Abstract
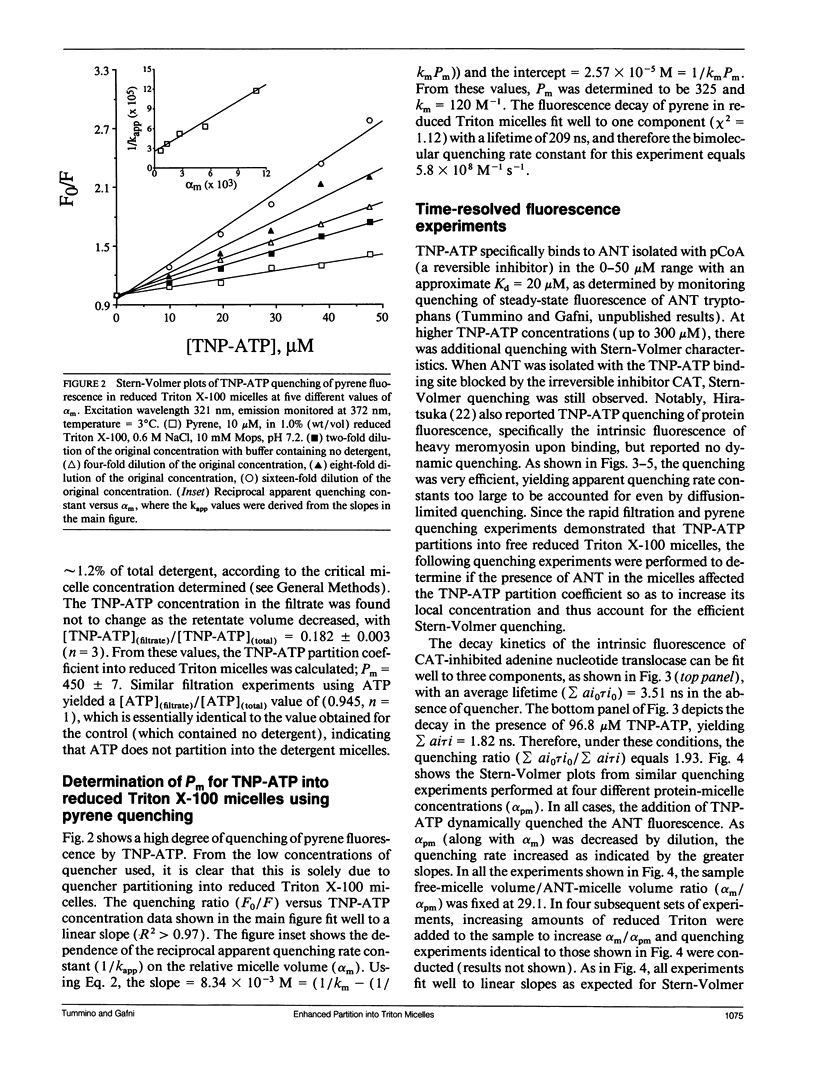
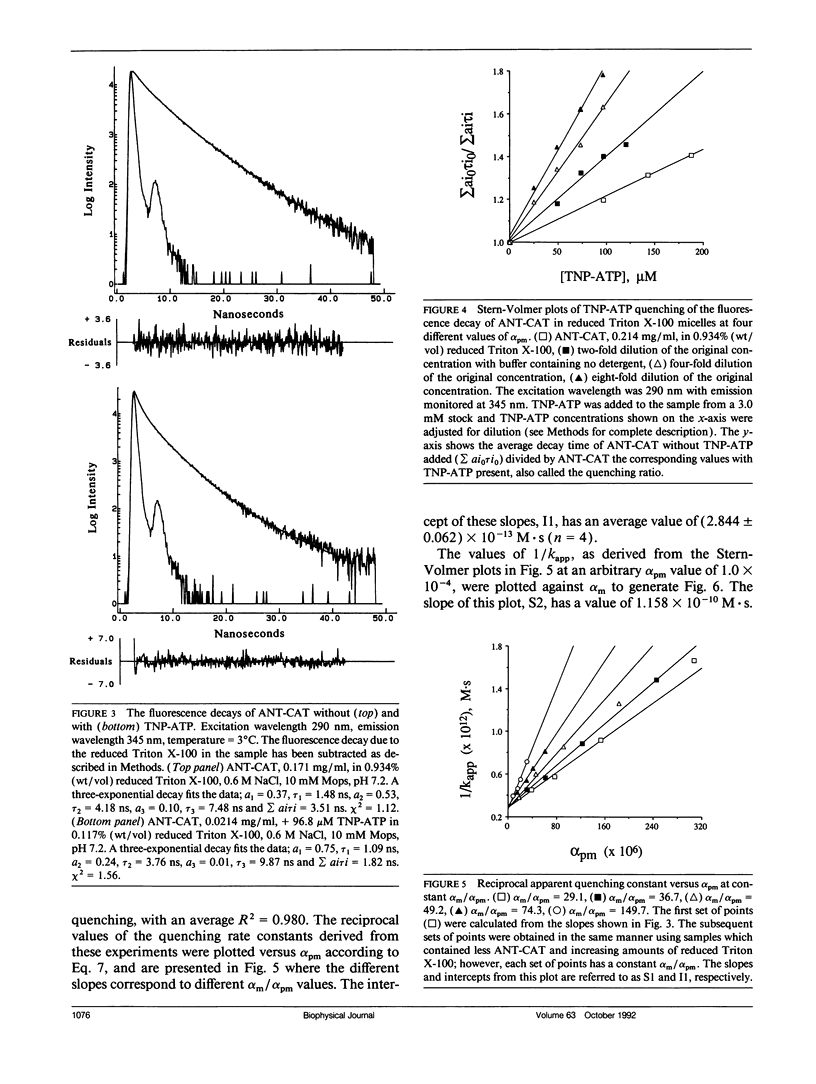
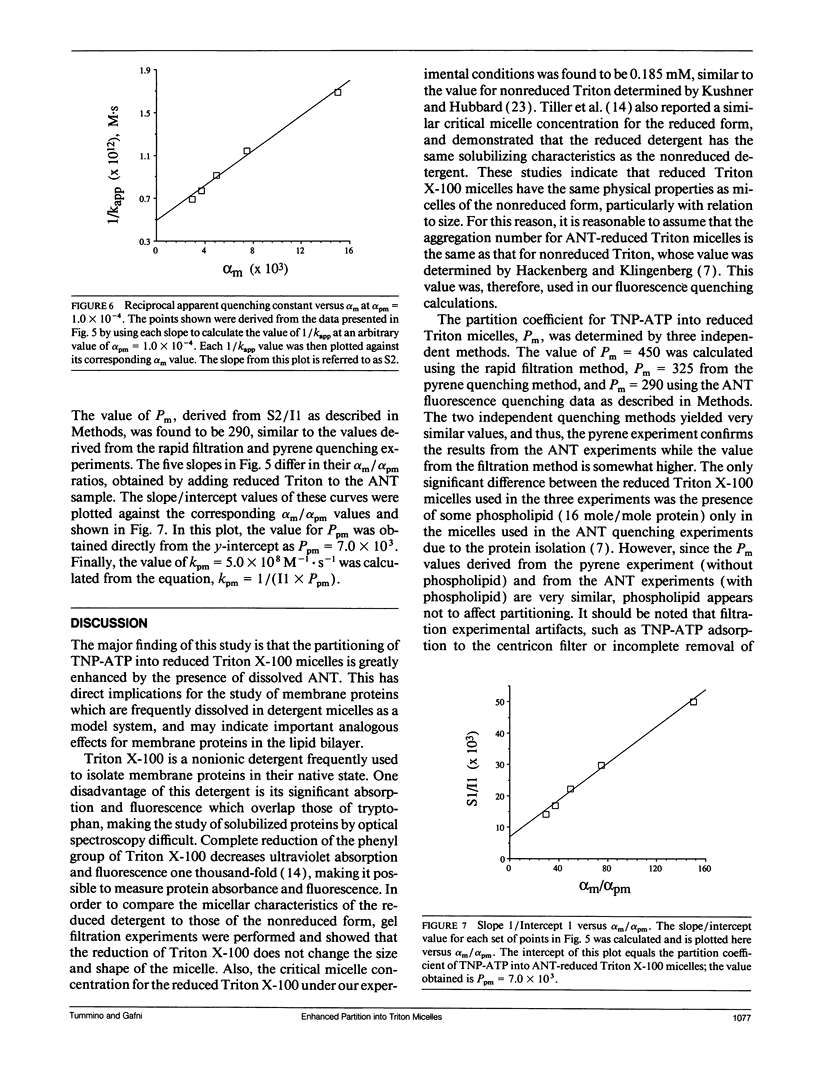
The presence of adenine nucleotide translocase (ANT) was found to greatly enhance the partitioning of the ATP analog 2',3'-O-(2,4,6-trinitrophenyl)-adenosine 5'-triphosphate (TNP-ATP) into reduced Triton X-100 micelles. The protein's effect was studied through the quenching of fluorescence of purified ANT, irreversibly inhibited by carboxyatractyloside (CAT), solubilized in reduced Triton X-100 micelles. The dependence of quenching of the protein's time-resolved tryptophan fluorescence on TNP-ATP concentration was measured and found to follow a Stern-Volmer mechanism. However, the calculated quenching constant was too large to be accounted for by the aqueous TNP-ATP concentration. Experiments were therefore conducted to determine the partitioning of the quencher between the three phases present: aqueous, protein-free micelle, and protein micelle; a system also described by the equation of Omann, G. M., and M. Glaser (1985. Biophys. J. 47:623-627.). By measuring the dependence of the apparent quenching rate constant on the protein concentration and protein/micelle ratios, this equation was used to calculate both the quencher partition coefficient into protein-free micelles (Pm) and into protein-micelles (Ppm), as well as the bimolecular quenching rate constant (kpm) in protein micelles. From the quenching experiments, kpm = 5.0 x 10(8)M-1s-1,Pm = 290 and pyrene quenching experiment to be 325, and by a rapid filtration experiment to be 450. Clearly, the presence of the integral membrane protein ANT-CAT in reduced Triton X-100 micelles greatly increases the partition of TNP-ATP into the micelle. ANT alters the properties and thus, the structure of the detergent micelle, which has direct implications for the use of detergent micelles as a model system for membrane proteins and may indicate that analogous effects occur in the mitochondrial membrane.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antunes-Madeira M. C., Madeira V. M. Partition of lindane in synthetic and native membranes. Biochim Biophys Acta. 1985 Nov 7;820(2):165–172. doi: 10.1016/0005-2736(85)90109-9. [DOI] [PubMed] [Google Scholar]
- Aquila H., Eiermann W., Babel W., Klingenberg M. Isolation of the ADP/ATP translocator from beef heart mitochondria as the bongkrekate-protein complex. Eur J Biochem. 1978 Apr 17;85(2):549–560. doi: 10.1111/j.1432-1033.1978.tb12270.x. [DOI] [PubMed] [Google Scholar]
- Beyer K., Klingenberg M. ADP/ATP carrier protein from beef heart mitochondria has high amounts of tightly bound cardiolipin, as revealed by 31P nuclear magnetic resonance. Biochemistry. 1985 Jul 16;24(15):3821–3826. doi: 10.1021/bi00336a001. [DOI] [PubMed] [Google Scholar]
- Conrad M. J., Singer S. J. The solubility of amphipathic molecules in biological membranes and lipid bilayers and its implications for membrane structure. Biochemistry. 1981 Feb 17;20(4):808–818. doi: 10.1021/bi00507a024. [DOI] [PubMed] [Google Scholar]
- De Vendittis E., Palumbo G., Parlato G., Bocchini V. A fluorimetric method for the estimation of the critical micelle concentration of surfactants. Anal Biochem. 1981 Aug;115(2):278–286. doi: 10.1016/0003-2697(81)90006-3. [DOI] [PubMed] [Google Scholar]
- Gains N., Dawson A. P. Evidence against protein-induced 'internal pressure' in biological membranes. Partition of 8-anilinonaphthalene-1-sulphonate into Triton X-100 micelles and submitochondrial particles. Biochem J. 1982 Dec 1;207(3):567–572. doi: 10.1042/bj2070567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garewal H. S. A procedure for the estimation of microgram quantities of triton X-100. Anal Biochem. 1973 Aug;54(2):319–324. doi: 10.1016/0003-2697(73)90359-x. [DOI] [PubMed] [Google Scholar]
- Hackenberg H., Klingenberg M. Molecular weight and hydrodynamic parameters of the adenosine 5'-diphosphate--adenosine 5'-triphosphate carrier in Triton X-100. Biochemistry. 1980 Feb 5;19(3):548–555. doi: 10.1021/bi00544a024. [DOI] [PubMed] [Google Scholar]
- Hiratsuka T. 2' (or 3')-O-(2, 4, 6-trinitrophenyl)adenosine 5'-triphosphate as a probe for the binding site of heavy meromyosin ATPase. J Biochem. 1975 Dec;78(6):1135–1147. doi: 10.1093/oxfordjournals.jbchem.a131009. [DOI] [PubMed] [Google Scholar]
- Klingenberg M., Riccio P., Aquila H. Isolation of the ADP, ATP carrier as the carboxyatractylate . protein complex from mitochondria. Biochim Biophys Acta. 1978 Aug 8;503(2):193–210. doi: 10.1016/0005-2728(78)90182-2. [DOI] [PubMed] [Google Scholar]
- LaNoue K. F., Schoolwerth A. C. Metabolite transport in mitochondria. Annu Rev Biochem. 1979;48:871–922. doi: 10.1146/annurev.bi.48.070179.004255. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lakowicz J. R., Hogen D., Omann G. Diffusion and partitioning of a pesticide, lindane, into phosphatidylcholine bilayers. A new fluorescence quenching method to study chlorinated hydrocarbon-membrane interactions. Biochim Biophys Acta. 1977 Dec 15;471(3):401–411. doi: 10.1016/0005-2736(77)90045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard M., Noy N., Zakim D. The interactions of bilirubin with model and biological membranes. J Biol Chem. 1989 Apr 5;264(10):5648–5652. [PubMed] [Google Scholar]
- Omann G. M., Glaser M. Dynamic quenchers in fluorescently labeled membranes. Theory for quenching in a three-phase system. Biophys J. 1985 May;47(5):623–627. doi: 10.1016/S0006-3495(85)83958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste M., Walker J. E. Internal sequence repeats and the path of polypeptide in mitochondrial ADP/ATP translocase. FEBS Lett. 1982 Aug 2;144(2):250–254. doi: 10.1016/0014-5793(82)80648-0. [DOI] [PubMed] [Google Scholar]
- Schauerte J. A., Gafni A. Long-lived tryptophan fluorescence in phosphoglycerate mutase. Biochemistry. 1989 May 2;28(9):3948–3954. doi: 10.1021/bi00435a048. [DOI] [PubMed] [Google Scholar]
- Schlimme E., Boos K. S., Onur G., Ponse G. Inhibition study of ADP,ATP transport in mitochondria with trinitrophenyl-modified substrates. FEBS Lett. 1983 May 2;155(1):6–10. doi: 10.1016/0014-5793(83)80197-5. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence energy transfer as a spectroscopic ruler. Annu Rev Biochem. 1978;47:819–846. doi: 10.1146/annurev.bi.47.070178.004131. [DOI] [PubMed] [Google Scholar]
- Tanford C., Nozaki Y., Reynolds J. A., Makino S. Molecular characterization of proteins in detergent solutions. Biochemistry. 1974 May 21;13(11):2369–2376. doi: 10.1021/bi00708a021. [DOI] [PubMed] [Google Scholar]
- Tanford C., Reynolds J. A. Characterization of membrane proteins in detergent solutions. Biochim Biophys Acta. 1976 Oct 26;457(2):133–170. doi: 10.1016/0304-4157(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Tiller G. E., Mueller T. J., Dockter M. E., Struve W. G. Hydrogenation of triton X-100 eliminates its fluorescence and ultraviolet light absorption while preserving its detergent properties. Anal Biochem. 1984 Aug 15;141(1):262–266. doi: 10.1016/0003-2697(84)90455-x. [DOI] [PubMed] [Google Scholar]
- Woldegiorgis G., Yousufzai S. Y., Shrago E. Studies on the interaction of palmitoyl coenzyme A with the adenine nucleotide translocase. J Biol Chem. 1982 Dec 25;257(24):14783–14787. [PubMed] [Google Scholar]


