Abstract
The picosecond time-domain incoherent singlet excitation transfer and trapping kinetics in core antenna of photosynthetic bacteria are studied in case of low excitation intensities by numerical integration of the appropriate master equation in a wide temperature range of 4-300 K. The essential features of our two-dimensional-lattice model are as follows: Förster excitation transfer theory, spectral heterogeneity of both the light-harvesting antenna and the reaction center, treatment of temperature effects through temperature dependence of spectral bands, inclusion of inner structure of the trap, and transition dipole moment orientation. The fluorescence kinetics is analyzed in terms of distributions of various kinetic components, and the influence of different inhomogeneities (orientational, spectral) is studied.
A reasonably good agreement between theoretical and experimental fluorescence decay kinetics for purple photosynthetic bacterium Rhodospirillum rubrum is achieved at high temperatures by assuming relatively large antenna spectral inhomogeneity: 20 nm at the whole bandwidth of 40 nm. The mean residence time in the antenna lattice site (it is assumed to be the aggregate of four bacteriochlorophyll a molecules bound to proteins) is estimated to be ∼12 ps. At 4 K only qualitative agreement between model and experiment is gained. The failure of quantitative fitting is perhaps due to the lack of knowledge about the real structure of antenna or local heating and cooling effects not taken into account.
Full text
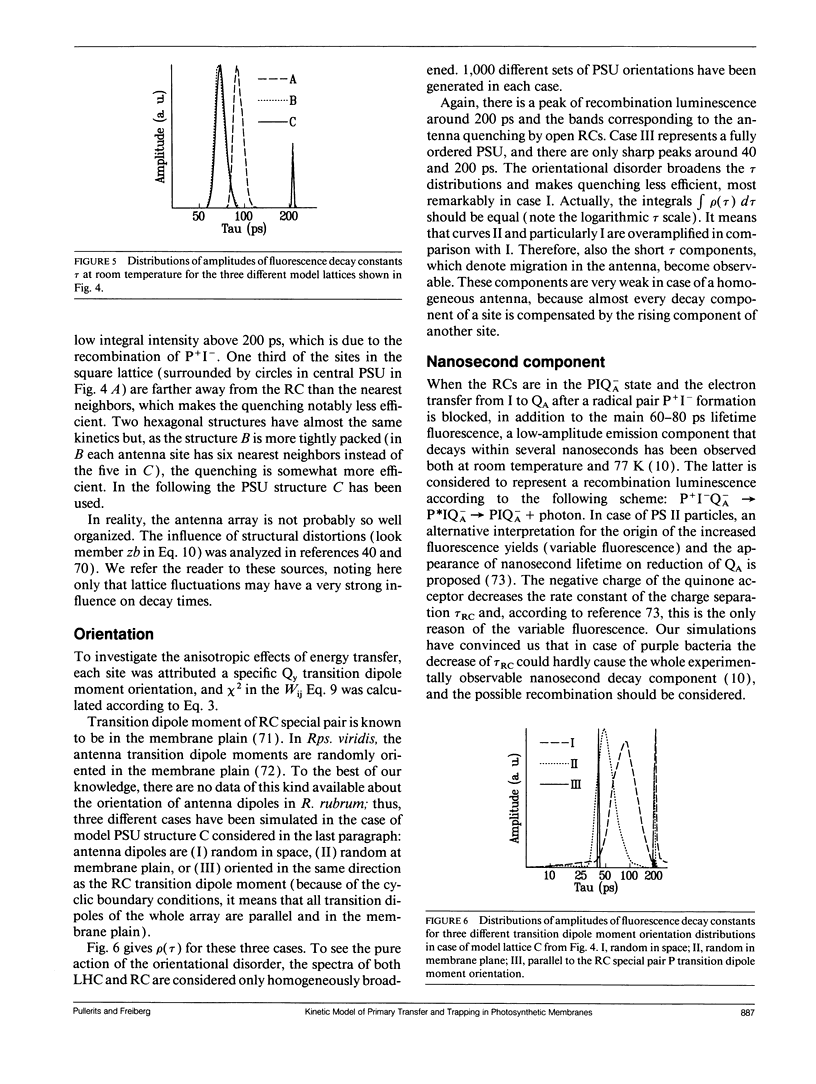
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
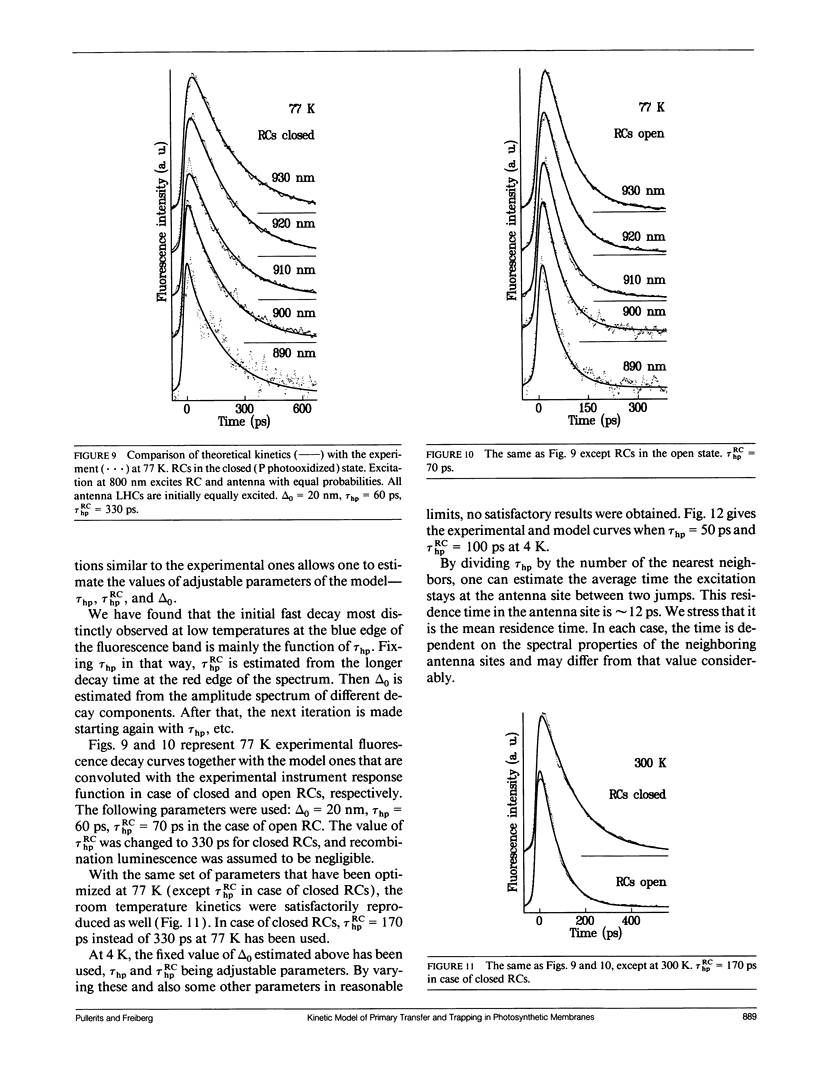
- Chang M. C., Callahan P. M., Parkes-Loach P. S., Cotton T. M., Loach P. A. Spectroscopic characterization of the light-harvesting complex of Rhodospirillum rubrum and its structural subunit. Biochemistry. 1990 Jan 16;29(2):421–429. doi: 10.1021/bi00454a017. [DOI] [PubMed] [Google Scholar]
- Friesner R. A., Won Y. D. Spectroscopy and electron transfer dynamics of the bacterial photosynthetic reaction center. Biochim Biophys Acta. 1989 Nov 23;977(2):99–122. doi: 10.1016/s0005-2728(89)80062-3. [DOI] [PubMed] [Google Scholar]
- Gingras G., Picorel R. Supramolecular arrangement of Rhodospirillum rubrum B880 holochrome as studied by radiation inactivation and electron paramagnetic resonance. Proc Natl Acad Sci U S A. 1990 May;87(9):3405–3409. doi: 10.1073/pnas.87.9.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goedheer J. C. Fluorescence polarization and pigment orientation in photosynthetic bacteria. Biochim Biophys Acta. 1973 Apr 5;292(3):665–676. doi: 10.1016/0005-2728(73)90014-5. [DOI] [PubMed] [Google Scholar]
- Goedheer J. C. Temperature dependence of absorption and fluorescence spectra of bacteriochlorophylls in vivo and in vitro. Biochim Biophys Acta. 1972 Aug 17;275(2):169–176. doi: 10.1016/0005-2728(72)90037-0. [DOI] [PubMed] [Google Scholar]
- Grad J, Hernandez G, Mukamel S. Radiative decay and energy transfer in molecular aggregates: The role of intermolecular dephasing. Phys Rev A Gen Phys. 1988 May 15;37(10):3835–3846. doi: 10.1103/physreva.37.3835. [DOI] [PubMed] [Google Scholar]
- Henry E. R., Eaton W. A., Hochstrasser R. M. Molecular dynamics simulations of cooling in laser-excited heme proteins. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8982–8986. doi: 10.1073/pnas.83.23.8982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzwarth A. R., Bittersmann E., Reuter W., Wehrmeyer W. Studies on chromophore coupling in isolated phycobiliproteins: III. Picosecond excited state kinetics and time-resolved fluorescence spectra of different allophycocyanins from Mastigocladus laminosus. Biophys J. 1990 Jan;57(1):133–145. doi: 10.1016/S0006-3495(90)82514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber DL, Ching WY. Time-domain analysis of the dynamics of Frenkel excitons in disordered systems. Phys Rev B Condens Matter. 1990 Nov 1;42(13):7718–7724. doi: 10.1103/physrevb.42.7718. [DOI] [PubMed] [Google Scholar]
- Hunter C. N., van Grondelle R., Olsen J. D. Photosynthetic antenna proteins: 100 ps before photochemistry starts. Trends Biochem Sci. 1989 Feb;14(2):72–76. doi: 10.1016/0968-0004(89)90047-9. [DOI] [PubMed] [Google Scholar]
- Jean J. M., Chan C. K., Fleming G. R., Owens T. G. Excitation transport and trapping on spectrally disordered lattices. Biophys J. 1989 Dec;56(6):1203–1215. doi: 10.1016/S0006-3495(89)82767-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkre VM, Schmid D. Coherence in singlet-exciton motion in anthracene crystals. Phys Rev B Condens Matter. 1985 Feb 15;31(4):2430–2436. doi: 10.1103/physrevb.31.2430. [DOI] [PubMed] [Google Scholar]
- Kirmaier C., Holten D. Evidence that a distribution of bacterial reaction centers underlies the temperature and detection-wavelength dependence of the rates of the primary electron-transfer reactions. Proc Natl Acad Sci U S A. 1990 May;87(9):3552–3556. doi: 10.1073/pnas.87.9.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudzmauskas S., Valkunas L., Borisov A. Y. A theory of excitation transfer in photosynthetic units. J Theor Biol. 1983 Nov 7;105(1):13–23. doi: 10.1016/0022-5193(83)90421-6. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Hinchigeri S. B., Parkes-Loach P. S., Callahan P. M., Sprinkle J. R., Riccobono J. R., Loach P. A. Isolation and characterization of a subunit form of the light-harvesting complex of Rhodospirillum rubrum. Biochemistry. 1987 Aug 11;26(16):5055–5062. doi: 10.1021/bi00390a026. [DOI] [PubMed] [Google Scholar]
- Monger T. G., Parson W. W. Singlet-triplet fusion in Rhodopseudomonas sphaeroides chromatophores. A probe of the organization of the photosynthetic apparatus. Biochim Biophys Acta. 1977 Jun 9;460(3):393–407. doi: 10.1016/0005-2728(77)90080-9. [DOI] [PubMed] [Google Scholar]
- Movaghar B, Grünewald M, Ries B, Bassler H, Würtz D. Diffusion and relaxation of energy in disordered organic and inorganic materials. Phys Rev B Condens Matter. 1986 Apr 15;33(8):5545–5554. doi: 10.1103/physrevb.33.5545. [DOI] [PubMed] [Google Scholar]
- Ormos P., Ansari A., Braunstein D., Cowen B. R., Frauenfelder H., Hong M. K., Iben I. E., Sauke T. B., Steinbach P. J., Young R. D. Inhomogeneous broadening in spectral bands of carbonmonoxymyoglobin. The connection between spectral and functional heterogeneity. Biophys J. 1990 Feb;57(2):191–199. doi: 10.1016/S0006-3495(90)82522-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein R. M. Migration and trapping of excitation quanta in photosynthetic units. Brookhaven Symp Biol. 1966;19:8–15. [PubMed] [Google Scholar]
- Petrich J. W., Martin J. L., Houde D., Poyart C., Orszag A. Time-resolved Raman spectroscopy with subpicosecond resolution: vibrational cooling and delocalization of strain energy in photodissociated (carbonmonoxy)hemoglobin. Biochemistry. 1987 Dec 1;26(24):7914–7923. doi: 10.1021/bi00398a056. [DOI] [PubMed] [Google Scholar]
- Robinson G. W. Excitation transfer and trapping in photosynthesis. Brookhaven Symp Biol. 1966;19:16–48. [PubMed] [Google Scholar]
- Schenck C. C., Parson W. W., Holten D., Windsor M. W., Sarai A. Temperature dependence of electron transfer between bacteriopheophytin and ubiquinone in protonated and deuterated reaction centers of Rhodopseudomonas sphaeroides. Biophys J. 1981 Dec;36(3):479–489. doi: 10.1016/S0006-3495(81)84747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seely G. R. Effects of spectral variety and molecular orientation on energy trapping in the photosynthetic unit: a model calculation. J Theor Biol. 1973 Jul;40(1):173–187. doi: 10.1016/0022-5193(73)90170-7. [DOI] [PubMed] [Google Scholar]
- Tronrud D. E., Schmid M. F., Matthews B. W. Structure and X-ray amino acid sequence of a bacteriochlorophyll A protein from Prosthecochloris aestuarii refined at 1.9 A resolution. J Mol Biol. 1986 Apr 5;188(3):443–454. doi: 10.1016/0022-2836(86)90167-1. [DOI] [PubMed] [Google Scholar]
- Woodbury N. W., Parson W. W. Nanosecond fluorescence from isolated photosynthetic reaction centers of Rhodopseudomonas sphaeroides. Biochim Biophys Acta. 1984 Nov 26;767(2):345–361. doi: 10.1016/0005-2728(84)90205-6. [DOI] [PubMed] [Google Scholar]


