Abstract
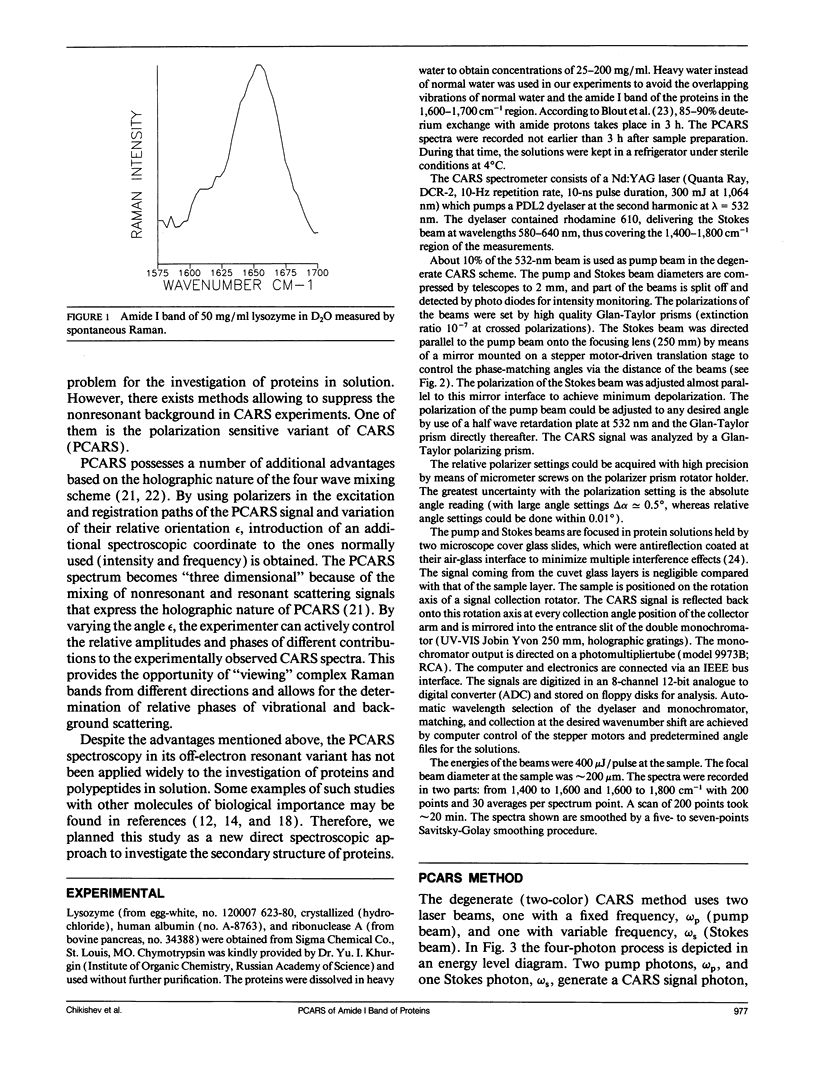
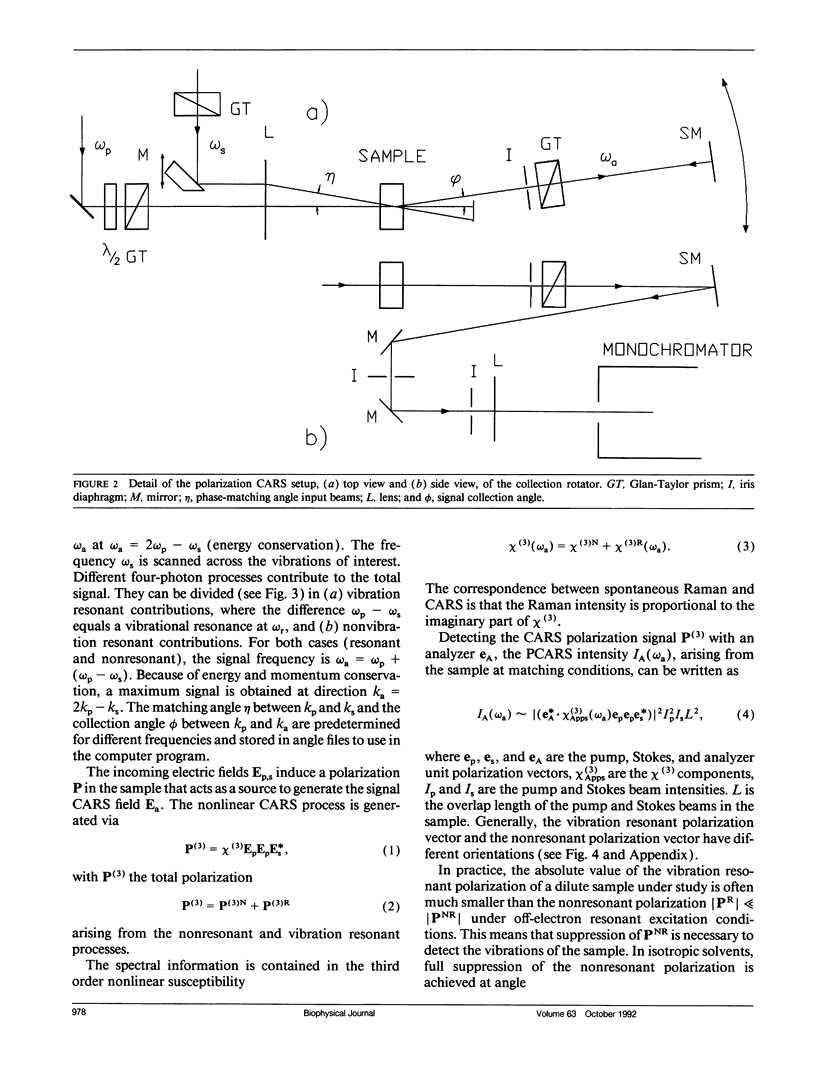
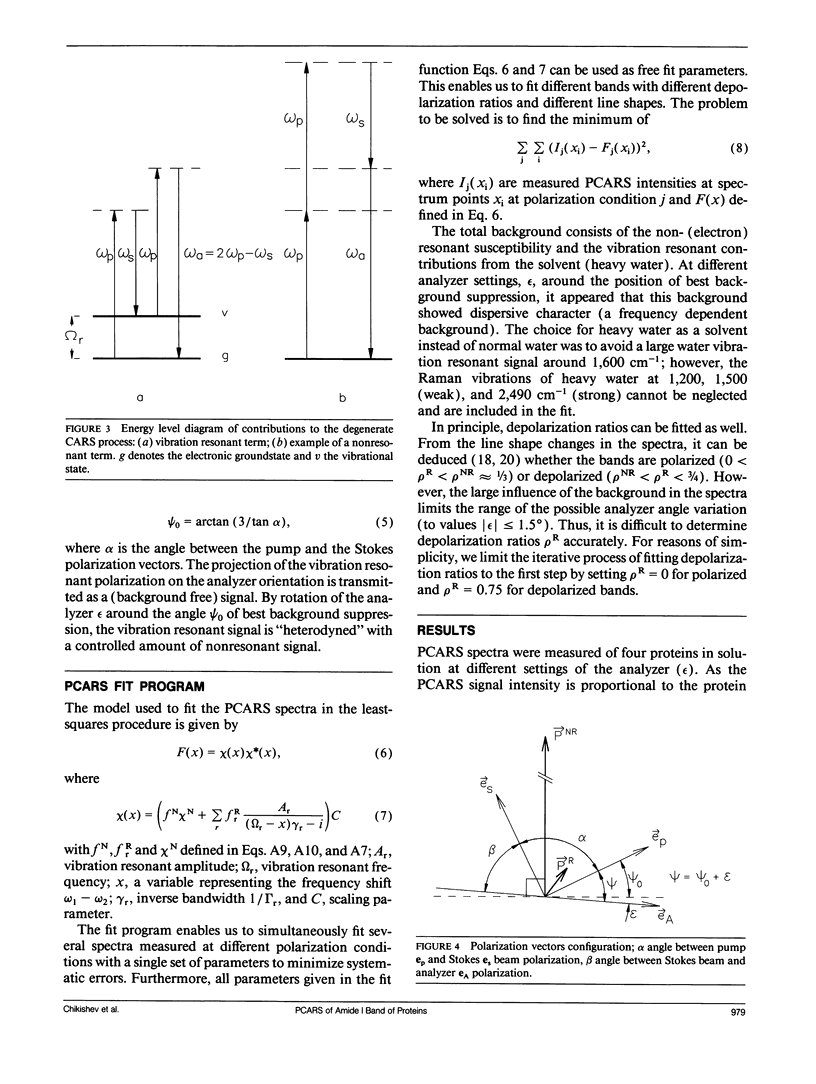
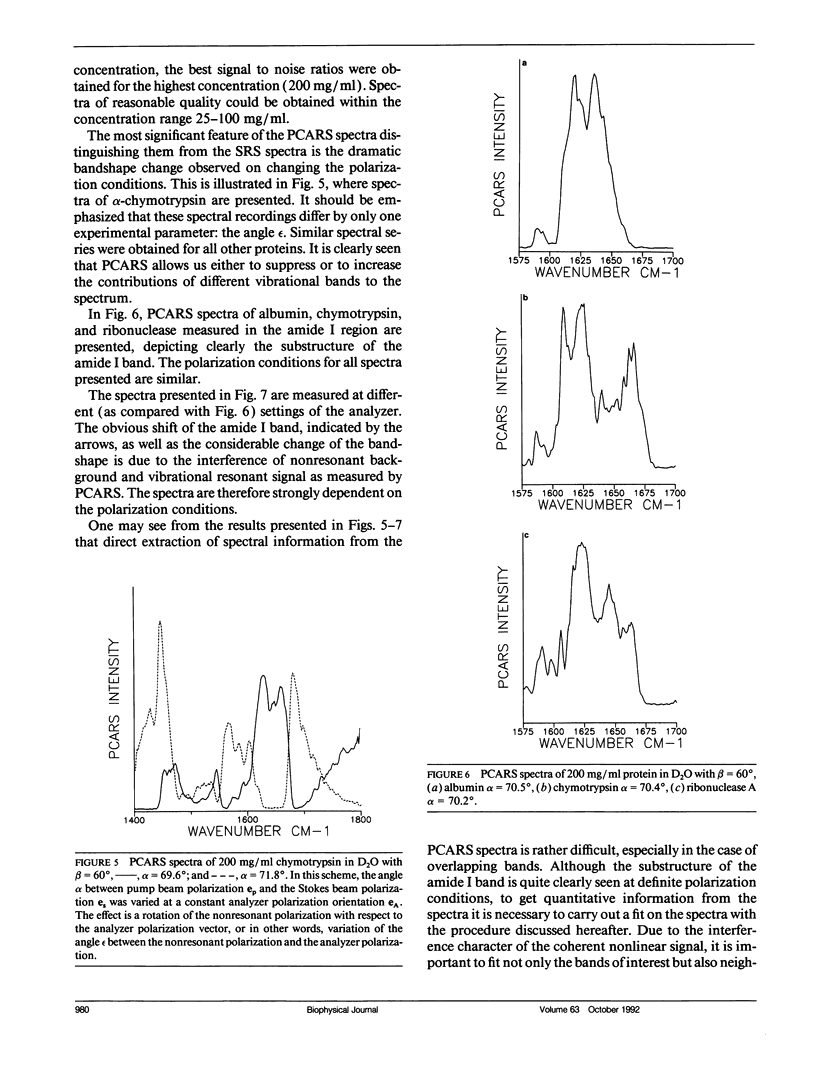
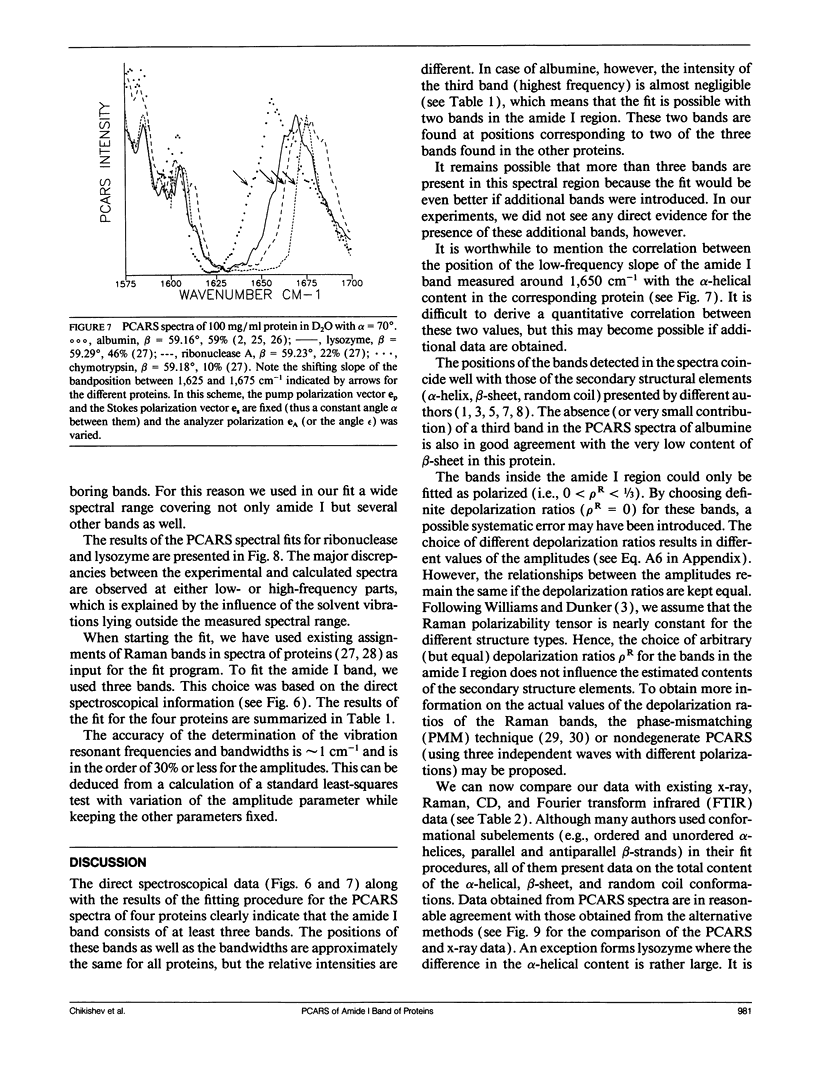
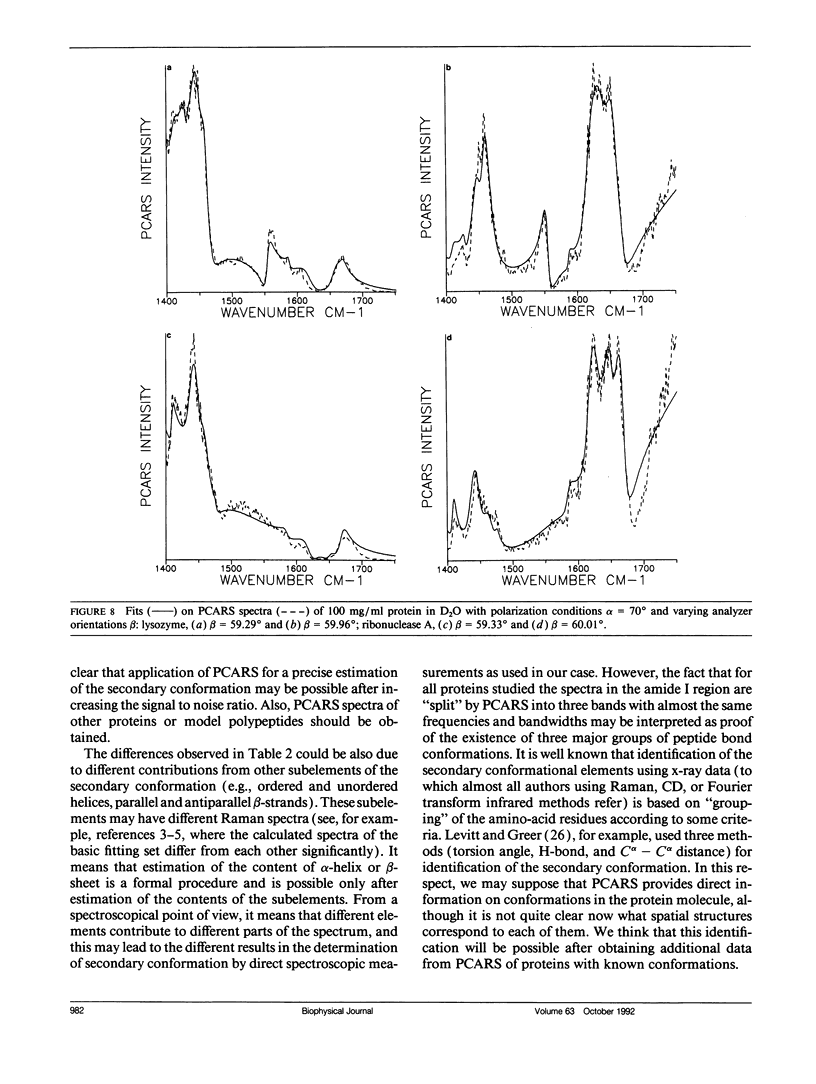
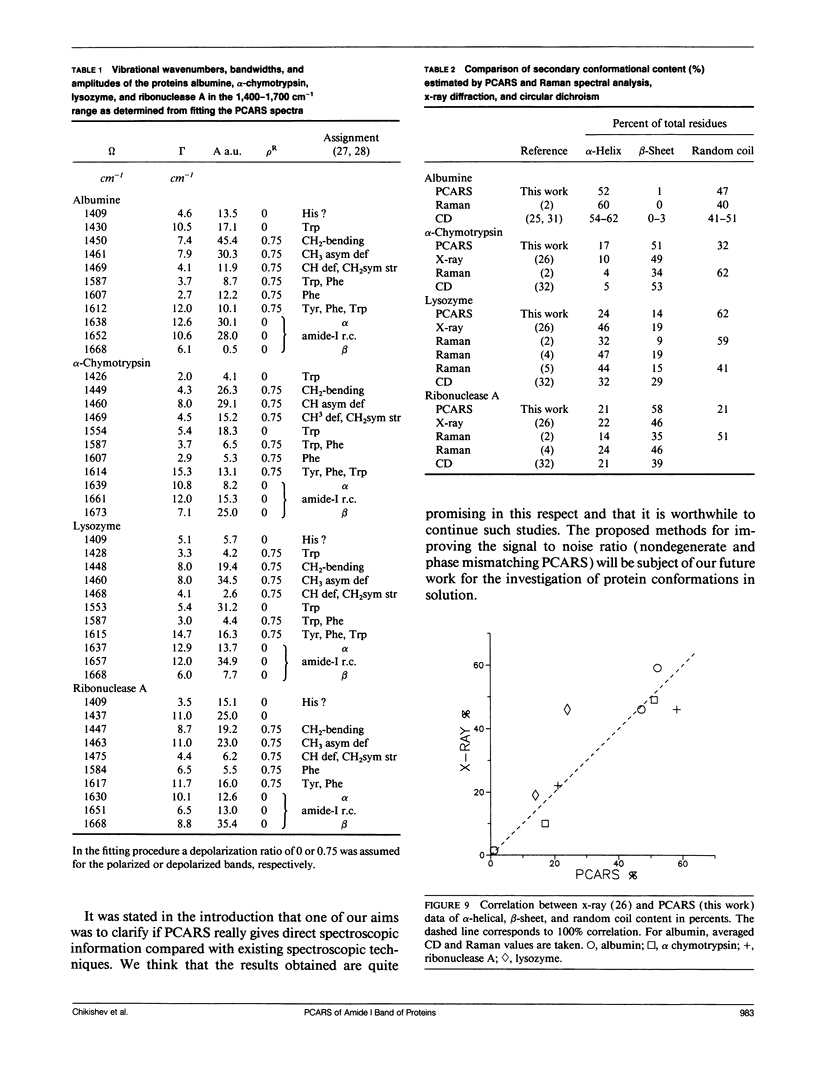
Polarization sensitive coherent anti-Stokes Raman scattering (PCARS) spectroscopy is a fruitful technique to study Raman vibrations of diluted molecules under off-electron resonant conditions. We apply PCARS as a direct spectroscopic method to investigate the broad amide I band of proteins in heavy water. In spontaneous Raman spectroscopy, this band is not well resolved. We fit a number of spectra taken of each protein under different polarization conditions, with a single set of parameters. It then appears that some substructure is observed in the amide I band. From this substructure, we determine the percentage of alpha-helix, beta-sheet, and random coil for the proteins lysozyme, albumin, ribonuclease A, and alpha-chymotrypsin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barela T. D., Darnall D. W. Practical aspects of calculating protein secondary structure from circular dichroism spectra. Biochemistry. 1974 Apr 9;13(8):1694–1700. doi: 10.1021/bi00705a022. [DOI] [PubMed] [Google Scholar]
- Byler D. M., Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers. 1986 Mar;25(3):469–487. doi: 10.1002/bip.360250307. [DOI] [PubMed] [Google Scholar]
- Chang C. T., Wu C. S., Yang J. T. Circular dichroic analysis of protein conformation: inclusion of the beta-turns. Anal Biochem. 1978 Nov;91(1):13–31. doi: 10.1016/0003-2697(78)90812-6. [DOI] [PubMed] [Google Scholar]
- Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969 Oct;8(10):4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- KRIMM S. Infrared spectra and chain conformation of proteins. J Mol Biol. 1962 Jun;4:528–540. doi: 10.1016/s0022-2836(62)80107-7. [DOI] [PubMed] [Google Scholar]
- Levitt M., Greer J. Automatic identification of secondary structure in globular proteins. J Mol Biol. 1977 Aug 5;114(2):181–239. doi: 10.1016/0022-2836(77)90207-8. [DOI] [PubMed] [Google Scholar]
- Lippert J. L., Tyminski D., Desmeules P. J. Determination of the secondary structure of proteins by laser Raman spectroscopy. J Am Chem Soc. 1976 Oct 27;98(22):7075–7080. doi: 10.1021/ja00438a057. [DOI] [PubMed] [Google Scholar]
- Lord R. C., Yu N. T. Laser-excited Raman spectroscopy of biomolecules. II. Native ribonuclease and alpha-chymotrypsin. J Mol Biol. 1970 Jul 28;51(2):203–213. doi: 10.1016/0022-2836(70)90137-3. [DOI] [PubMed] [Google Scholar]
- Marx J., Jacquot J., Berjot M., Puchelle E., Alix A. J. Characterization and conformational analysis by Raman spectroscopy of human airway lysozyme. Biochim Biophys Acta. 1986 Apr 22;870(3):488–494. doi: 10.1016/0167-4838(86)90257-8. [DOI] [PubMed] [Google Scholar]
- Nestor J., Spiro T. G., Klauminzer G. Coherent anti-Stokes Raman scattering (CARS) spectra, with resonance enhancement, of cytochrome c and vitamin B12 in dilute aqueous solution. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3329–3332. doi: 10.1073/pnas.73.10.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto C., de Mul F. F., Harmsen B. J., Greve J. A Raman scattering study of the helix-destabilizing gene-5 protein with adenine-containing nucleotides. Nucleic Acids Res. 1987 Sep 25;15(18):7605–7625. doi: 10.1093/nar/15.18.7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pézolet M., Pigeon M., Ménard D., Caillé J. P. Raman spectroscopy of cytoplasmic muscle fiber proteins. Orientational order. Biophys J. 1988 Mar;53(3):319–325. doi: 10.1016/S0006-3495(88)83109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. W., Dunker A. K. Determination of the secondary structure of proteins from the amide I band of the laser Raman spectrum. J Mol Biol. 1981 Nov 15;152(4):783–813. doi: 10.1016/0022-2836(81)90127-3. [DOI] [PubMed] [Google Scholar]
- Williams R. W. Estimation of protein secondary structure from the laser Raman amide I spectrum. J Mol Biol. 1983 Jun 5;166(4):581–603. doi: 10.1016/s0022-2836(83)80285-x. [DOI] [PubMed] [Google Scholar]
- Yu T. J., Lippert J. L., Peticolas W. L. Laser Raman studies of conformational variations of poly-L-lysine. Biopolymers. 1973;12(9):2161–2175. doi: 10.1002/bip.1973.360120919. [DOI] [PubMed] [Google Scholar]


