Abstract
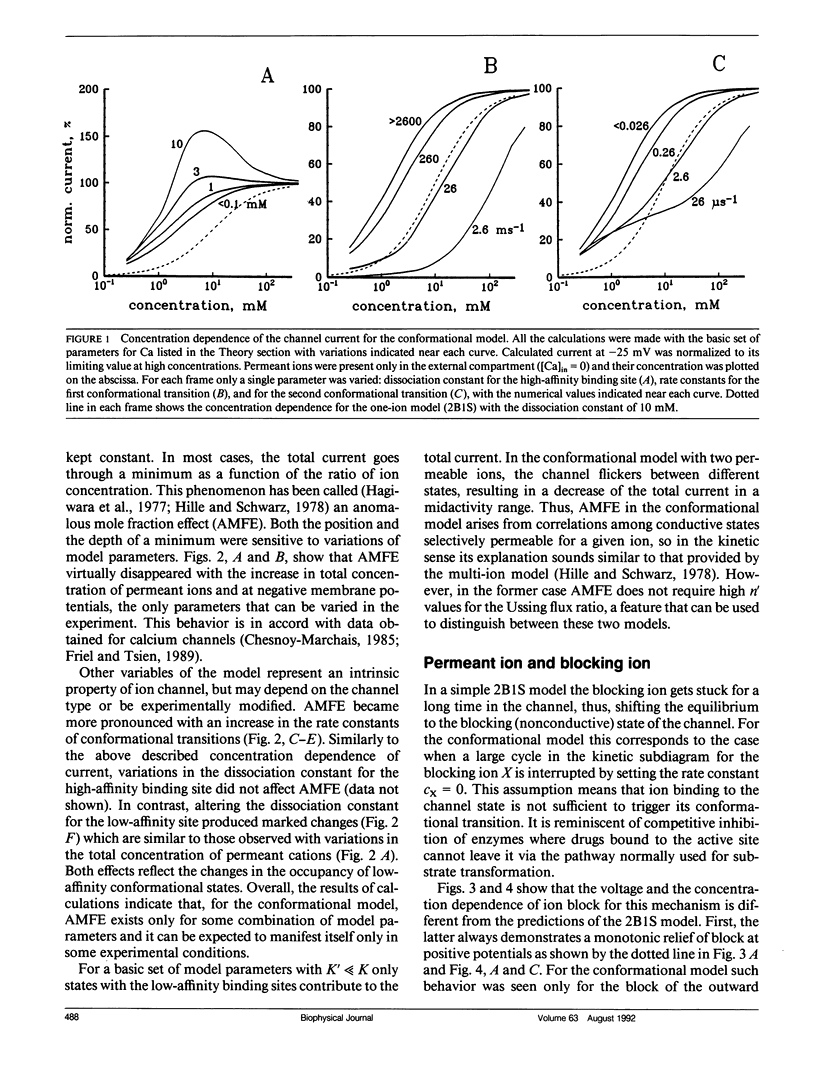
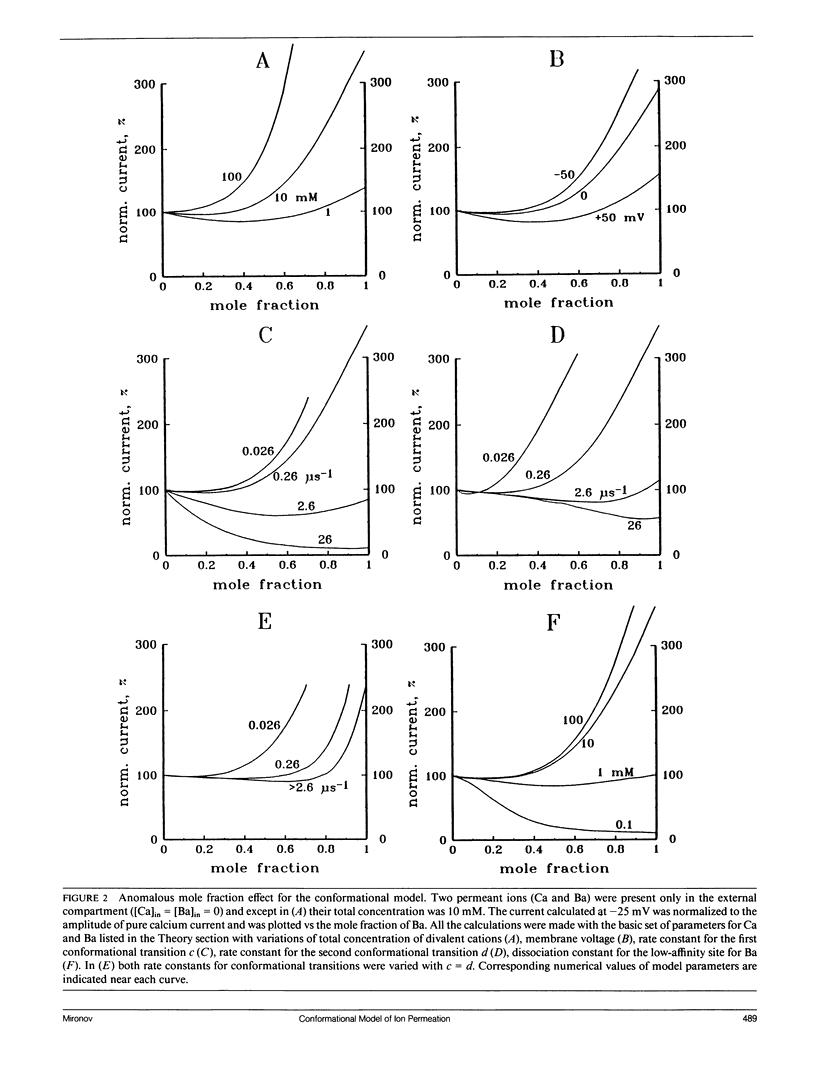
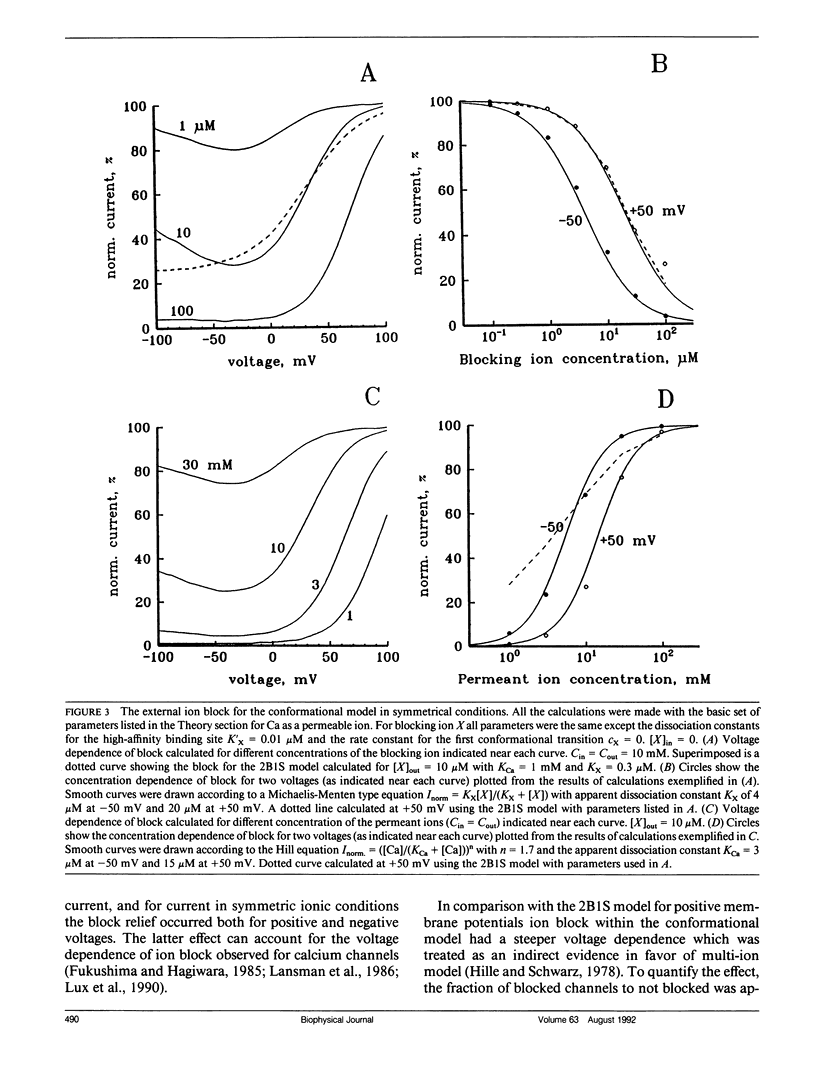
The permeation properties of ion channels existing in several conductive states were analyzed. Each state was represented by the one-ion model. A special emphasis was placed on features, assumed to be indicative of a multi-ion mode of channel occupancy such as a deviation of concentration dependence of channel conductance from the Michaelis-Menten equation, an anomalous mole fraction effect, a strong voltage dependence of ion block and coupling of unidirectional fluxes (anomalous Ussing flux ratio). The conformational model was shown to have all these properties. The ion permeation through voltage-sensitive calcium channels fulfilled all the characteristics of the model proposed.
Full text
PDF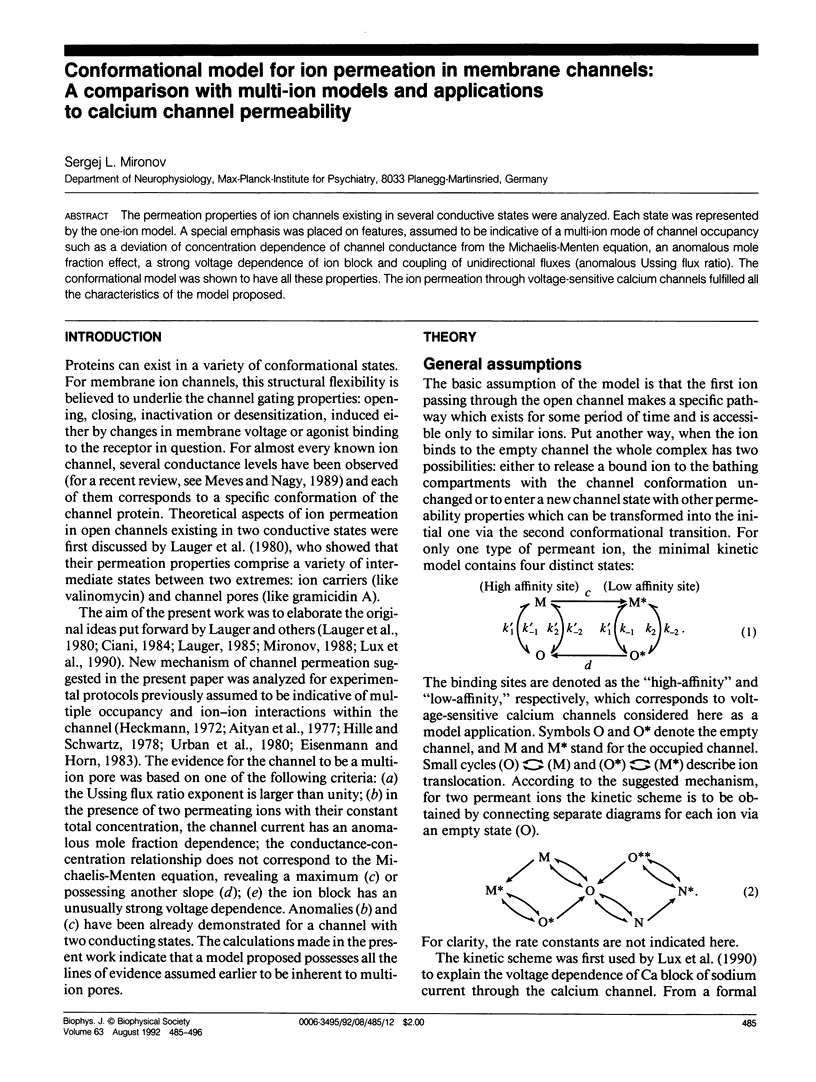






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., McCleskey E. W. Non-selective conductance in calcium channels of frog muscle: calcium selectivity in a single-file pore. J Physiol. 1984 Aug;353:585–608. doi: 10.1113/jphysiol.1984.sp015352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer S. J., Cafiso D. S. Voltage-dependent conductance for alamethicin in phospholipid vesicles. A test for the mechanism of gating. Biophys J. 1991 Aug;60(2):380–388. doi: 10.1016/S0006-3495(91)82063-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begenisich T., De Weer P. Potassium flux ratio in voltage-clamped squid giant axons. J Gen Physiol. 1980 Jul;76(1):83–98. doi: 10.1085/jgp.76.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickmann J., Fischer W. Entropy effects on the ion-diffusion rate in transmembrane protein channels. Biophys Chem. 1983 Apr;17(3):245–258. doi: 10.1016/0301-4622(83)87007-0. [DOI] [PubMed] [Google Scholar]
- Carbone E., Lux H. D. Omega-conotoxin blockade distinguishes Ca from Na permeable states in neuronal calcium channels. Pflugers Arch. 1988 Nov;413(1):14–22. doi: 10.1007/BF00581223. [DOI] [PubMed] [Google Scholar]
- Chesnoy-Marchais D. Kinetic properties and selectivity of calcium-permeable single channels in Aplysia neurones. J Physiol. 1985 Oct;367:457–488. doi: 10.1113/jphysiol.1985.sp015835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciani S. Coupling between fluxes in one-particle pores with fluctuating energy profiles. A theoretical study. Biophys J. 1984 Aug;46(2):249–252. doi: 10.1016/S0006-3495(84)84017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draber S., Schultze R., Hansen U. P. Patch-clamp studies on the anomalous mole fraction effect of the K+ channel in cytoplasmic droplets of Nitella: an attempt to distinguish between a multi-ion single-file pore and an enzyme kinetic model with lazy state. J Membr Biol. 1991 Aug;123(2):183–190. doi: 10.1007/BF01998088. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. S. Channels as enzymes. J Membr Biol. 1990 Apr;115(1):1–12. doi: 10.1007/BF01869101. [DOI] [PubMed] [Google Scholar]
- Eisenman G., Horn R. Ionic selectivity revisited: the role of kinetic and equilibrium processes in ion permeation through channels. J Membr Biol. 1983;76(3):197–225. doi: 10.1007/BF01870364. [DOI] [PubMed] [Google Scholar]
- Erijman L., Weber G. Oligomeric protein associations: transition from stochastic to deterministic equilibrium. Biochemistry. 1991 Feb 12;30(6):1595–1599. doi: 10.1021/bi00220a022. [DOI] [PubMed] [Google Scholar]
- Finkelstein A., Andersen O. S. The gramicidin A channel: a review of its permeability characteristics with special reference to the single-file aspect of transport. J Membr Biol. 1981 Apr 30;59(3):155–171. doi: 10.1007/BF01875422. [DOI] [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- Friel D. D., Tsien R. W. Voltage-gated calcium channels: direct observation of the anomalous mole fraction effect at the single-channel level. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5207–5211. doi: 10.1073/pnas.86.13.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y., Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. J Physiol. 1985 Jan;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. The potassium permeability of a giant nerve fibre. J Physiol. 1955 Apr 28;128(1):61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Krasne S., Ciani S. Anomalous permeabilities of the egg cell membrane of a starfish in K+-Tl+ mixtures. J Gen Physiol. 1977 Sep;70(3):269–281. doi: 10.1085/jgp.70.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckmann K. Single file diffusion. Biomembranes. 1972;3:127–153. doi: 10.1007/978-1-4684-0961-1_9. [DOI] [PubMed] [Google Scholar]
- Hess P., Tsien R. W. Mechanism of ion permeation through calcium channels. 1984 May 31-Jun 6Nature. 309(5967):453–456. doi: 10.1038/309453a0. [DOI] [PubMed] [Google Scholar]
- Hille B., Schwarz W. Potassium channels as multi-ion single-file pores. J Gen Physiol. 1978 Oct;72(4):409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson E., Chiu S. W. Stochastic theory of ion movement in channels with single-ion occupancy. Application to sodium permeation of gramicidin channels. Biophys J. 1987 Jul;52(1):33–45. doi: 10.1016/S0006-3495(87)83186-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Mironov S. L. Some predictions concerning the calcium channel model with different conformational states. Gen Physiol Biophys. 1986 Dec;5(6):649–654. [PubMed] [Google Scholar]
- Lansman J. B., Hess P., Tsien R. W. Blockade of current through single calcium channels by Cd2+, Mg2+, and Ca2+. Voltage and concentration dependence of calcium entry into the pore. J Gen Physiol. 1986 Sep;88(3):321–347. doi: 10.1085/jgp.88.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn J. M. Supramolecular chemistry: receptors, catalysts, and carriers. Science. 1985 Feb 22;227(4689):849–856. doi: 10.1126/science.227.4689.849. [DOI] [PubMed] [Google Scholar]
- Lux H. D., Carbone E., Zucker H. Na+ currents through low-voltage-activated Ca2+ channels of chick sensory neurones: block by external Ca2+ and Mg2+. J Physiol. 1990 Nov;430:159–188. doi: 10.1113/jphysiol.1990.sp018287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P. Ion transport through pores: a rate-theory analysis. Biochim Biophys Acta. 1973 Jul 6;311(3):423–441. doi: 10.1016/0005-2736(73)90323-4. [DOI] [PubMed] [Google Scholar]
- Läuger P. Ionic channels with conformational substates. Biophys J. 1985 May;47(5):581–590. doi: 10.1016/S0006-3495(85)83954-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P., Stephan W., Frehland E. Fluctuations of barrier structure in ionic channels. Biochim Biophys Acta. 1980 Oct 16;602(1):167–180. doi: 10.1016/0005-2736(80)90299-0. [DOI] [PubMed] [Google Scholar]
- Meves H., Nagy K. Multiple conductance states of the sodium channel and of other ion channels. Biochim Biophys Acta. 1989 Jan 18;988(1):99–105. doi: 10.1016/0304-4157(89)90005-1. [DOI] [PubMed] [Google Scholar]
- Miller C. 1990: annus mirabilis of potassium channels. Science. 1991 May 24;252(5009):1092–1096. doi: 10.1126/science.252.5009.1092. [DOI] [PubMed] [Google Scholar]
- Millhauser G. L. Reptation theory of ion channel gating. Biophys J. 1990 Apr;57(4):857–864. doi: 10.1016/S0006-3495(90)82605-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov S. L., Sokolov YuV, Chanturiya A. N., Lishko V. K. Channels produced by spider venoms in bilayer lipid membrane: mechanisms of ion transport and toxic action. Biochim Biophys Acta. 1986 Nov 6;862(1):185–198. doi: 10.1016/0005-2736(86)90482-7. [DOI] [PubMed] [Google Scholar]
- Nelson M. T. Interactions of divalent cations with single calcium channels from rat brain synaptosomes. J Gen Physiol. 1986 Feb;87(2):201–222. doi: 10.1085/jgp.87.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard E. A., Miller C. Steady-state coupling of ion-channel conformations to a transmembrane ion gradient. Science. 1990 Mar 9;247(4947):1208–1210. doi: 10.1126/science.2156338. [DOI] [PubMed] [Google Scholar]
- Sigworth F. J. Open channel noise. I. Noise in acetylcholine receptor currents suggests conformational fluctuations. Biophys J. 1985 May;47(5):709–720. doi: 10.1016/S0006-3495(85)83968-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D. L. A model of protein conformational substates. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3670–3672. doi: 10.1073/pnas.82.11.3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strynadka N. C., James M. N. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- Urban B. W., Hladky S. B., Haydon D. A. Ion movements in gramicidin pores. An example of single-file transport. Biochim Biophys Acta. 1980 Nov 4;602(2):331–354. doi: 10.1016/0005-2736(80)90316-8. [DOI] [PubMed] [Google Scholar]
- Urry D. W., Trapane T. L., Venkatachalam C. M., McMichens R. B. Ion interactions at membranous polypeptide sites using nuclear magnetic resonance: determining rate and binding constants and site locations. Methods Enzymol. 1989;171:286–342. doi: 10.1016/s0076-6879(89)71018-1. [DOI] [PubMed] [Google Scholar]
- Yamashita N., Ciani S., Hagiwara S. Effects of internal Na+ on the Ca channel outward current in mouse neoplastic B lymphocytes. J Gen Physiol. 1990 Sep;96(3):559–579. doi: 10.1085/jgp.96.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue D. T., Marban E. Permeation in the dihydropyridine-sensitive calcium channel. Multi-ion occupancy but no anomalous mole-fraction effect between Ba2+ and Ca2+. J Gen Physiol. 1990 May;95(5):911–939. doi: 10.1085/jgp.95.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]


