Abstract
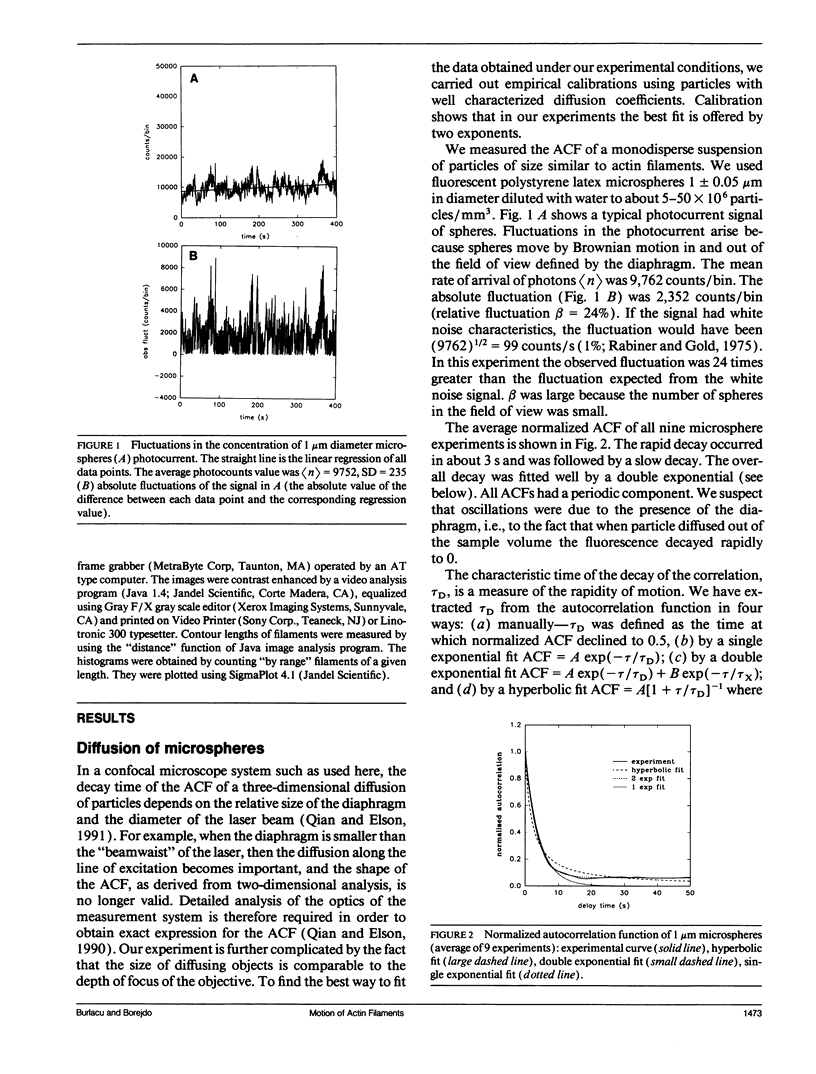
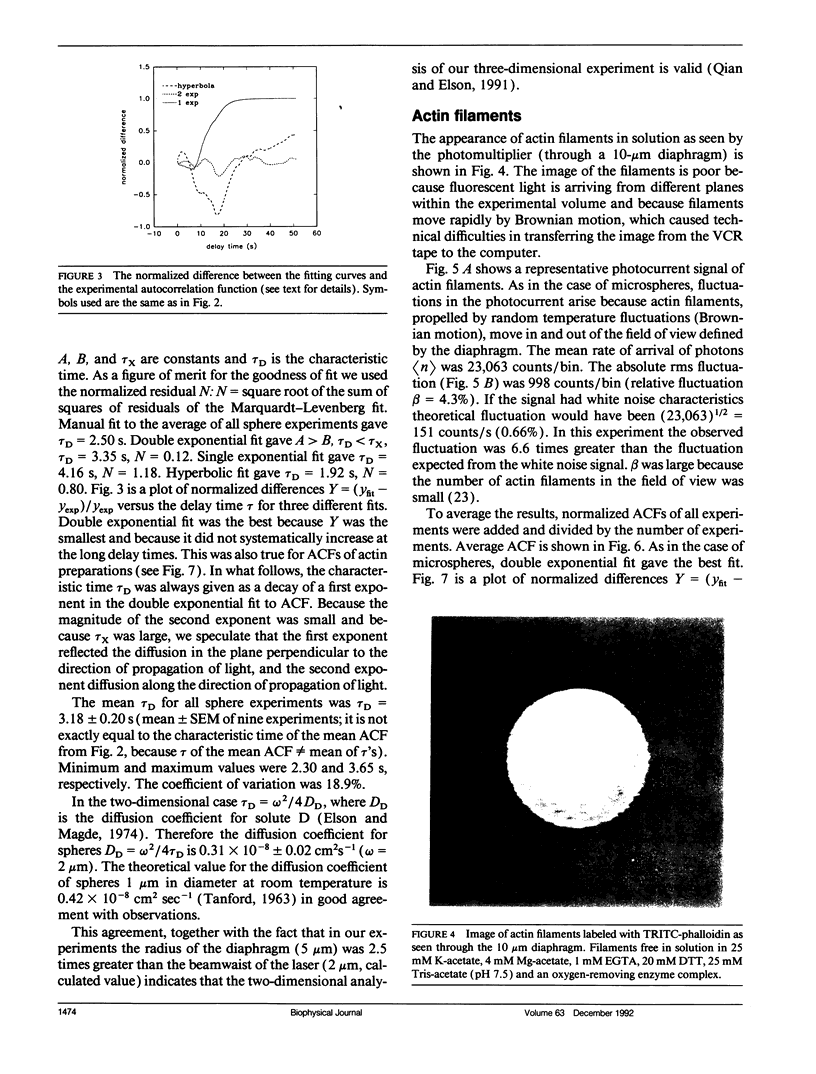
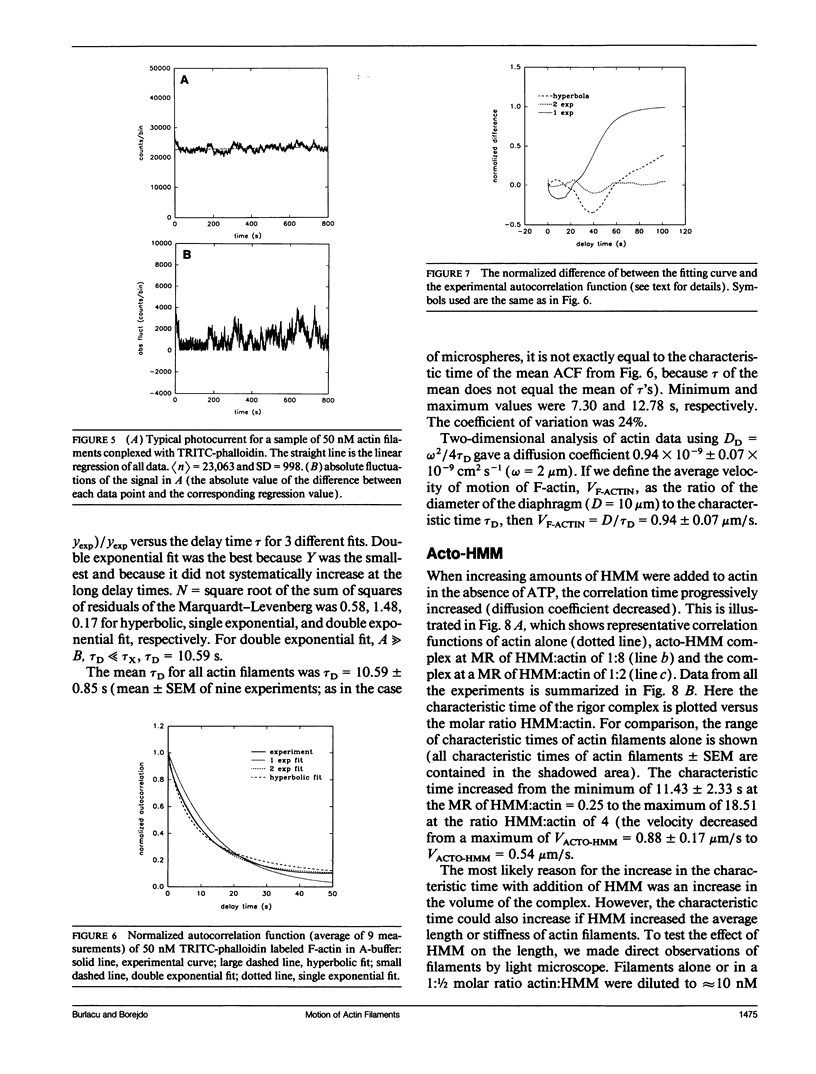
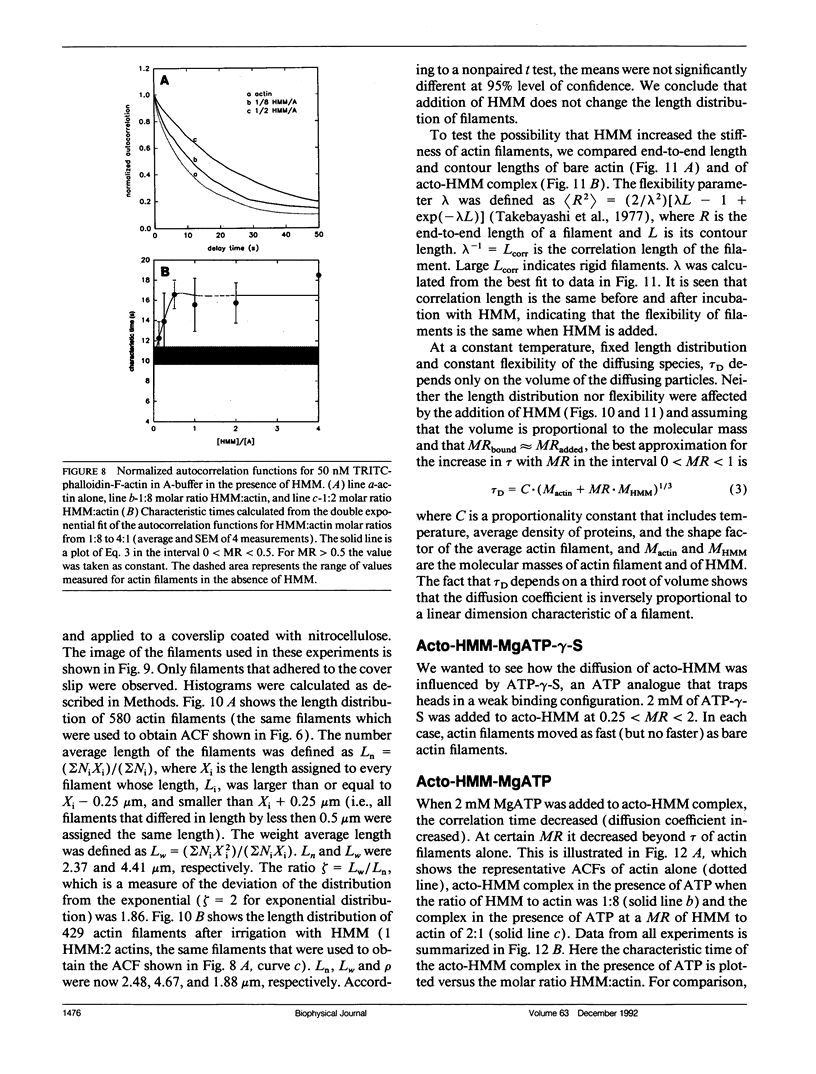
We measured, by fluorescence correlation spectroscopy, the motion of actin filaments in solution during hydrolysis of ATP by acto-heavy meromyosin (acto-HMM). The method relies on the fact that the intensity of fluorescence fluctuates as fluorescently labeled actin filaments enter and leave a small sample volume. The rapidity of these number fluctuations is characterized by the autocorrelation function, which decays to 0 in time that is related to the average velocity of translation of filaments. The time of decay of the autocorrelation function of bare actin filaments in solution was 10.59 +/- 0.85 s. Strongly bound (rigor) heads slowed down the diffusion. Direct observation of filaments under an optical microscope showed that addition of HMM did not change the average length or flexibility of actin filaments, suggesting that the decrease in diffusion was not due to a HMM-induced change in the shape of filaments. Rather, slowing down of translational motion was caused by an increase in the volume of the diffusing complex. Surprisingly, the addition of ATP to acto-HMM accelerated the motion of actin filaments. The acceleration was the greatest at the low molar ratios of HMM:actin. Direct observation of filaments under an optical microscope showed that in the presence of ATP the average length of filaments did not change and that the filaments became stiffer, suggesting that acceleration of diffusion was not due to an ATP-induced increase in flexibility of filaments. These results show that some of the energy of splitting of ATP is impaired to actin filaments and suggest that 0.06 +/- 0.02 of HMM interferes with the diffusion of actin filaments during hydrolysis of ATP.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borejdo J., Burlacu S. Diffusion of heavy meromyosin in the presence of F-actin and ATP. J Muscle Res Cell Motil. 1992 Feb;13(1):106–116. doi: 10.1007/BF01738434. [DOI] [PubMed] [Google Scholar]
- Borejdo J., Burlacu S. Velocity of movement of actin filaments in in vitro motility assay. Measured by fluorescence correlation spectroscopy. Biophys J. 1992 May;61(5):1267–1280. doi: 10.1016/S0006-3495(92)81935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borejdo J., Morales M. F. Fluctuations in tension during contraction of single muscle fibers. Biophys J. 1977 Dec;20(3):315–334. doi: 10.1016/S0006-3495(77)85552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borejdo J. Motion of myosin fragments during actin-activated ATPase: fluorescence correlation spectroscopy study. Biopolymers. 1979 Nov;18(11):2807–2820. doi: 10.1002/bip.1979.360181111. [DOI] [PubMed] [Google Scholar]
- Burlacu S., Janmey P. A., Borejdo J. Distribution of actin filament lengths measured by fluorescence microscopy. Am J Physiol. 1992 Mar;262(3 Pt 1):C569–C577. doi: 10.1152/ajpcell.1992.262.3.C569. [DOI] [PubMed] [Google Scholar]
- Dantzig J. A., Walker J. W., Trentham D. R., Goldman Y. E. Relaxation of muscle fibers with adenosine 5'-[gamma-thio]triphosphate (ATP[gamma S]) and by laser photolysis of caged ATP[gamma S]: evidence for Ca2+-dependent affinity of rapidly detaching zero-force cross-bridges. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6716–6720. doi: 10.1073/pnas.85.18.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson E. L., Qian H. Interpretation of fluorescence correlation spectroscopy and photobleaching recovery in terms of molecular interactions. Methods Cell Biol. 1989;30:307–332. doi: 10.1016/s0091-679x(08)60984-x. [DOI] [PubMed] [Google Scholar]
- Elson E. L., Webb W. W. Concentration correlation spectroscopy: a new biophysical probe based on occupation number fluctuations. Annu Rev Biophys Bioeng. 1975;4(00):311–334. doi: 10.1146/annurev.bb.04.060175.001523. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Calhoun D. B., Englander J. J. Biochemistry without oxygen. Anal Biochem. 1987 Mar;161(2):300–306. doi: 10.1016/0003-2697(87)90454-4. [DOI] [PubMed] [Google Scholar]
- Fraser A. B., Eisenberg E., Kielley W. W., Carlson F. D. The interaction of heavy meromyosin and subfragment 1 with actin. Physical measurements in the presence and absence of adenosine triphosphate. Biochemistry. 1975 May 20;14(10):2207–2214. doi: 10.1021/bi00681a025. [DOI] [PubMed] [Google Scholar]
- Fujime S., Ishiwata S. Dynamic study of F-actin by quasielastic scattering of laser light. J Mol Biol. 1971 Nov 28;62(1):251–265. doi: 10.1016/0022-2836(71)90144-6. [DOI] [PubMed] [Google Scholar]
- Fujime S., Ishiwata S., Maeda T. F-actin-heavy meromyosin complex studied by optical homodyne and heterodyne methods. Biochim Biophys Acta. 1972 Nov 17;283(2):351–363. doi: 10.1016/0005-2728(72)90251-4. [DOI] [PubMed] [Google Scholar]
- Fujime S. Quasi-elastic scattering of laser light. A new tool for the dynamic study of biological macromolecules. Adv Biophys. 1972;3:1–43. [PubMed] [Google Scholar]
- Goldman Y. E., Hibberd M. G., Trentham D. R. Initiation of active contraction by photogeneration of adenosine-5'-triphosphate in rabbit psoas muscle fibres. J Physiol. 1984 Sep;354:605–624. doi: 10.1113/jphysiol.1984.sp015395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada Y., Sakurada K., Aoki T., Thomas D. D., Yanagida T. Mechanochemical coupling in actomyosin energy transduction studied by in vitro movement assay. J Mol Biol. 1990 Nov 5;216(1):49–68. doi: 10.1016/S0022-2836(05)80060-9. [DOI] [PubMed] [Google Scholar]
- Highsmith S., Mendelson R. A., Morales M. F. Affinity of myosin S-1 for F-actin, measured by time-resolved fluorescence anisotropy. Proc Natl Acad Sci U S A. 1976 Jan;73(1):133–137. doi: 10.1073/pnas.73.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseal T. W., Bustamante C., Stump R. F., Maestre M. F. Real-time imaging of single DNA molecules with fluorescence microscopy. Biophys J. 1989 Sep;56(3):507–516. doi: 10.1016/S0006-3495(89)82697-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Janmey P. A., Hvidt S., Oster G. F., Lamb J., Stossel T. P., Hartwig J. H. Effect of ATP on actin filament stiffness. Nature. 1990 Sep 6;347(6288):95–99. doi: 10.1038/347095a0. [DOI] [PubMed] [Google Scholar]
- Kron S. J., Spudich J. A. Fluorescent actin filaments move on myosin fixed to a glass surface. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6272–6276. doi: 10.1073/pnas.83.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymn R. W., Taylor E. W. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochemistry. 1971 Dec 7;10(25):4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- Magde D., Elson E. L., Webb W. W. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974 Jan;13(1):29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- Newman J., Carlson F. D. Dynamic light-scattering evidence for the flexibility of native muscle thin filaments. Biophys J. 1980 Jan;29(1):37–47. doi: 10.1016/S0006-3495(80)85116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oplatka A., Gadasi H., Tirosh R., Lamed Y., Muhlrad A., Liron N. Demonstration of mechanochemical coupling in systems containing actin, atp and non-aggregating active myosin derivatives. J Mechanochem Cell Motil. 1974 Mar;2(4):295–306. [PubMed] [Google Scholar]
- Podolsky R. J. The rate-limiting step in muscle contraction. Basic Res Cardiol. 1980 Jan-Feb;75(1):34–39. doi: 10.1007/BF02001391. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Takebayashi T., Morita Y., Oosawa F. Electronmicroscopic investigation of the flexibility of F-actin. Biochim Biophys Acta. 1977 Jun 24;492(2):357–363. doi: 10.1016/0005-2795(77)90086-1. [DOI] [PubMed] [Google Scholar]
- Thomas D. D., Seidel J. C., Gergely J. Rotational dynamics of spin-labeled F-actin in the sub-millisecond time range. J Mol Biol. 1979 Aug 15;132(3):257–273. doi: 10.1016/0022-2836(79)90259-6. [DOI] [PubMed] [Google Scholar]
- Tirosh R., Low W. Z., Oplatka A. Translational motion of actin filaments in the presence of heavy meromyosin and MgATP as measured by Doppler broadening of laser light scattering. Biochim Biophys Acta. 1990 Mar 1;1037(3):274–280. doi: 10.1016/0167-4838(90)90025-b. [DOI] [PubMed] [Google Scholar]
- Uyeda T. Q., Warrick H. M., Kron S. J., Spudich J. A. Quantized velocities at low myosin densities in an in vitro motility assay. Nature. 1991 Jul 25;352(6333):307–311. doi: 10.1038/352307a0. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Studies on the chymotryptic digestion of myosin. Effects of divalent cations on proteolytic susceptibility. J Mol Biol. 1977 Apr;111(2):129–157. doi: 10.1016/s0022-2836(77)80119-8. [DOI] [PubMed] [Google Scholar]
- Yanagida T., Arata T., Oosawa F. Sliding distance of actin filament induced by a myosin crossbridge during one ATP hydrolysis cycle. Nature. 1985 Jul 25;316(6026):366–369. doi: 10.1038/316366a0. [DOI] [PubMed] [Google Scholar]
- Yanagida T., Nakase M., Nishiyama K., Oosawa F. Direct observation of motion of single F-actin filaments in the presence of myosin. Nature. 1984 Jan 5;307(5946):58–60. doi: 10.1038/307058a0. [DOI] [PubMed] [Google Scholar]
- Yanagida T., Oosawa F. Polarized fluorescence from epsilon-ADP incorporated into F-actin in a myosin-free single fiber: conformation of F-actin and changes induced in it by heavy meromyosin. J Mol Biol. 1978 Dec 15;126(3):507–524. doi: 10.1016/0022-2836(78)90056-6. [DOI] [PubMed] [Google Scholar]




