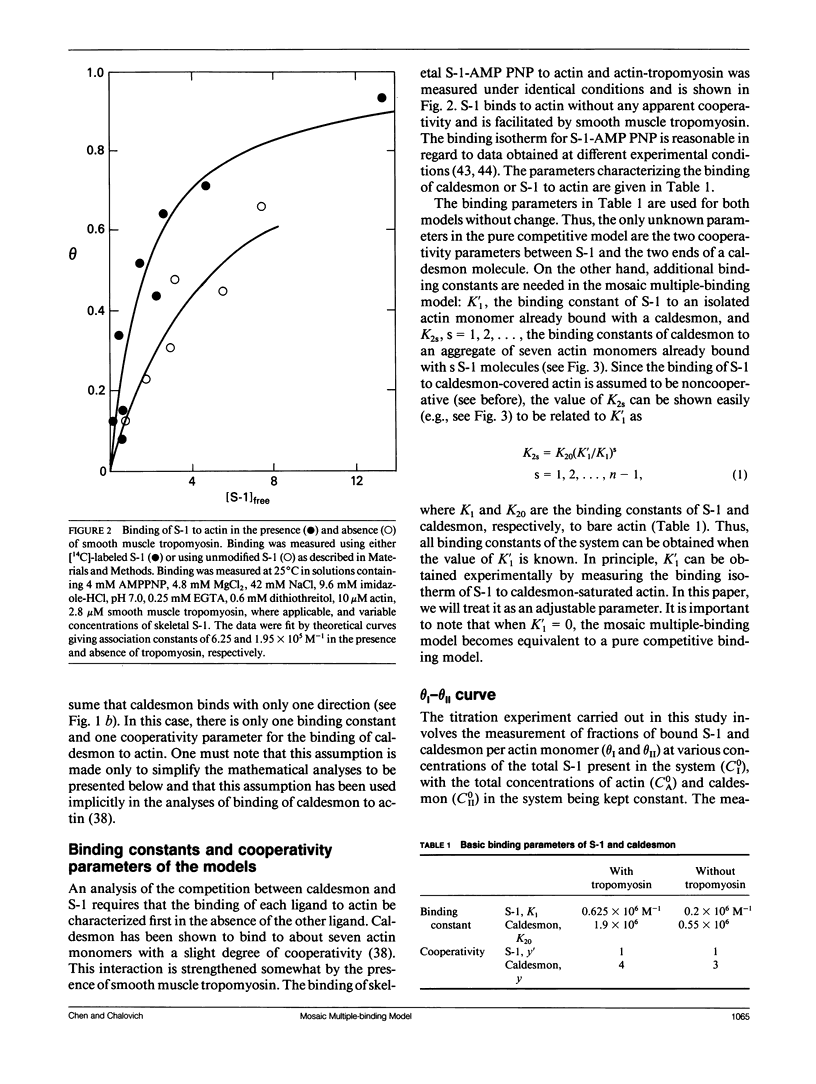
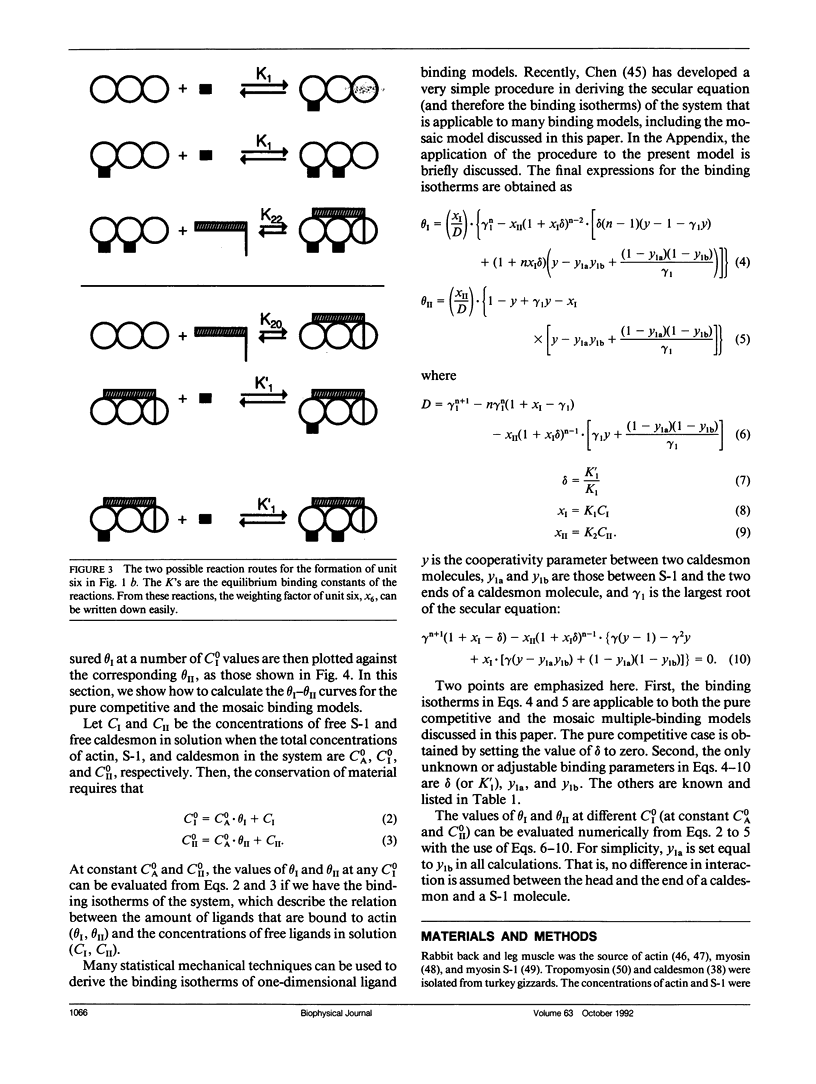
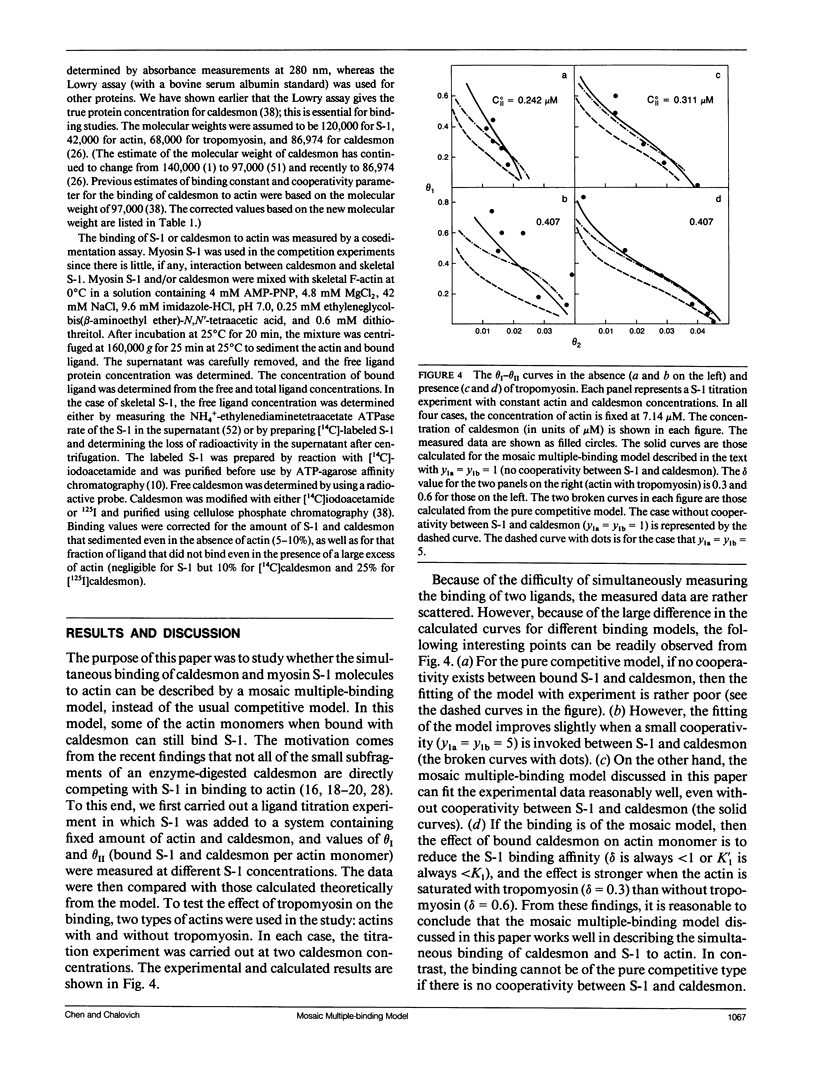
Abstract
Binding of caldesmon to actin causes a decrease in the quantity of bound myosin and results in a reduction in the rate of actin-activated adenosine triphosphate hydrolysis. It is generally assumed that the binding of caldesmon and myosin to actin is a pure competitive interaction. However, recent binding studies of enzyme digested caldesmon subfragments directed at mapping the actin binding site of caldesmon have shown that a small 8-kD fragment around the COOH-terminal can compete directly with the myosin subfragment 1 (S-1) binding to actin; at least one other fragment that binds to actin does not inhibit the actin-activated adenosine triphosphate activity of myosin. That is, only a part of the caldesmon sequence may be responsible for directly blocking the binding of S-1 to actin. This prompts us to question the actual mode of binding of intact caldesmon and myosin S-1 to actin: whether the entire intact caldesmon molecule is competing with S-1 binding (pure competitive model) or just a small part of it (mosaic multiple-binding model). To answer this question, we measured the amount of myosin S-1 and caldesmon bound per actin monomer as a function of the total concentration of S-1 added to the system at constant concentrations of actin and caldesmon. A formalism for calculating the titration data based on the pure competitive model and a mosaic multiple-binding model was then developed. When compared with theoretical calculations, it is found that the binding of caldesmon and S-1 to actin cannot be pure competitive if no cooperativity exists between S-1 and caldesmon. In contrast, the mosaic multiple-binding model can fit the binding data rather well regardless of the existence of cooperativity between S-1 and caldesmon.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S., DasGupta G., Chalovich J. M., Reisler E. Immunochemical evidence for the binding of caldesmon to the NH2-terminal segment of actin. J Biol Chem. 1990 Nov 15;265(32):19652–19657. [PubMed] [Google Scholar]
- Bartegi A., Fattoum A., Derancourt J., Kassab R. Characterization of the carboxyl-terminal 10-kDa cyanogen bromide fragment of caldesmon as an actin-calmodulin-binding region. J Biol Chem. 1990 Sep 5;265(25):15231–15238. [PubMed] [Google Scholar]
- Bartegi A., Fattoum A., Kassab R. Cross-linking of smooth muscle caldesmon to the NH2-terminal region of skeletal F-actin. J Biol Chem. 1990 Feb 5;265(4):2231–2237. [PubMed] [Google Scholar]
- Bertrand R., Chaussepied P., Audemard E., Kassab R. Functional characterization of skeletal F-actin labeled on the NH2-terminal segment of residues 1-28. Eur J Biochem. 1989 May 15;181(3):747–754. doi: 10.1111/j.1432-1033.1989.tb14787.x. [DOI] [PubMed] [Google Scholar]
- Brenner B., Yu L. C., Chalovich J. M. Parallel inhibition of active force and relaxed fiber stiffness in skeletal muscle by caldesmon: implications for the pathway to force generation. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5739–5743. doi: 10.1073/pnas.88.13.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A. Smooth muscle caldesmon. Rapid purification and F-actin cross-linking properties. J Biol Chem. 1984 Oct 25;259(20):12873–12880. [PubMed] [Google Scholar]
- Bryan J., Imai M., Lee R., Moore P., Cook R. G., Lin W. G. Cloning and expression of a smooth muscle caldesmon. J Biol Chem. 1989 Aug 15;264(23):13873–13879. [PubMed] [Google Scholar]
- Chalovich J. M., Cornelius P., Benson C. E. Caldesmon inhibits skeletal actomyosin subfragment-1 ATPase activity and the binding of myosin subfragment-1 to actin. J Biol Chem. 1987 Apr 25;262(12):5711–5716. [PubMed] [Google Scholar]
- Chalovich J. M., Eisenberg E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem. 1982 Mar 10;257(5):2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Greene L. E., Eisenberg E. Crosslinked myosin subfragment 1: a stable analogue of the subfragment-1.ATP complex. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4909–4913. doi: 10.1073/pnas.80.16.4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. D. A general secular equation for cooperative binding of n-mer ligands to a one-dimensional lattice. Biopolymers. 1990;30(11-12):1113–1121. doi: 10.1002/bip.360301111. [DOI] [PubMed] [Google Scholar]
- Dabrowska R., Goch A., Gałazkiewicz B., Osińska H. The influence of caldesmon on ATPase activity of the skeletal muscle actomyosin and bundling of actin filaments. Biochim Biophys Acta. 1985 Sep 27;842(1):70–75. doi: 10.1016/0304-4165(85)90295-8. [DOI] [PubMed] [Google Scholar]
- DasGupta G., Reisler E. Antibody against the amino terminus of alpha-actin inhibits actomyosin interactions in the presence of ATP. J Mol Biol. 1989 Jun 20;207(4):833–836. doi: 10.1016/0022-2836(89)90249-0. [DOI] [PubMed] [Google Scholar]
- Ebashi S., Endo M., Otsuki I. Control of muscle contraction. Q Rev Biophys. 1969 Nov;2(4):351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- Fujii T., Imai M., Rosenfeld G. C., Bryan J. Domain mapping of chicken gizzard caldesmon. J Biol Chem. 1987 Feb 25;262(6):2757–2763. [PubMed] [Google Scholar]
- Fujii T., Ozawa J., Ogoma Y., Kondo Y. Interaction between chicken gizzard caldesmon and tropomyosin. J Biochem. 1988 Nov;104(5):734–737. doi: 10.1093/oxfordjournals.jbchem.a122542. [DOI] [PubMed] [Google Scholar]
- Graceffa P., Wang C. L., Stafford W. F. Caldesmon. Molecular weight and subunit composition by analytical ultracentrifugation. J Biol Chem. 1988 Oct 5;263(28):14196–14202. [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Dissociation of the actin.subfragment 1 complex by adenyl-5'-yl imidodiphosphate, ADP, and PPi. J Biol Chem. 1980 Jan 25;255(2):543–548. [PubMed] [Google Scholar]
- Hayashi K., Fujio Y., Kato I., Sobue K. Structural and functional relationships between h- and l-caldesmons. J Biol Chem. 1991 Jan 5;266(1):355–361. [PubMed] [Google Scholar]
- Hayashi K., Yamada S., Kanda K., Kimizuka F., Kato I., Sobue K. 35 kDa fragment of h-caldesmon conserves two consensus sequences of the tropomyosin-binding domain in troponin T. Biochem Biophys Res Commun. 1989 May 30;161(1):38–45. doi: 10.1016/0006-291x(89)91556-8. [DOI] [PubMed] [Google Scholar]
- Hemric M. E., Chalovich J. M. Characterization of caldesmon binding to myosin. J Biol Chem. 1990 Nov 15;265(32):19672–19678. [PMC free article] [PubMed] [Google Scholar]
- Hemric M. E., Chalovich J. M. Effect of caldesmon on the ATPase activity and the binding of smooth and skeletal myosin subfragments to actin. J Biol Chem. 1988 Feb 5;263(4):1878–1885. [PubMed] [Google Scholar]
- Horiuchi K. Y., Chacko S. Caldesmon inhibits the cooperative turning-on of the smooth muscle heavy meromyosin by tropomyosin-actin. Biochemistry. 1989 Nov 14;28(23):9111–9116. doi: 10.1021/bi00449a023. [DOI] [PubMed] [Google Scholar]
- Horiuchi K. Y., Chacko S. Interaction between caldesmon and tropomyosin in the presence and absence of smooth muscle actin. Biochemistry. 1988 Nov 1;27(22):8388–8393. doi: 10.1021/bi00422a014. [DOI] [PubMed] [Google Scholar]
- Horiuchi K. Y., Samuel M., Chacko S. Mechanism for the inhibition of acto-heavy meromyosin ATPase by the actin/calmodulin binding domain of caldesmon. Biochemistry. 1991 Jan 22;30(3):712–717. doi: 10.1021/bi00217a019. [DOI] [PubMed] [Google Scholar]
- Ikebe M., Reardon S. Binding of caldesmon to smooth muscle myosin. J Biol Chem. 1988 Mar 5;263(7):3055–3058. [PubMed] [Google Scholar]
- KIELLEY W. W., HARRINGTON W. F. A model for the myosin molecule. Biochim Biophys Acta. 1960 Jul 15;41:401–421. doi: 10.1016/0006-3002(60)90037-8. [DOI] [PubMed] [Google Scholar]
- Lash J. A., Sellers J. R., Hathaway D. R. The effects of caldesmon on smooth muscle heavy actomeromyosin ATPase activity and binding of heavy meromyosin to actin. J Biol Chem. 1986 Dec 5;261(34):16155–16160. [PubMed] [Google Scholar]
- Leszyk J., Mornet D., Audemard E., Collins J. H. Caldesmon structure and function: sequence analysis of a 35 kilodalton actin- and calmodulin-binding fragment from the C-terminus of the turkey gizzard protein. Biochem Biophys Res Commun. 1989 May 15;160(3):1371–1378. doi: 10.1016/s0006-291x(89)80155-x. [DOI] [PubMed] [Google Scholar]
- Levine B. A., Moir A. J., Audemard E., Mornet D., Patchell V. B., Perry S. V. Structural study of gizzard caldesmon and its interaction with actin. Binding involves residues of actin also recognised by myosin subfragment 1. Eur J Biochem. 1990 Nov 13;193(3):687–696. doi: 10.1111/j.1432-1033.1990.tb19388.x. [DOI] [PubMed] [Google Scholar]
- Marston S. Aorta caldesmon inhibits actin activation of thiophosphorylated heavy meromyosin Mg2+-ATPase activity by slowing the rate of product release. FEBS Lett. 1988 Sep 26;238(1):147–150. doi: 10.1016/0014-5793(88)80245-x. [DOI] [PubMed] [Google Scholar]
- Mornet D., Audemard E., Derancourt J. Identification of a 15 kilodalton actin binding region on gizzard caldesmon probed by chemical cross-linking. Biochem Biophys Res Commun. 1988 Jul 29;154(2):564–571. doi: 10.1016/0006-291x(88)90177-5. [DOI] [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. Properties of caldesmon isolated from chicken gizzard. Biochem J. 1985 Sep 15;230(3):695–707. doi: 10.1042/bj2300695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riseman V. M., Lynch W. P., Nefsky B., Bretscher A. The calmodulin and F-actin binding sites of smooth muscle caldesmon lie in the carboxyl-terminal domain whereas the molecular weight heterogeneity lies in the middle of the molecule. J Biol Chem. 1989 Feb 15;264(5):2869–2875. [PubMed] [Google Scholar]
- Smith C. W., Marston S. B. Disassembly and reconstitution of the Ca2+-sensitive thin filaments of vascular smooth muscle. FEBS Lett. 1985 May 6;184(1):115–119. doi: 10.1016/0014-5793(85)80665-7. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Pritchard K., Marston S. B. The mechanism of Ca2+ regulation of vascular smooth muscle thin filaments by caldesmon and calmodulin. J Biol Chem. 1987 Jan 5;262(1):116–122. [PubMed] [Google Scholar]
- Sobue K., Kanda K., Adachi J., Kakiuchi S. Calmodulin-binding proteins that interact with actin filaments in a Ca2+-dependent flip-flop manner: survey in brain and secretory tissues. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6868–6871. doi: 10.1073/pnas.80.22.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K., Kanda K., Tanaka T., Ueki N. Caldesmon: a common actin-linked regulatory protein in the smooth muscle and nonmuscle contractile system. J Cell Biochem. 1988 Jul;37(3):317–325. doi: 10.1002/jcb.240370306. [DOI] [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5652–5655. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobue K., Takahashi K., Wakabayashi I. Caldesmon150 regulates the tropomyosin-enhanced actin-myosin interaction in gizzard smooth muscle. Biochem Biophys Res Commun. 1985 Oct 30;132(2):645–651. doi: 10.1016/0006-291x(85)91181-7. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stafford W. F., Jancso A., Graceffa P. Caldesmon from rabbit liver: molecular weight and length by analytical ultracentrifugation. Arch Biochem Biophys. 1990 Aug 15;281(1):66–69. doi: 10.1016/0003-9861(90)90413-s. [DOI] [PubMed] [Google Scholar]
- Sutherland C., Walsh M. P. Phosphorylation of caldesmon prevents its interaction with smooth muscle myosin. J Biol Chem. 1989 Jan 5;264(1):578–583. [PubMed] [Google Scholar]
- Szpacenko A., Dabrowska R. Functional domain of caldesmon. FEBS Lett. 1986 Jul 7;202(2):182–186. doi: 10.1016/0014-5793(86)80683-4. [DOI] [PubMed] [Google Scholar]
- Velaz L., Hemric M. E., Benson C. E., Chalovich J. M. The binding of caldesmon to actin and its effect on the ATPase activity of soluble myosin subfragments in the presence and absence of tropomyosin. J Biol Chem. 1989 Jun 5;264(16):9602–9610. [PubMed] [Google Scholar]
- Velaz L., Ingraham R. H., Chalovich J. M. Dissociation of the effect of caldesmon on the ATPase activity and on the binding of smooth heavy meromyosin to actin by partial digestion of caldesmon. J Biol Chem. 1990 Feb 15;265(5):2929–2934. [PubMed] [Google Scholar]
- Wang C. L., Chalovich J. M., Graceffa P., Lu R. C., Mabuchi K., Stafford W. F. A long helix from the central region of smooth muscle caldesmon. J Biol Chem. 1991 Jul 25;266(21):13958–13963. [PMC free article] [PubMed] [Google Scholar]
- Wang C. L., Wang L. W., Lu R. C. Caldesmon has two calmodulin-binding domains. Biochem Biophys Res Commun. 1989 Jul 31;162(2):746–752. doi: 10.1016/0006-291x(89)92373-5. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Taylor R. S. Separation of subfragment-1 isoenzymes from rabbit skeletal muscle myosin. Nature. 1975 Sep 4;257(5521):54–56. doi: 10.1038/257054a0. [DOI] [PubMed] [Google Scholar]
- Yazawa M., Yagi K., Sobue K. Isolation and characterization of a calmodulin binding fragment of chicken gizzard caldesmon. J Biochem. 1987 Nov;102(5):1065–1073. doi: 10.1093/oxfordjournals.jbchem.a122144. [DOI] [PubMed] [Google Scholar]


