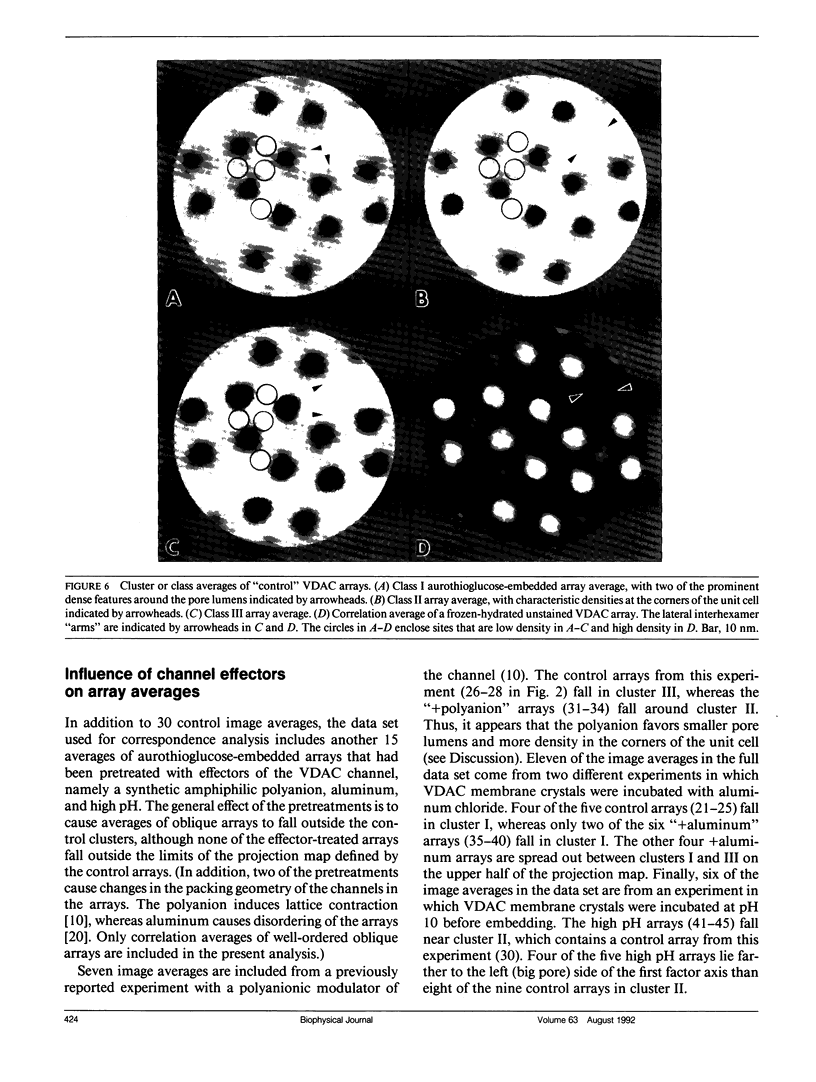
Abstract
Low-dose electron microscopic images have been recorded from membrane crystals of the mitochondrial, voltage-dependent anion-selective channel, embedded in aurothioglucose. There is considerable variation in the high-resolution detail present in correlation averages computed from these images. Correspondence analysis reveals three classes of "control" averages, with main components of variation involving projected size of the pores and density modulations around the pores and in the corners of the unit cells away from the pores. Pretreatments that affect the functional state of the channel also affect the array averages. In particular, there appears to be a general correlation between the expected effector-induced state (i.e., open and closed) and the projected diameter of the channel lumens in the crystalline arrays.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R. Porin from bacterial and mitochondrial outer membranes. CRC Crit Rev Biochem. 1985;19(2):145–190. doi: 10.3109/10409238509082542. [DOI] [PubMed] [Google Scholar]
- Benz R., Wojtczak L., Bosch W., Brdiczka D. Inhibition of adenine nucleotide transport through the mitochondrial porin by a synthetic polyanion. FEBS Lett. 1988 Apr 11;231(1):75–80. doi: 10.1016/0014-5793(88)80706-3. [DOI] [PubMed] [Google Scholar]
- Colombini M. A candidate for the permeability pathway of the outer mitochondrial membrane. Nature. 1979 Jun 14;279(5714):643–645. doi: 10.1038/279643a0. [DOI] [PubMed] [Google Scholar]
- Colombini M. Regulation of the mitochondrial outer membrane channel, VDAC. J Bioenerg Biomembr. 1987 Aug;19(4):309–320. doi: 10.1007/BF00768534. [DOI] [PubMed] [Google Scholar]
- Colombini M. Voltage gating in the mitochondrial channel, VDAC. J Membr Biol. 1989 Oct;111(2):103–111. doi: 10.1007/BF01871775. [DOI] [PubMed] [Google Scholar]
- Colombini M., Yeung C. L., Tung J., König T. The mitochondrial outer membrane channel, VDAC, is regulated by a synthetic polyanion. Biochim Biophys Acta. 1987 Dec 11;905(2):279–286. doi: 10.1016/0005-2736(87)90456-1. [DOI] [PubMed] [Google Scholar]
- Dill E. T., Holden M. J., Colombini M. Voltage gating in VDAC is markedly inhibited by micromolar quantities of aluminum. J Membr Biol. 1987;99(3):187–196. doi: 10.1007/BF01995699. [DOI] [PubMed] [Google Scholar]
- Engel A., Massalski A., Schindler H., Dorset D. L., Rosenbusch J. P. Porin channel triplets merge into single outlets in Escherichia coli outer membranes. Nature. 1985 Oct 17;317(6038):643–645. doi: 10.1038/317643a0. [DOI] [PubMed] [Google Scholar]
- Frank J., Goldfarb W., Eisenberg D., Baker T. S. Reconstruction of glutamine synthetase using computer averaging. Ultramicroscopy. 1978;3(3):283–290. doi: 10.1016/s0304-3991(78)80038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J., Verschoor A., Boublik M. Multivariate statistical analysis of ribosome electron micrographs. L and R lateral views of the 40 S subunit from HeLa cells. J Mol Biol. 1982 Oct 15;161(1):107–133. doi: 10.1016/0022-2836(82)90281-9. [DOI] [PubMed] [Google Scholar]
- Holden M. J., Colombini M. The mitochondrial outer membrane channel, VDAC, is modulated by a soluble protein. FEBS Lett. 1988 Dec 5;241(1-2):105–109. doi: 10.1016/0014-5793(88)81040-8. [DOI] [PubMed] [Google Scholar]
- Kessel M., Radermacher M., Frank J. The structure of the stalk surface layer of a brine pond microorganism: correlation averaging applied to a double layered lattice structure. J Microsc. 1985 Jul;139(Pt 1):63–74. doi: 10.1111/j.1365-2818.1985.tb04662.x. [DOI] [PubMed] [Google Scholar]
- Kühlbrandt W. Discrimination of protein and nucleic acids by electron microscopy using contrast variation. Ultramicroscopy. 1982;7(3):221–232. doi: 10.1016/0304-3991(82)90169-3. [DOI] [PubMed] [Google Scholar]
- Mannella C. A. Electron microscopy and image analysis of the mitochondrial outer membrane channel, VDAC. J Bioenerg Biomembr. 1987 Aug;19(4):329–340. doi: 10.1007/BF00768536. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Forte M., Colombini M. Toward the molecular structure of the mitochondrial channel, VDAC. J Bioenerg Biomembr. 1992 Feb;24(1):7–19. doi: 10.1007/BF00769525. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Frank J. Negative staining characteristics of arrays of mitochondrial pore protein: use of correspondence analysis to classify different staining patterns. Ultramicroscopy. 1984;13(1-2):93–102. doi: 10.1016/0304-3991(84)90060-3. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Guo X. W., Dias J. Binding of a synthetic targeting peptide to a mitochondrial channel protein. J Bioenerg Biomembr. 1992 Feb;24(1):55–61. doi: 10.1007/BF00769531. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Guo X. W. Interaction between the VDAC channel and a polyanionic effector. An electron microscopic study. Biophys J. 1990 Jan;57(1):23–31. doi: 10.1016/S0006-3495(90)82503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella C. A. Mitochondrial outer membrane channel (VDAC, porin) two-dimensional crystals from Neurospora. Methods Enzymol. 1986;125:595–610. doi: 10.1016/s0076-6879(86)25048-x. [DOI] [PubMed] [Google Scholar]
- Mannella C. A. Phospholipase-induced crystallization of channels in mitochondrial outer membranes. Science. 1984 Apr 13;224(4645):165–166. doi: 10.1126/science.6322311. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Ribeiro A. J., Frank J. Cytochrome c binds to lipid domains in arrays of mitochondrial outer membrane channels. Biophys J. 1987 Feb;51(2):221–226. doi: 10.1016/S0006-3495(87)83327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella C. A., Ribeiro A., Frank J. Structure of the Channels in the Outer Mitochondrial Membrane: Electron Microscopic Studies of the Periodic Arrays Induced by Phospholipase a(2) Treatment of the Neurospora membrane. Biophys J. 1986 Jan;49(1):307–317. doi: 10.1016/s0006-3495(86)83643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella C. A. Structural analysis of mitochondrial pores. Experientia. 1990 Feb 15;46(2):137–145. doi: 10.1007/BF02027309. [DOI] [PubMed] [Google Scholar]
- Mannella C. A. Structure of the outer mitochondrial membrane: ordered arrays of porelike subunits in outer-membrane fractions from Neurospora crassa mitochondria. J Cell Biol. 1982 Sep;94(3):680–687. doi: 10.1083/jcb.94.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton W. O., Baumeister W. The correlation averaging of a regularly arranged bacterial cell envelope protein. J Microsc. 1982 Aug;127(Pt 2):127–138. doi: 10.1111/j.1365-2818.1982.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Thomas L., Kocsis E., Colombini M., Erbe E., Trus B. L., Steven A. C. Surface topography and molecular stoichiometry of the mitochondrial channel, VDAC, in crystalline arrays. J Struct Biol. 1991 Apr;106(2):161–171. doi: 10.1016/1047-8477(91)90085-b. [DOI] [PubMed] [Google Scholar]
- Unser M., Trus B. L., Steven A. C. Normalization procedures and factorial representations for classification of correlation-aligned images: a comparative study. Ultramicroscopy. 1989 Jul-Aug;30(3):299–310. doi: 10.1016/0304-3991(89)90058-2. [DOI] [PubMed] [Google Scholar]