in culture: a correlative physiological and ultrastructural study. J Membr Biol. 1990 Nov;118(2):179–192. doi: 10.1007/BF01868475. [DOI] [PubMed] [Google Scholar]
- Rüdisüli A., Weingart R. Electrical properties of gap junction channels in guinea-pig ventricular cell pairs revealed by exposure to heptanol. Pflugers Arch. 1989 Oct;415(1):12–21. doi: 10.1007/BF00373136. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981 Jan;77(1):77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson K. I., Jordan J. R., Beyer E. C., Paul D. L. Formation of gap junctions by expression of connexins in Xenopus oocyte pairs. Cell. 1989 Apr 7;57(1):145–155. doi: 10.1016/0092-8674(89)90180-3. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D., DeHaan R. L. Cardiac gap junction channel activity in embryonic chick ventricle cells. Am J Physiol. 1988 Jan;254(1 Pt 2):H170–H180. doi: 10.1152/ajpheart.1988.254.1.H170. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D., DeHaan R. L. Measurement of single channel currents from cardiac gap junctions. Science. 1986 Aug 29;233(4767):972–974. doi: 10.1126/science.2426781. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D. Developmental changes in regulation of embryonic chick heart gap junctions. J Membr Biol. 1991 Feb;119(3):253–265. doi: 10.1007/BF01868730. [DOI] [PubMed] [Google Scholar]
- Veenstra R. D. Voltage-dependent gating of gap junction channels in embryonic chick ventricular cell pairs. Am J Physiol. 1990 Apr;258(4 Pt 1):C662–C672. doi: 10.1152/ajpcell.1990.258.4.C662. [DOI] [PubMed] [Google Scholar]
- Weingart R. Electrical properties of the nexal membrane studied in rat ventricular cell pairs. J Physiol. 1986 Jan;370:267–284. doi: 10.1113/jphysiol.1986.sp015934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner R., Levine E., Rabadan-Diehl C., Dahl G. Formation of hybrid cell-cell channels. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5380–5384. doi: 10.1073/pnas.86.14.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. L., Spray D. C., Campos de Carvalho A. C., Wittenberg B. A., Bennett M. V. Some electrical and pharmacological properties of gap junctions between adult ventricular myocytes. Am J Physiol. 1985 Nov;249(5 Pt 1):C447–C455. doi: 10.1152/ajpcell.1985.249.5.C447. [DOI] [PubMed] [Google Scholar]
- Yancey S. B., John S. A., Lal R., Austin B. J., Revel J. P. The 43-kD polypeptide of heart gap junctions: immunolocalization, topology, and functional domains. J Cell Biol. 1989 Jun;108(6):2241–2254. doi: 10.1083/jcb.108.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. T., Nicholson B. J. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol. 1989 Dec;109(6 Pt 2):3391–3401. doi: 10.1083/jcb.109.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el Aoumari A., Fromaget C., Dupont E., Reggio H., Durbec P., Briand J. P., Böller K., Kreitman B., Gros D. Conservation of a cytoplasmic carboxy-terminal domain of connexin 43, a gap junctional protein, in mammal heart and brain. J Membr Biol. 1990 May;115(3):229–240. doi: 10.1007/BF01868638. [DOI] [PubMed] [Google Scholar]