Abstract
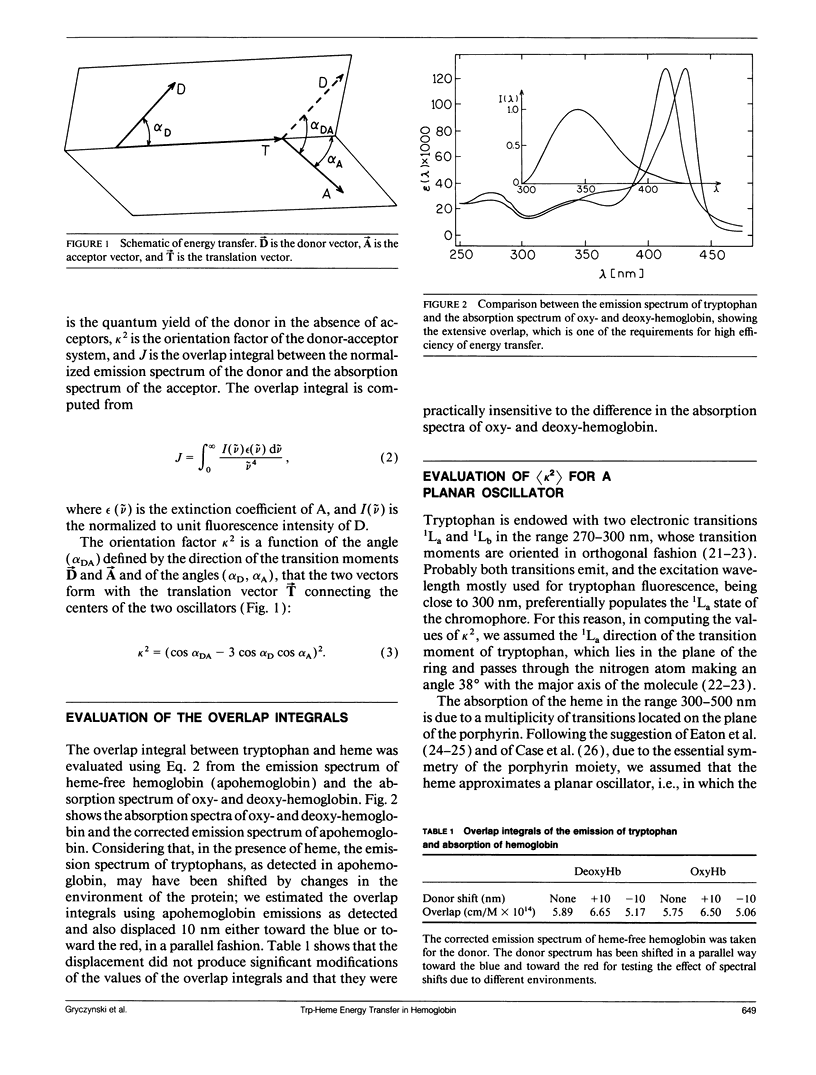
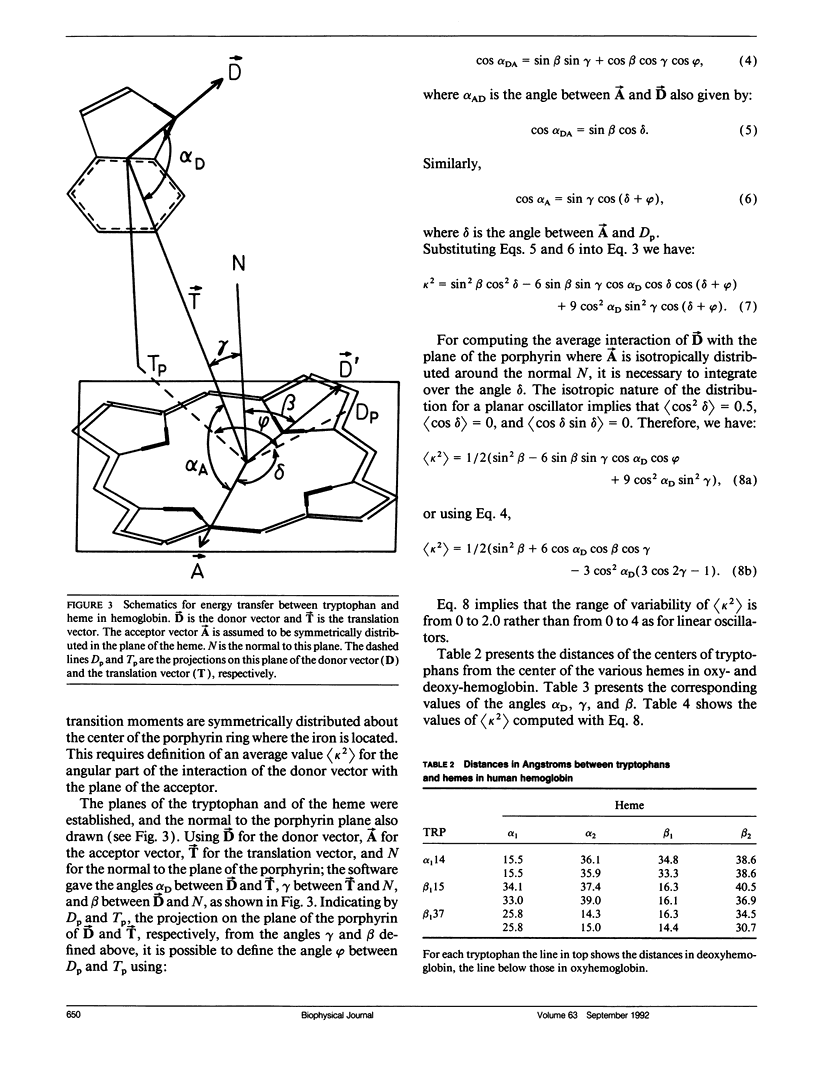
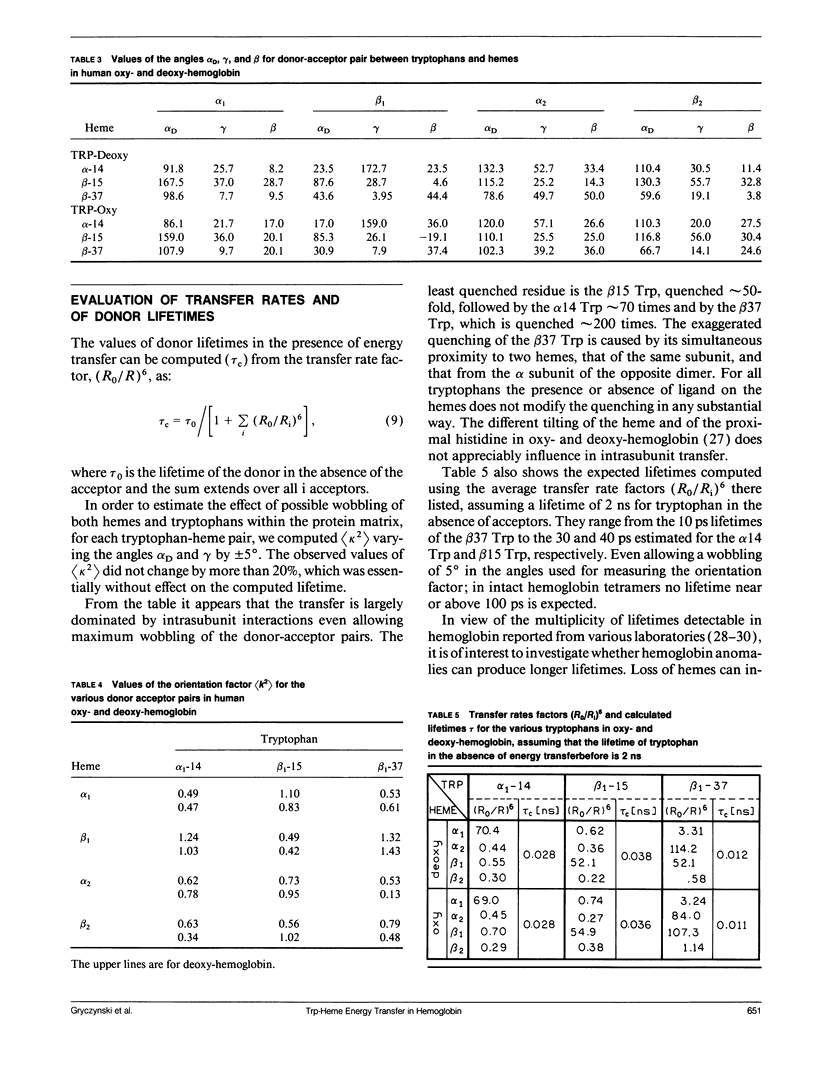
Using the Förster equations we have estimated the rate of energy transfer from tryptophans to hemes in hemoglobin. Assuming an isotropic distribution of the transition moments of the heme in the plane of the porphyrin, we computed the orientation factors and the consequent transfer rates from the crystallographic coordinates of human oxy- and deoxy-hemoglobin. It appears that the orientation factors do not play a limiting role in regulating the energy transfer and that the rates are controlled almost exclusively by the intrasubunit separations between tryptophans and hemes. In intact hemoglobin tetramers the intrasubunit separations are such as to reduce lifetimes to 5 and 15 ps/ns of tryptophan lifetime. Lifetimes of several hundred picoseconds would be allowed by the intersubunit separations, but intersubunits transfer becomes important only when one heme per tetramer is absent or does not accept transfer. If more than one heme per tetramer is absent lifetimes of more than 1 ns would appear.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J., Chothia C. Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J Mol Biol. 1979 Apr 5;129(2):175–220. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
- Bucci E., Malak H., Fronticelli C., Gryczynski I., Laczko G., Lakowicz J. R. Time-resolved emission spectra of hemoglobin on the picosecond time scale. Biophys Chem. 1988 Dec;32(2-3):187–198. doi: 10.1016/0301-4622(88)87006-6. [DOI] [PubMed] [Google Scholar]
- Bucci E., Malak H., Fronticelli C., Gryczynski I., Lakowicz J. R. Resolution of the lifetimes and correlation times of the intrinsic tryptophan fluorescence of human hemoglobin solutions using 2 GHz frequency-domain fluorometry. J Biol Chem. 1988 May 25;263(15):6972–6977. [PubMed] [Google Scholar]
- Dale R. E., Eisinger J., Blumberg W. E. The orientational freedom of molecular probes. The orientation factor in intramolecular energy transfer. Biophys J. 1979 May;26(2):161–193. doi: 10.1016/S0006-3495(79)85243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. E., Eisinger J. Intramolecular energy transfer and molecular conformation. Proc Natl Acad Sci U S A. 1976 Feb;73(2):271–273. doi: 10.1073/pnas.73.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton W. A., Hofrichter J. Polarized absorption and linear dichroism spectroscopy of hemoglobin. Methods Enzymol. 1981;76:175–261. doi: 10.1016/0076-6879(81)76126-3. [DOI] [PubMed] [Google Scholar]
- Fermi G., Perutz M. F., Shaanan B., Fourme R. The crystal structure of human deoxyhaemoglobin at 1.74 A resolution. J Mol Biol. 1984 May 15;175(2):159–174. doi: 10.1016/0022-2836(84)90472-8. [DOI] [PubMed] [Google Scholar]
- Haas E., Wilchek M., Katchalski-Katzir E., Steinberg I. Z. Distribution of end-to-end distances of oligopeptides in solution as estimated by energy transfer. Proc Natl Acad Sci U S A. 1975 May;72(5):1807–1811. doi: 10.1073/pnas.72.5.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaanan B. Structure of human oxyhaemoglobin at 2.1 A resolution. J Mol Biol. 1983 Nov 25;171(1):31–59. doi: 10.1016/s0022-2836(83)80313-1. [DOI] [PubMed] [Google Scholar]
- Steinberg I. Z. Long-range nonradiative transfer of electronic excitation energy in proteins and polypeptides. Annu Rev Biochem. 1971;40:83–114. doi: 10.1146/annurev.bi.40.070171.000503. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- TEALE F. W. Cleavage of the haem-protein link by acid methylethylketone. Biochim Biophys Acta. 1959 Oct;35:543–543. doi: 10.1016/0006-3002(59)90407-x. [DOI] [PubMed] [Google Scholar]
- Valeur B., Weber G. Resolution of the fluorescence excitation spectrum of indole into the 1La and 1Lb excitation bands. Photochem Photobiol. 1977 May;25(5):441–444. doi: 10.1111/j.1751-1097.1977.tb09168.x. [DOI] [PubMed] [Google Scholar]
- Willis K. J., Szabo A. G., Zuker M., Ridgeway J. M., Alpert B. Fluorescence decay kinetics of the tryptophyl residues of myoglobin: effect of heme ligation and evidence for discrete lifetime components. Biochemistry. 1990 Jun 5;29(22):5270–5275. doi: 10.1021/bi00474a008. [DOI] [PubMed] [Google Scholar]


