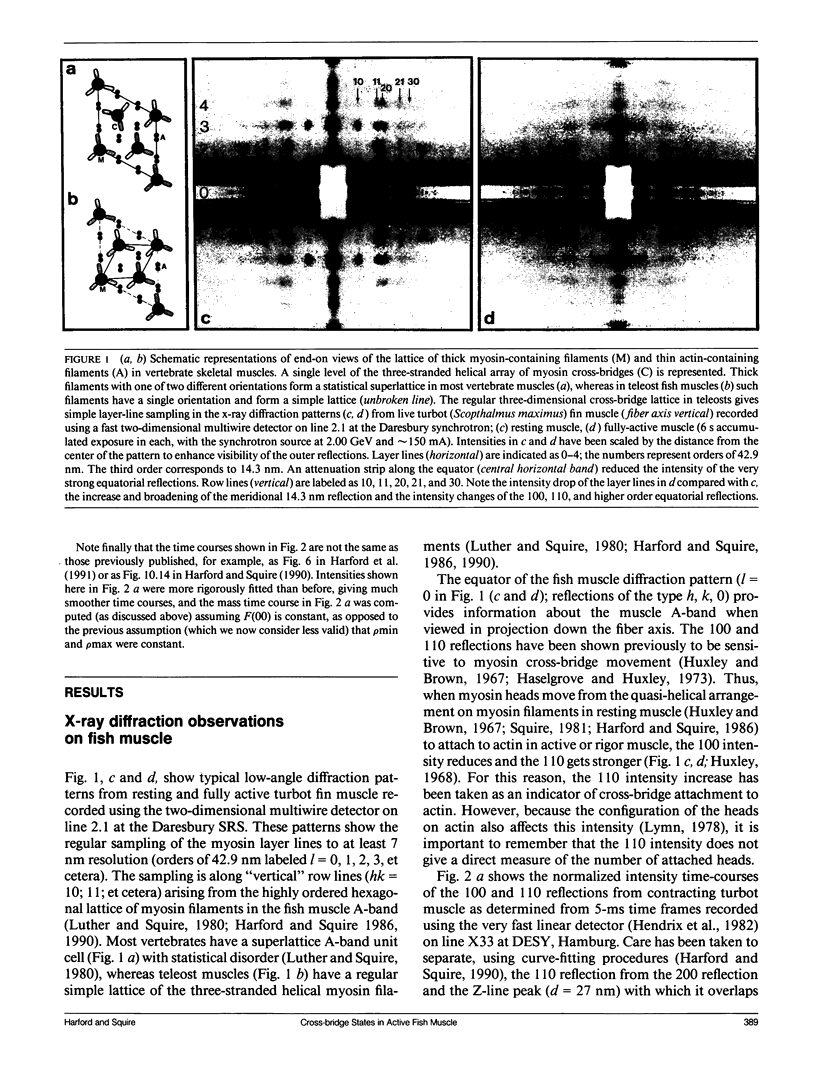
Abstract
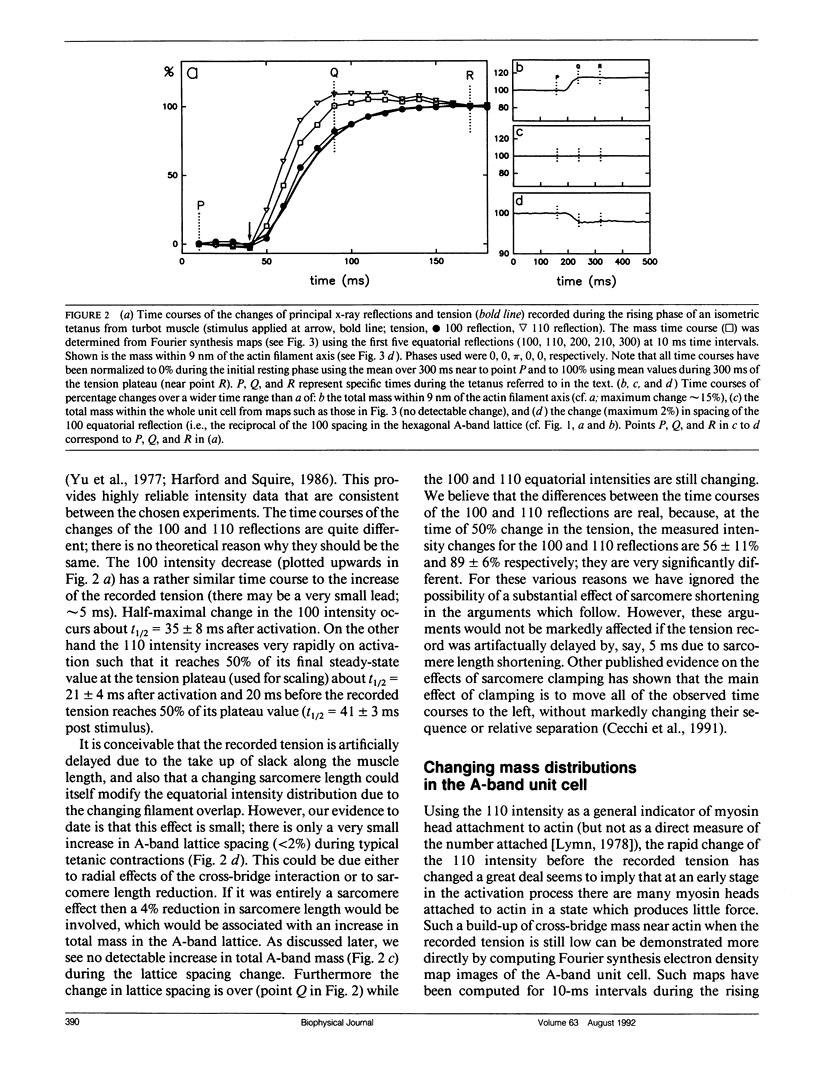
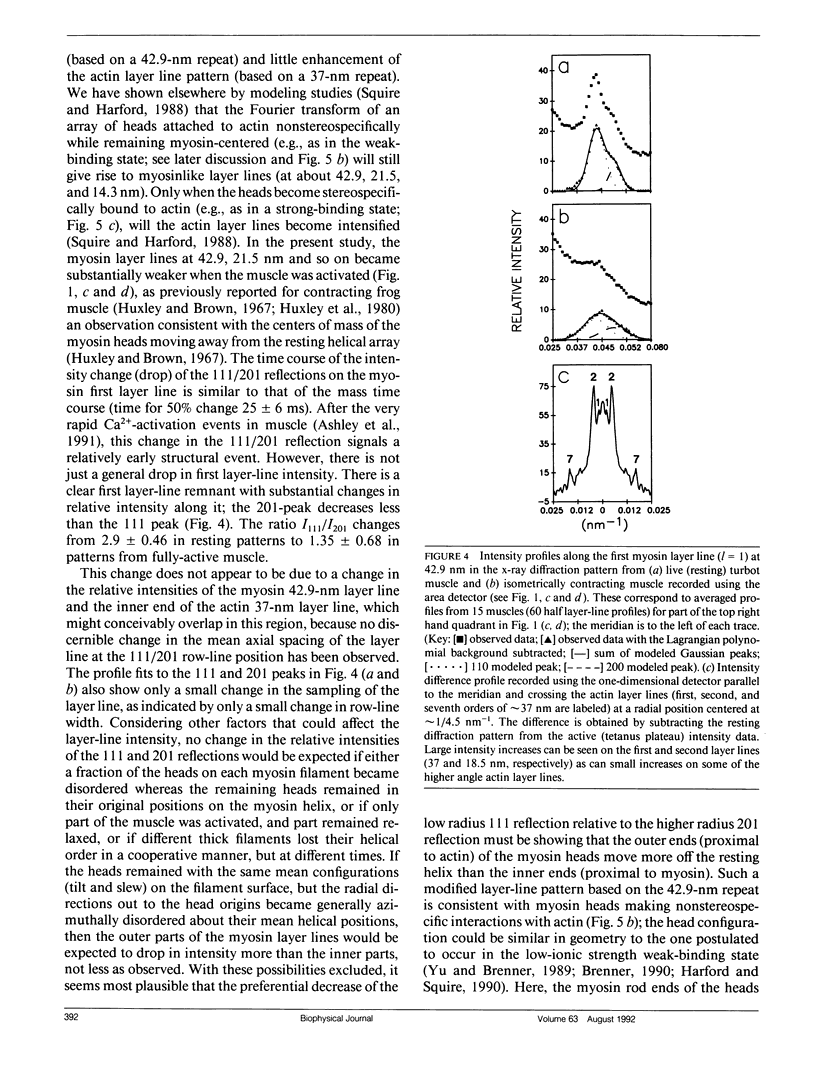
Using data from fast time-resolved x-ray diffraction experiments on the synchrotrons at Daresbury and (Deutsches Elektronen Synchrotron [DESY]), it is shown that during contraction of fish muscle there are at least two distinct configurations of myosin cross-bridges on actin, that they appear to have different tension producing properties and that they probably differ in the axial tilt of the cross-bridges on actin. Evidence is presented for newly observed myosin-based layer lines in patterns from active fish muscle, together with intensity changes of the actin layer lines. On the equator, the 110 reflection changes much faster (time for 50% change t1/2 = 21 +/- 4 ms after activation) than the 100 reflection (t1/2 = 35 +/- 8 ms) and tension (t1/2 = 41 +/- 3 ms) during the rising phase of tetanic contractions. These and higher order reflections have been used to show the time course of mass attachment at actin during this rising phase. Mass arrival (t1/2 = 25 ms) precedes tension by approximately 15 ms. Analysis has been carried out to evaluate the effects of changes in sarcomere length during the tetanus. It is shown that any such effects are very small. Difference "equatorial" electron density maps between active muscle at a time when mass arrival at actin is just complete, but the tension is still rising, and at a later time well into the tension plateau, show that the structural difference between the lower and higher force states corresponds to mass movement consistent with axial swinging of heads from a nonstereospecific actin attached state (low force) to a more stereospecific (high force) state.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Mulligan I. P., Lea T. J. Ca2+ and activation mechanisms in skeletal muscle. Q Rev Biophys. 1991 Feb;24(1):1–73. doi: 10.1017/s0033583500003267. [DOI] [PubMed] [Google Scholar]
- Barnett V. A., Thomas D. D. Microsecond rotational motion of spin-labeled myosin heads during isometric muscle contraction. Saturation transfer electron paramagnetic resonance. Biophys J. 1989 Sep;56(3):517–523. doi: 10.1016/S0006-3495(89)82698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger C. L., Svensson E. C., Thomas D. D. Photolysis of a photolabile precursor of ATP (caged ATP) induces microsecond rotational motions of myosin heads bound to actin. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8753–8757. doi: 10.1073/pnas.86.22.8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordas J., Diakun G. P., Harries J. E., Lewis R. A., Mant G. R., Martin-Fernandez M. L., Towns-Andrews E. Two-dimensional time resolved X-ray diffraction of muscle: recent results. Adv Biophys. 1991;27:15–33. doi: 10.1016/0065-227x(91)90005-x. [DOI] [PubMed] [Google Scholar]
- Brenner B., Schoenberg M., Chalovich J. M., Greene L. E., Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Yu L. C., Podolsky R. J. X-ray diffraction evidence for cross-bridge formation in relaxed muscle fibers at various ionic strengths. Biophys J. 1984 Sep;46(3):299–306. doi: 10.1016/S0006-3495(84)84026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi G., Griffiths P. J., Bagni M. A., Ashley C. C., Maeda Y. Time-resolved changes in equatorial x-ray diffraction and stiffness during rise of tetanic tension in intact length-clamped single muscle fibers. Biophys J. 1991 Jun;59(6):1273–1283. doi: 10.1016/S0006-3495(91)82342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Crowder M. S., Thomas D. D. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982 Dec 23;300(5894):776–778. doi: 10.1038/300776a0. [DOI] [PubMed] [Google Scholar]
- Curmi P. M., Stone D. B., Schneider D. K., Spudich J. A., Mendelson R. A. Comparison of the structure of myosin subfragment 1 bound to actin and free in solution. A neutron scattering study using actin made "invisible" by deuteration. J Mol Biol. 1988 Oct 5;203(3):781–798. doi: 10.1016/0022-2836(88)90209-4. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L. A cross-bridge model of muscle contraction. Prog Biophys Mol Biol. 1978;33(1):55–82. doi: 10.1016/0079-6107(79)90025-7. [DOI] [PubMed] [Google Scholar]
- Eisenberg E., Hill T. L. Muscle contraction and free energy transduction in biological systems. Science. 1985 Mar 1;227(4690):999–1006. doi: 10.1126/science.3156404. [DOI] [PubMed] [Google Scholar]
- Fajer P. G., Fajer E. A., Schoenberg M., Thomas D. D. Orientational disorder and motion of weakly attached cross-bridges. Biophys J. 1991 Sep;60(3):642–649. doi: 10.1016/S0006-3495(91)82093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Huxley A. F., Simmons R. M. Tension transients during the rise of tetanic tension in frog muscle fibres. J Physiol. 1986 Mar;372:595–609. doi: 10.1113/jphysiol.1986.sp016027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N. P., Melchior V., Kirshner D. A., Caspar D. L. Structure of myelin lipid bilayers. Changes during maturation. J Mol Biol. 1982 Feb 25;155(2):133–153. doi: 10.1016/0022-2836(82)90441-7. [DOI] [PubMed] [Google Scholar]
- Harford J. J., Chew M. W., Squire J. M., Towns-Andrews E. Crossbridge states in isometrically contracting fish muscle: evidence for swinging of myosin heads on actin. Adv Biophys. 1991;27:45–61. doi: 10.1016/0065-227x(91)90007-z. [DOI] [PubMed] [Google Scholar]
- Harford J., Squire J. "Crystalline" myosin cross-bridge array in relaxed bony fish muscle. Low-angle x-ray diffraction from plaice fin muscle and its interpretation. Biophys J. 1986 Jul;50(1):145–155. doi: 10.1016/S0006-3495(86)83447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Huxley A. F. A note suggesting that the cross-bridge attachment during muscle contraction may take place in two stages. Proc R Soc Lond B Biol Sci. 1973 Feb 27;183(1070):83–86. doi: 10.1098/rspb.1973.0006. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Brown W. The low-angle x-ray diagram of vertebrate striated muscle and its behaviour during contraction and rigor. J Mol Biol. 1967 Dec 14;30(2):383–434. doi: 10.1016/s0022-2836(67)80046-9. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Faruqi A. R., Bordas J., Koch M. H., Milch J. R. The use of synchrotron radiation in time-resolved X-ray diffraction studies of myosin layer-line reflections during muscle contraction. Nature. 1980 Mar 13;284(5752):140–143. doi: 10.1038/284140a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Faruqi A. R., Kress M., Bordas J., Koch M. H. Time-resolved X-ray diffraction studies of the myosin layer-line reflections during muscle contraction. J Mol Biol. 1982 Jul 15;158(4):637–684. doi: 10.1016/0022-2836(82)90253-4. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Kress M. Crossbridge behaviour during muscle contraction. J Muscle Res Cell Motil. 1985 Apr;6(2):153–161. doi: 10.1007/BF00713057. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. Structural difference between resting and rigor muscle; evidence from intensity changes in the lowangle equatorial x-ray diagram. J Mol Biol. 1968 Nov 14;37(3):507–520. doi: 10.1016/0022-2836(68)90118-6. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The structural basis of contraction and regulation in skeletal muscle. Kaibogaku Zasshi. 1975 Dec;50(6):310–325. [PubMed] [Google Scholar]
- Kabsch W., Mannherz H. G., Suck D., Pai E. F., Holmes K. C. Atomic structure of the actin:DNase I complex. Nature. 1990 Sep 6;347(6288):37–44. doi: 10.1038/347037a0. [DOI] [PubMed] [Google Scholar]
- Kress M., Huxley H. E., Faruqi A. R., Hendrix J. Structural changes during activation of frog muscle studied by time-resolved X-ray diffraction. J Mol Biol. 1986 Apr 5;188(3):325–342. doi: 10.1016/0022-2836(86)90158-0. [DOI] [PubMed] [Google Scholar]
- Luther P. K., Squire J. M. Three-dimensional structure of the vertebrate muscle A-band. II. The myosin filament superlattice. J Mol Biol. 1980 Aug 25;141(4):409–439. doi: 10.1016/0022-2836(80)90254-5. [DOI] [PubMed] [Google Scholar]
- Lymn R. W. Myosin subfragment-1 attachment to actin. Expected effect on equatorial reflections. Biophys J. 1978 Jan;21(1):93–98. doi: 10.1016/S0006-3495(78)85510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara I., Yagi N., Miura H., Ozeki M., Izumi T. Intensification of the 5.9-nm actin layer line in contracting muscle. 1984 Nov 29-Dec 5Nature. 312(5993):471–473. doi: 10.1038/312471a0. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Podolsky R. J. X-ray evidence for two structural states of the actomyosin cross-bridge in muscle fibers. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2364–2368. doi: 10.1073/pnas.81.8.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A., Tregear R. T. Structure of insect fibrillar flight muscle in the presence and absence of ATP. J Mol Biol. 1972 Sep 14;70(1):85–104. doi: 10.1016/0022-2836(72)90165-9. [DOI] [PubMed] [Google Scholar]
- Nagano H., Yanagida T. Predominant attached state of myosin cross-bridges during contraction and relaxation at low ionic strength. J Mol Biol. 1984 Aug 25;177(4):769–785. doi: 10.1016/0022-2836(84)90048-2. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Squire J. M. Structural role of tropomyosin in muscle regulation: analysis of the x-ray diffraction patterns from relaxed and contracting muscles. J Mol Biol. 1973 Mar 25;75(1):33–55. doi: 10.1016/0022-2836(73)90527-5. [DOI] [PubMed] [Google Scholar]
- Reedy M. K., Holmes K. C., Tregear R. T. Induced changes in orientation of the cross-bridges of glycerinated insect flight muscle. Nature. 1965 Sep 18;207(5003):1276–1280. doi: 10.1038/2071276a0. [DOI] [PubMed] [Google Scholar]
- Squire J. M., Harford J. J. Actin filament organization and myosin head labelling patterns in vertebrate skeletal muscles in the rigor and weak binding states. J Muscle Res Cell Motil. 1988 Aug;9(4):344–358. doi: 10.1007/BF01773878. [DOI] [PubMed] [Google Scholar]
- Squire J. Muscle contraction. Invisible actin makes its debut. Nature. 1988 Oct 13;335(6191):590–591. doi: 10.1038/335590a0. [DOI] [PubMed] [Google Scholar]
- Stein R. A., Ludescher R. D., Dahlberg P. S., Fajer P. G., Bennett R. L., Thomas D. D. Time-resolved rotational dynamics of phosphorescent-labeled myosin heads in contracting muscle fibers. Biochemistry. 1990 Oct 30;29(43):10023–10031. doi: 10.1021/bi00495a003. [DOI] [PubMed] [Google Scholar]
- Vibert P. J. Domain structure of the myosin head in correlation-averaged images of shadowed molecules. J Muscle Res Cell Motil. 1988 Apr;9(2):147–155. doi: 10.1007/BF01773736. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Tanaka H., Saito H., Moriwaki N., Ueno Y., Amemiya Y. Dynamic X-ray diffraction of skeletal muscle contraction: structural change of actin filaments. Adv Biophys. 1991;27:3–13. doi: 10.1016/0065-227x(91)90004-w. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K., Ueno Y., Amemiya Y., Tanaka H. Intensity changes of actin-based layer lines from frog skeletal muscles during an isometric contraction. Adv Exp Med Biol. 1988;226:353–367. [PubMed] [Google Scholar]
- Xu S. G., Kress M., Huxley H. E. X-ray diffraction studies of the structural state of crossbridges in skinned frog sartorius muscle at low ionic strength. J Muscle Res Cell Motil. 1987 Feb;8(1):39–54. doi: 10.1007/BF01767263. [DOI] [PubMed] [Google Scholar]
- Yagi N. Intensification of the first actin layer-line during contraction of frog skeletal muscle. Adv Biophys. 1991;27:35–43. doi: 10.1016/0065-227x(91)90006-y. [DOI] [PubMed] [Google Scholar]
- Yagi N., Matsubara I. Structural changes in the thin filament during activation studied by X-ray diffraction of highly stretched skeletal muscle. J Mol Biol. 1989 Jul 20;208(2):359–363. doi: 10.1016/0022-2836(89)90396-3. [DOI] [PubMed] [Google Scholar]
- Yu L. C. Analysis of equatorial x-ray diffraction patterns from skeletal muscle. Biophys J. 1989 Mar;55(3):433–440. doi: 10.1016/S0006-3495(89)82837-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L. C., Brenner B. Structures of actomyosin crossbridges in relaxed and rigor muscle fibers. Biophys J. 1989 Mar;55(3):441–453. doi: 10.1016/S0006-3495(89)82838-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L. C., Lymn R. W., Podolsky R. J. Characterization of a non-indexible equatorial x-ray reflection from frog sartorius muscle. J Mol Biol. 1977 Sep 25;115(3):455–464. doi: 10.1016/0022-2836(77)90165-6. [DOI] [PubMed] [Google Scholar]
- Yu L. C., Steven A. C., Naylor G. R., Gamble R. C., Podolsky R. J. Distribution of mass in relaxed frog skeletal muscle and its redistribution upon activation. Biophys J. 1985 Mar;47(3):311–321. doi: 10.1016/S0006-3495(85)83921-7. [DOI] [PMC free article] [PubMed] [Google Scholar]