Abstract
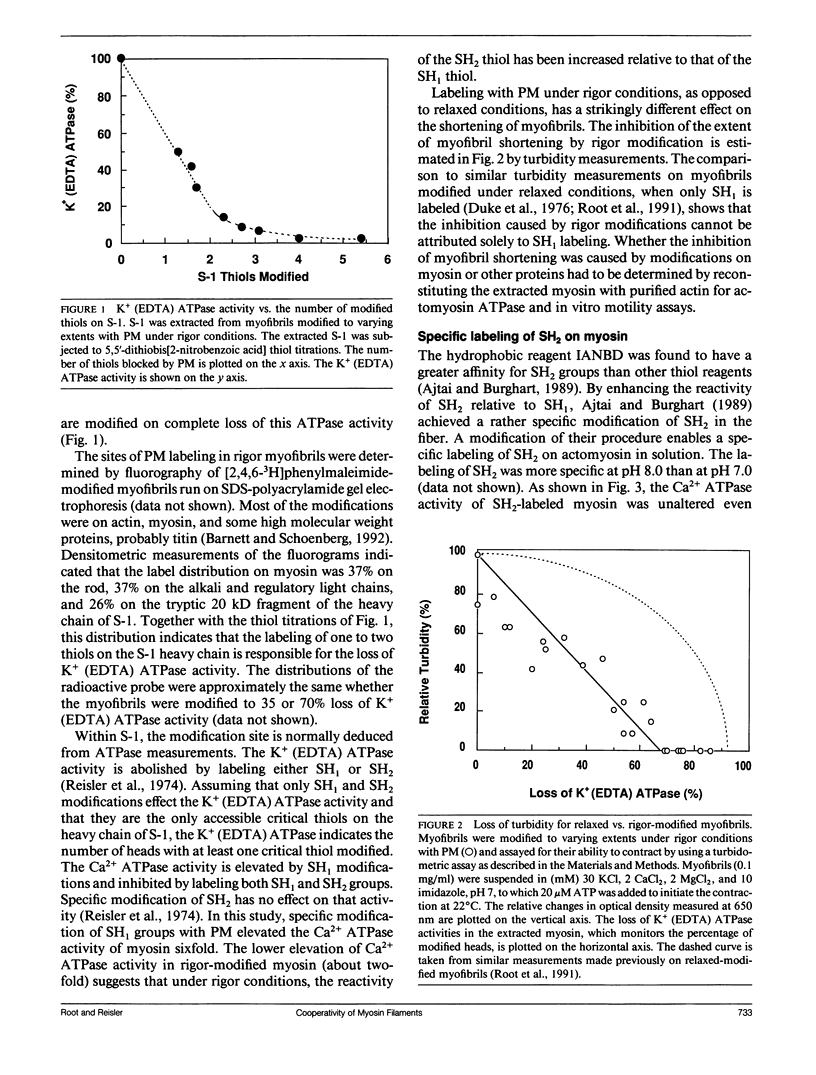
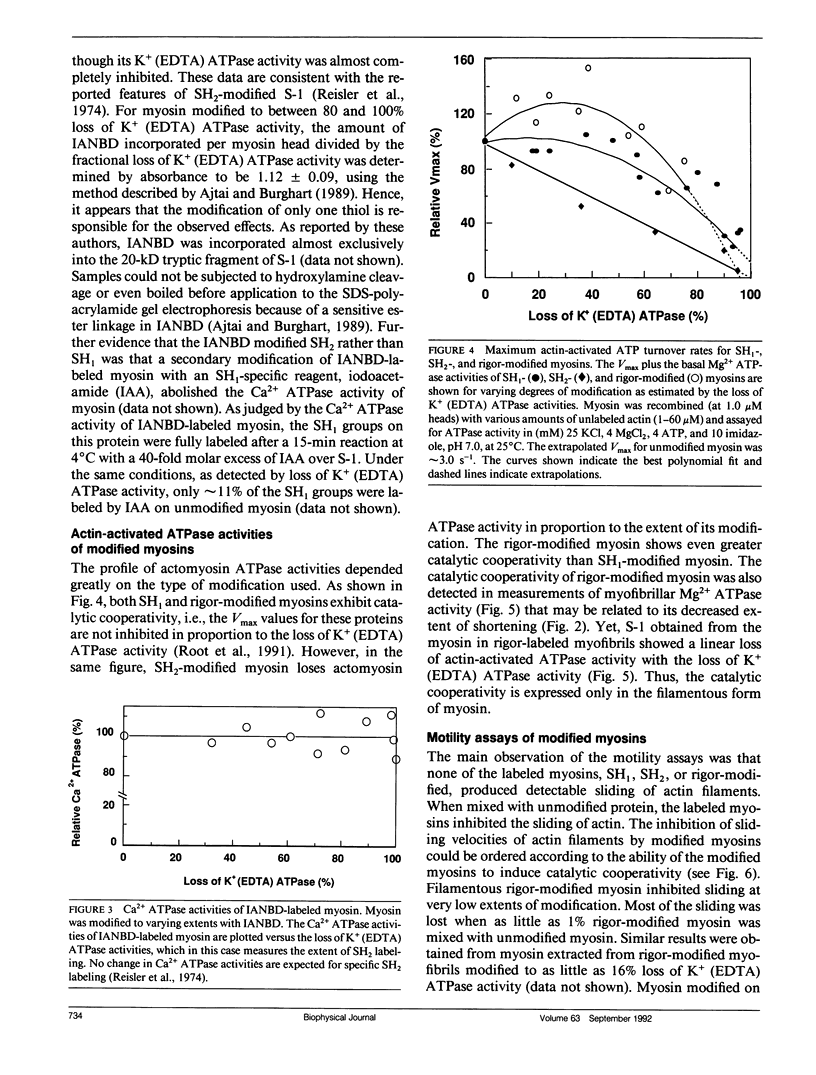
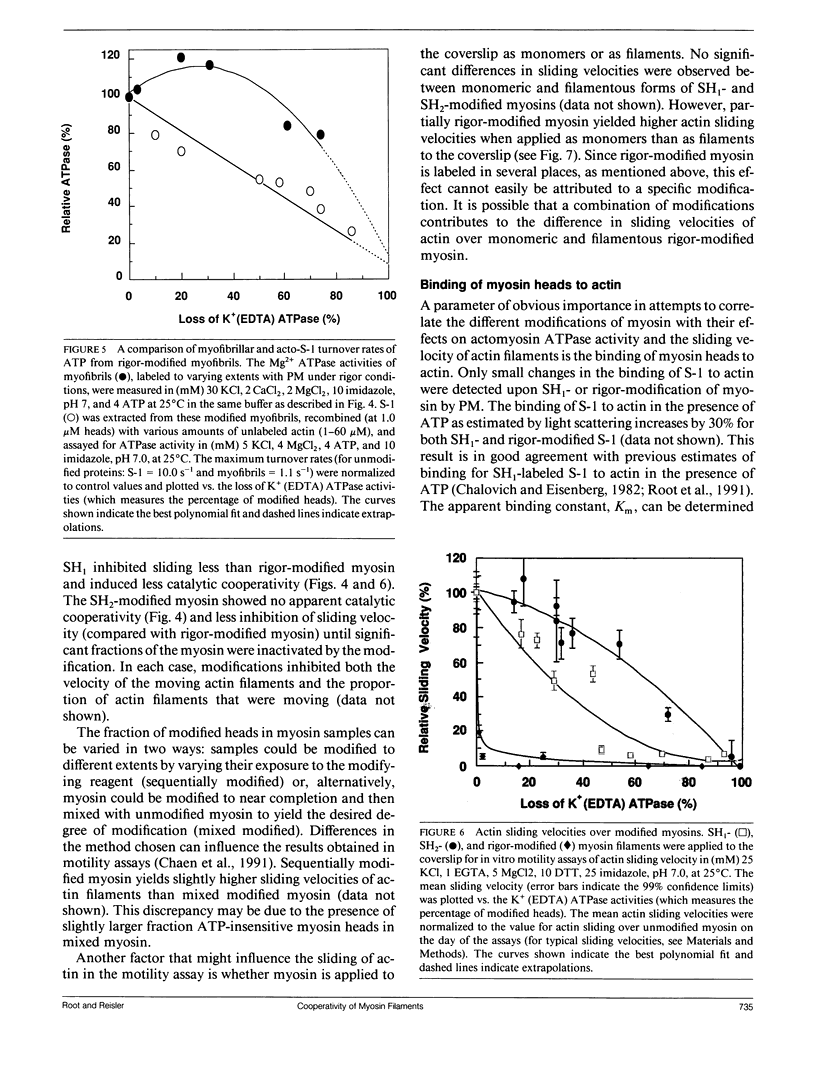
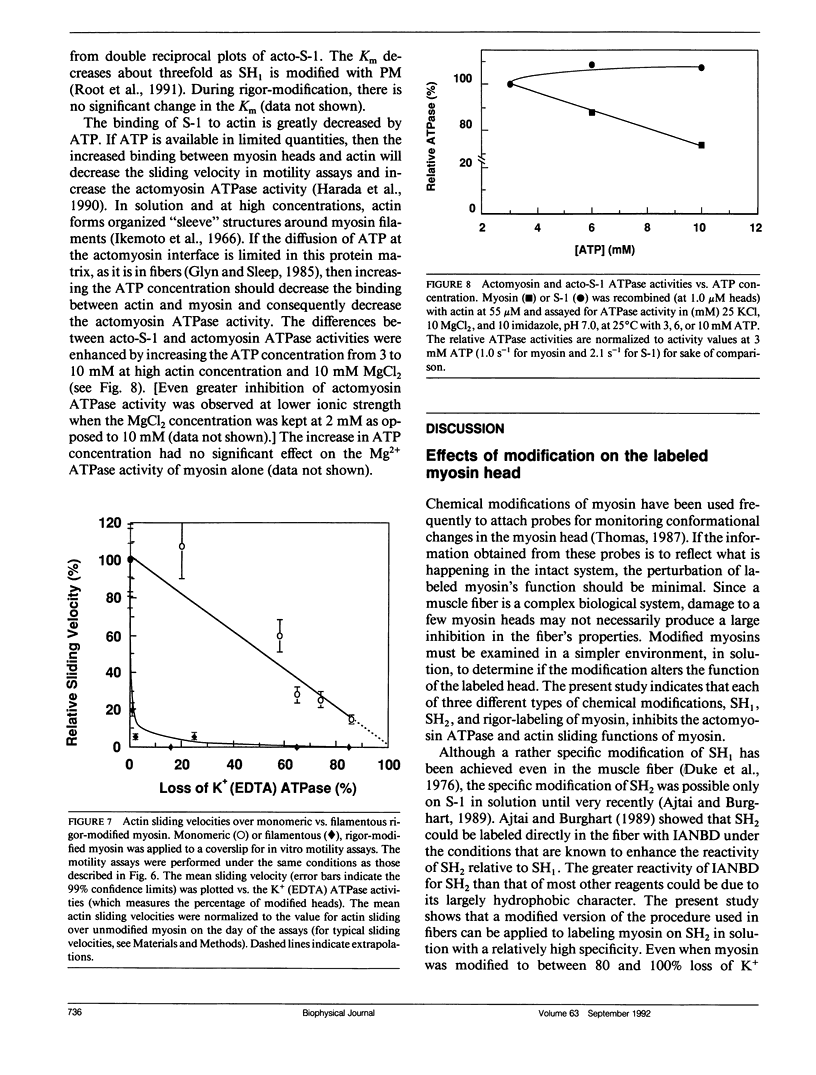
The effects of chemical modifications of myosin's reactive cysteines on actomyosin adenosine triphosphatase (ATPase) activities and sliding velocities in the in vitro motility assays were examined in this work. The three types of modifications studied were 4-[N-[(iodoacetoxy)ethyl]-N-methylamino]-7-nitrobenz-2-oxa-1,3- diazole labeling of SH2 (based on Ajtai and Burghart. 1989. Biochemistry. 28:2204-2210.), phenylmaleimide labeling of SH1, and phenylmaleimide labeling of myosin in myofibrils under rigor conditions. Each type of modified myosin inhibited the sliding of actin in motility assays. The sliding velocities of actin over copolymers of modified and unmodified myosins in the motility assay were slowest with rigor-modified myosin and most rapid with SH2-labeled myosin. The actin-activated ATPase activities of similarly copolymerized myosins were lowest with SH2-labeled myosin and highest with rigor-modified myosin. The actin-activated ATPase activities of myosin subfragment-1 obtained from these modified myosins decreased in the same linear manner with the fraction of modified heads. These results are interpreted using a model in which the sliding of actin filaments over myosin filaments decreases the probability of myosin activation by actin. The sliding velocity of actin over monomeric rigor-modified myosin exceeded that over the filamentous form, which suggests for this myosin that filament structure is important for the inhibition of actin sliding in motility assays. The fact that all cysteine modifications examined inhibited the actomyosin ATPase activities and sliding velocities of actin over myosin poses questions concerning the information about the activated crossbridge obtained from probes attached to SH1 or SH2 on myosin.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajtai K., Burghardt T. P. Fluorescent modification and orientation of myosin sulfhydryl 2 in skeletal muscle fibers. Biochemistry. 1989 Mar 7;28(5):2204–2210. doi: 10.1021/bi00431a035. [DOI] [PubMed] [Google Scholar]
- Applegate D., Reisler E. Protease-sensitive regions in myosin subfragment 1. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7109–7112. doi: 10.1073/pnas.80.23.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett V. A., Ehrlich A., Schoenberg M. Formation of ATP-insensitive weakly-binding crossbridges in single rabbit psoas fibers by treatment with phenylmaleimide or para-phenylenedimaleimide. Biophys J. 1992 Feb;61(2):358–367. doi: 10.1016/S0006-3495(92)81842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M. Fluorography for the detection of radioactivity in gels. Methods Enzymol. 1984;104:460–465. doi: 10.1016/s0076-6879(84)04115-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burke M., Reisler E. Effect of nucleotide binding on the proximity of the essential sulfhydryl groups of myosin. Chemical probing of movement of residues during conformational transitions. Biochemistry. 1977 Dec 13;16(25):5559–5563. doi: 10.1021/bi00644a026. [DOI] [PubMed] [Google Scholar]
- Chalovich J. M., Eisenberg E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem. 1982 Mar 10;257(5):2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Cooke R. The mechanism of muscle contraction. CRC Crit Rev Biochem. 1986;21(1):53–118. doi: 10.3109/10409238609113609. [DOI] [PubMed] [Google Scholar]
- Crowder M. S., Cooke R. The effect of myosin sulphydryl modification on the mechanics of fibre contraction. J Muscle Res Cell Motil. 1984 Apr;5(2):131–146. doi: 10.1007/BF00712152. [DOI] [PubMed] [Google Scholar]
- Duke J., Takashi R., Ue K., Morales M. F. Reciprocal reactivities of specific thiols when actin binds to myosin. Proc Natl Acad Sci U S A. 1976 Feb;73(2):302–306. doi: 10.1073/pnas.73.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duong A. M., Reisler E. Nucleotide-induced states of myosin subfragment 1 cross-linked to actin. Biochemistry. 1989 Apr 18;28(8):3502–3509. doi: 10.1021/bi00434a053. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fajer P. G., Fajer E. A., Brunsvold N. J., Thomas D. D. Effects of AMPPNP on the orientation and rotational dynamics of spin-labeled muscle cross-bridges. Biophys J. 1988 Apr;53(4):513–524. doi: 10.1016/S0006-3495(88)83131-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glyn H., Sleep J. Dependence of adenosine triphosphatase activity of rabbit psoas muscle fibres and myofibrils on substrate concentration. J Physiol. 1985 Aug;365:259–276. doi: 10.1113/jphysiol.1985.sp015770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey J. E., Harrington W. F. Self-association in the myosin system at high ionic strength. I. Sensitivity of the interaction to pH and ionic environment. Biochemistry. 1970 Feb 17;9(4):886–893. doi: 10.1021/bi00806a025. [DOI] [PubMed] [Google Scholar]
- HILL A. V. THE EFFECT OF LOAD ON THE HEAT OF SHORTENING OF MUSCLE. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:297–318. doi: 10.1098/rspb.1964.0004. [DOI] [PubMed] [Google Scholar]
- Harada Y., Noguchi A., Kishino A., Yanagida T. Sliding movement of single actin filaments on one-headed myosin filaments. Nature. 1987 Apr 23;326(6115):805–808. doi: 10.1038/326805a0. [DOI] [PubMed] [Google Scholar]
- Harada Y., Sakurada K., Aoki T., Thomas D. D., Yanagida T. Mechanochemical coupling in actomyosin energy transduction studied by in vitro movement assay. J Mol Biol. 1990 Nov 5;216(1):49–68. doi: 10.1016/S0022-2836(05)80060-9. [DOI] [PubMed] [Google Scholar]
- Harrington W. F., Karr T., Busa W. B., Lovell S. J. Contraction of myofibrils in the presence of antibodies to myosin subfragment 2. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7453–7456. doi: 10.1073/pnas.87.19.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington W. F., Reisler E., Burke M. An activation mechanism for ATP cleavage in muscle. J Supramol Struct. 1975;3(2):112–124. doi: 10.1002/jss.400030204. [DOI] [PubMed] [Google Scholar]
- Higuchi H., Goldman Y. E. Sliding distance between actin and myosin filaments per ATP molecule hydrolysed in skinned muscle fibres. Nature. 1991 Jul 25;352(6333):352–354. doi: 10.1038/352352a0. [DOI] [PubMed] [Google Scholar]
- Homsher E. Muscle enthalpy production and its relationship to actomyosin ATPase. Annu Rev Physiol. 1987;49:673–690. doi: 10.1146/annurev.ph.49.030187.003325. [DOI] [PubMed] [Google Scholar]
- Homsher E., Wang F., Sellers J. R. Factors affecting movement of F-actin filaments propelled by skeletal muscle heavy meromyosin. Am J Physiol. 1992 Mar;262(3 Pt 1):C714–C723. doi: 10.1152/ajpcell.1992.262.3.C714. [DOI] [PubMed] [Google Scholar]
- Houadjeto M., Barman T., Travers F. What is the true ATPase activity of contracting myofibrils? FEBS Lett. 1991 Apr 9;281(1-2):105–107. doi: 10.1016/0014-5793(91)80369-e. [DOI] [PubMed] [Google Scholar]
- Huxley A. F. A note suggesting that the cross-bridge attachment during muscle contraction may take place in two stages. Proc R Soc Lond B Biol Sci. 1973 Feb 27;183(1070):83–86. doi: 10.1098/rspb.1973.0006. [DOI] [PubMed] [Google Scholar]
- KIELLEY W. W., BRADLEY L. B. The relationship between sulfhydryl groups and the activation of myosin adenosinetriphosphatase. J Biol Chem. 1956 Feb;218(2):653–659. [PubMed] [Google Scholar]
- Kominz D. R. Studies of adenosine triphosphatase activity and turbidity in myofibril and actomyosin suspensions. Biochemistry. 1970 Apr 14;9(8):1792–1801. doi: 10.1021/bi00810a019. [DOI] [PubMed] [Google Scholar]
- Kron S. J., Toyoshima Y. Y., Uyeda T. Q., Spudich J. A. Assays for actin sliding movement over myosin-coated surfaces. Methods Enzymol. 1991;196:399–416. doi: 10.1016/0076-6879(91)96035-p. [DOI] [PubMed] [Google Scholar]
- Kushmerick M. J., Davies R. E. The chemical energetics of muscle contraction. II. The chemistry, efficiency and power of maximally working sartorius muscles. Appendix. Free energy and enthalpy of atp hydrolysis in the sarcoplasm. Proc R Soc Lond B Biol Sci. 1969 Dec 23;174(1036):315–353. doi: 10.1098/rspb.1969.0096. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Kominz D. R. Reversibility of myofibrillar turbidity and ATPase activity changes. J Biochem. 1969 Mar;65(3):465–470. doi: 10.1093/oxfordjournals.jbchem.a129035. [DOI] [PubMed] [Google Scholar]
- Mendelson R., Putnam S., Morales M. Time-dependent fluorescence depolarization and lifetime studies of myosin subfragment-one in the presence of nucleotide and actin. J Supramol Struct. 1975;3(2):162–168. doi: 10.1002/jss.400030209. [DOI] [PubMed] [Google Scholar]
- Mulhern S. A., Eisenberg E. Further studies on the interaction of actin with heavy meromyosin and subfragment 1 in the presence of ATP. Biochemistry. 1976 Dec 28;15(26):5702–5708. doi: 10.1021/bi00671a004. [DOI] [PubMed] [Google Scholar]
- Mulhern S. A., Eisenberg E. Interaction of spin-labeled and N-(iodacetylaminoethyl)-5-naphthylamine-1-sulfonic acid SH1-blocked heavy meromyosin and myosin with actin and adenosine triphosphate. Biochemistry. 1978 Oct 17;17(21):4419–4425. doi: 10.1021/bi00614a010. [DOI] [PubMed] [Google Scholar]
- Pope B., Wagner P. D., Weeds A. G. Studies on the actomyosin ATPase and the role of the alkali light chains. Eur J Biochem. 1981 Jun;117(1):201–206. doi: 10.1111/j.1432-1033.1981.tb06322.x. [DOI] [PubMed] [Google Scholar]
- Prochniewicz E., Yanagida T. Inhibition of sliding movement of F-actin by crosslinking emphasizes the role of actin structure in the mechanism of motility. J Mol Biol. 1990 Dec 5;216(3):761–772. doi: 10.1016/0022-2836(90)90397-5. [DOI] [PubMed] [Google Scholar]
- Reisler E., Burke M., Harrington W. F. Cooperative role of two sulfhydryl groups in myosin adenosine triphosphatase. Biochemistry. 1974 May 7;13(10):2014–2022. doi: 10.1021/bi00707a003. [DOI] [PubMed] [Google Scholar]
- Reisler E. On the question of co-operative interaction of myosin heads with F-actin in the presence of ATP. J Mol Biol. 1980 Mar 25;138(1):93–107. doi: 10.1016/s0022-2836(80)80006-4. [DOI] [PubMed] [Google Scholar]
- Root D. D., Cheung P., Reisler E. Catalytic cooperativity induced by SH1 labeling of myosin filaments. Biochemistry. 1991 Jan 8;30(1):286–294. doi: 10.1021/bi00215a039. [DOI] [PubMed] [Google Scholar]
- Schwyter D. H., Kron S. J., Toyoshima Y. Y., Spudich J. A., Reisler E. Subtilisin cleavage of actin inhibits in vitro sliding movement of actin filaments over myosin. J Cell Biol. 1990 Aug;111(2):465–470. doi: 10.1083/jcb.111.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel J. C. The effects of actin on the electron spin resonance of spin-labeled myosin. Arch Biochem Biophys. 1973 Aug;157(2):588–596. doi: 10.1016/0003-9861(73)90678-4. [DOI] [PubMed] [Google Scholar]
- Silverman R., Eisenberg E., Kielley W. W. Interaction of SH 1 -blocked HMM with actin and ATP. Nat New Biol. 1972 Dec 13;240(102):207–208. doi: 10.1038/newbio240207a0. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Srivastava S., Wikman-Coffelt J. An investigation into the role of SH1 and SH2 groups of myosin in calcium binding and tension generation. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1383–1388. doi: 10.1016/0006-291x(80)90439-8. [DOI] [PubMed] [Google Scholar]
- Sutoh K., Harrington W. F. Cross-linking of myosin thick filaments under activating and rigor conditions. A study of the radial disposition of cross-bridges. Biochemistry. 1977 May 31;16(11):2441–2449. doi: 10.1021/bi00630a020. [DOI] [PubMed] [Google Scholar]
- Svensson E. C., Thomas D. D. ATP induces microsecond rotational motions of myosin heads crosslinked to actin. Biophys J. 1986 Nov;50(5):999–1002. doi: 10.1016/S0006-3495(86)83541-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawada K., Sekimoto K. A physical model of ATP-induced actin-myosin movement in vitro. Biophys J. 1991 Feb;59(2):343–356. doi: 10.1016/S0006-3495(91)82228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Cooke R. Orientation of spin-labeled myosin heads in glycerinated muscle fibers. Biophys J. 1980 Dec;32(3):891–906. doi: 10.1016/S0006-3495(80)85024-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Ishiwata S., Seidel J. C., Gergely J. Submillisecond rotational dynamics of spin-labeled myosin heads in myofibrils. Biophys J. 1980 Dec;32(3):873–889. doi: 10.1016/S0006-3495(80)85023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D. Spectroscopic probes of muscle cross-bridge rotation. Annu Rev Physiol. 1987;49:691–709. doi: 10.1146/annurev.ph.49.030187.003355. [DOI] [PubMed] [Google Scholar]
- Titus M. A., Ashiba G., Szent-Györgyi A. G. SH-1 modification of rabbit myosin interferes with calcium regulation. J Muscle Res Cell Motil. 1989 Feb;10(1):25–33. doi: 10.1007/BF01739854. [DOI] [PubMed] [Google Scholar]
- Umemoto S., Sellers J. R. Characterization of in vitro motility assays using smooth muscle and cytoplasmic myosins. J Biol Chem. 1990 Sep 5;265(25):14864–14869. [PubMed] [Google Scholar]
- Uyeda T. Q., Kron S. J., Spudich J. A. Myosin step size. Estimation from slow sliding movement of actin over low densities of heavy meromyosin. J Mol Biol. 1990 Aug 5;214(3):699–710. doi: 10.1016/0022-2836(90)90287-V. [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Weeds A. G. Studies on the role of myosin alkali light chains. Recombination and hybridization of light chains and heavy chains in subfragment-1 preparations. J Mol Biol. 1977 Jan 25;109(3):455–470. doi: 10.1016/s0022-2836(77)80023-5. [DOI] [PubMed] [Google Scholar]
- Warshaw D. M., Desrosiers J. M., Work S. S., Trybus K. M. Smooth muscle myosin cross-bridge interactions modulate actin filament sliding velocity in vitro. J Cell Biol. 1990 Aug;111(2):453–463. doi: 10.1083/jcb.111.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]
- Wells J. A., Yount R. G. Reaction of 5,5'-dithiobis(2-nitrobenzoic acid) with myosin subfragment one: evidence for formation of a single protein disulfide with trapping of metal nucleotide at the active site. Biochemistry. 1980 Apr 15;19(8):1711–1717. doi: 10.1021/bi00549a030. [DOI] [PubMed] [Google Scholar]
- West J. J., Nagy B., Gergely J. Free adenosine diphosphate as an intermediary in the phosphorylation by creatine phosphate of adenosine diphosphate bound to actin. J Biol Chem. 1967 Mar 25;242(6):1140–1145. [PubMed] [Google Scholar]


