Abstract
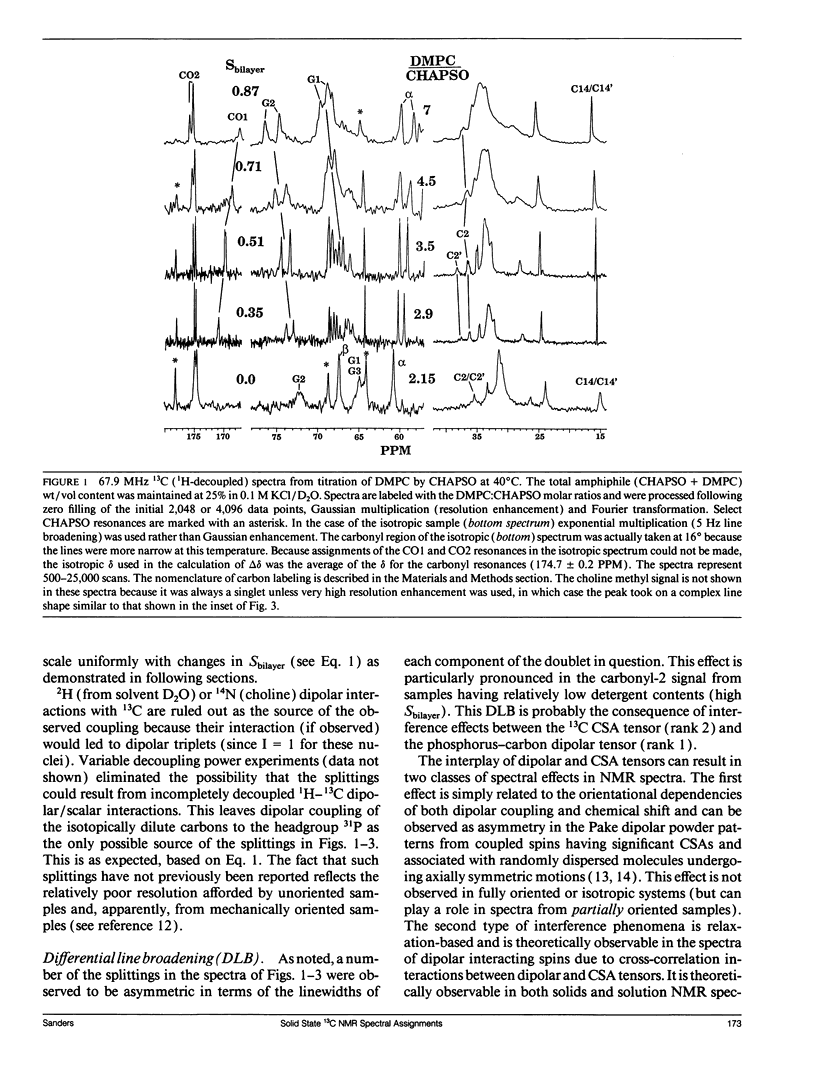
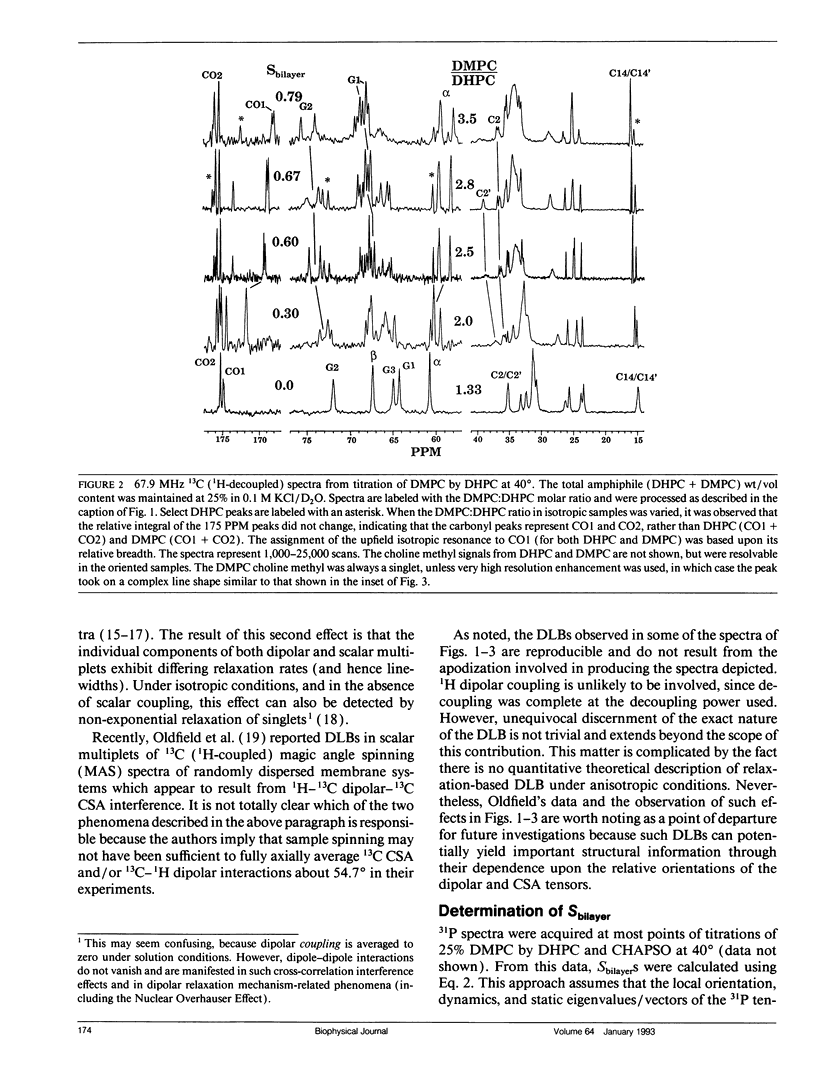
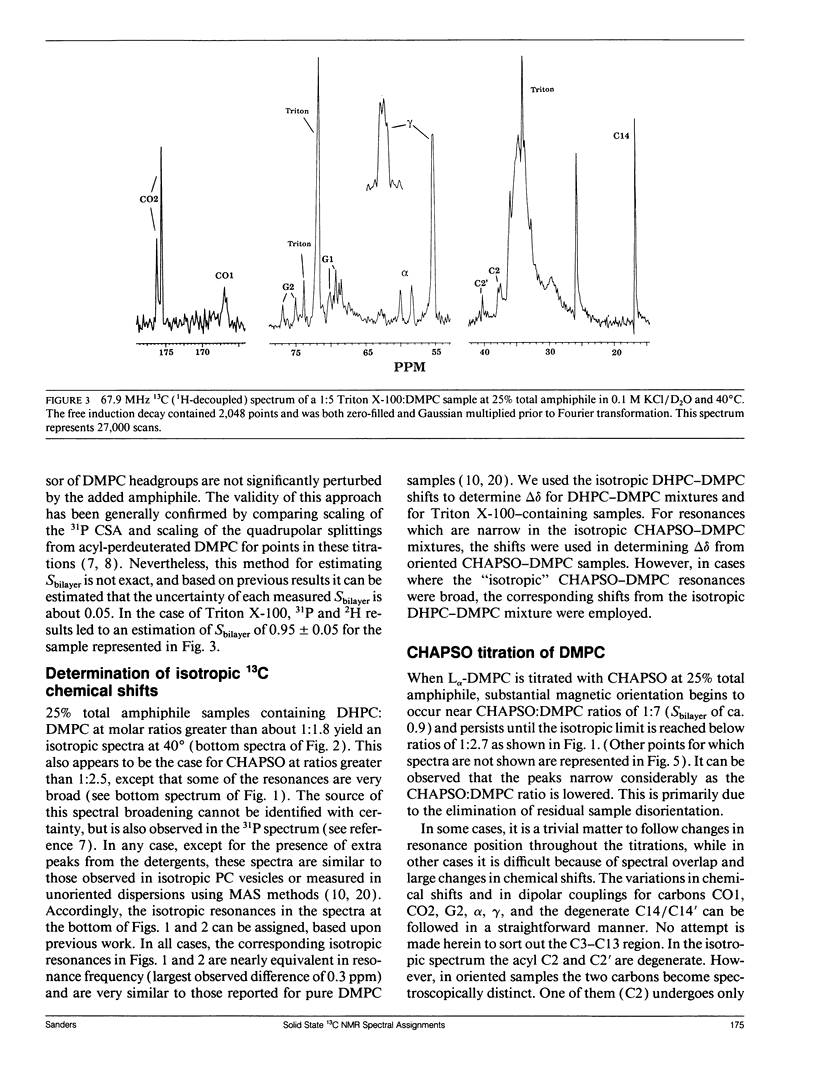
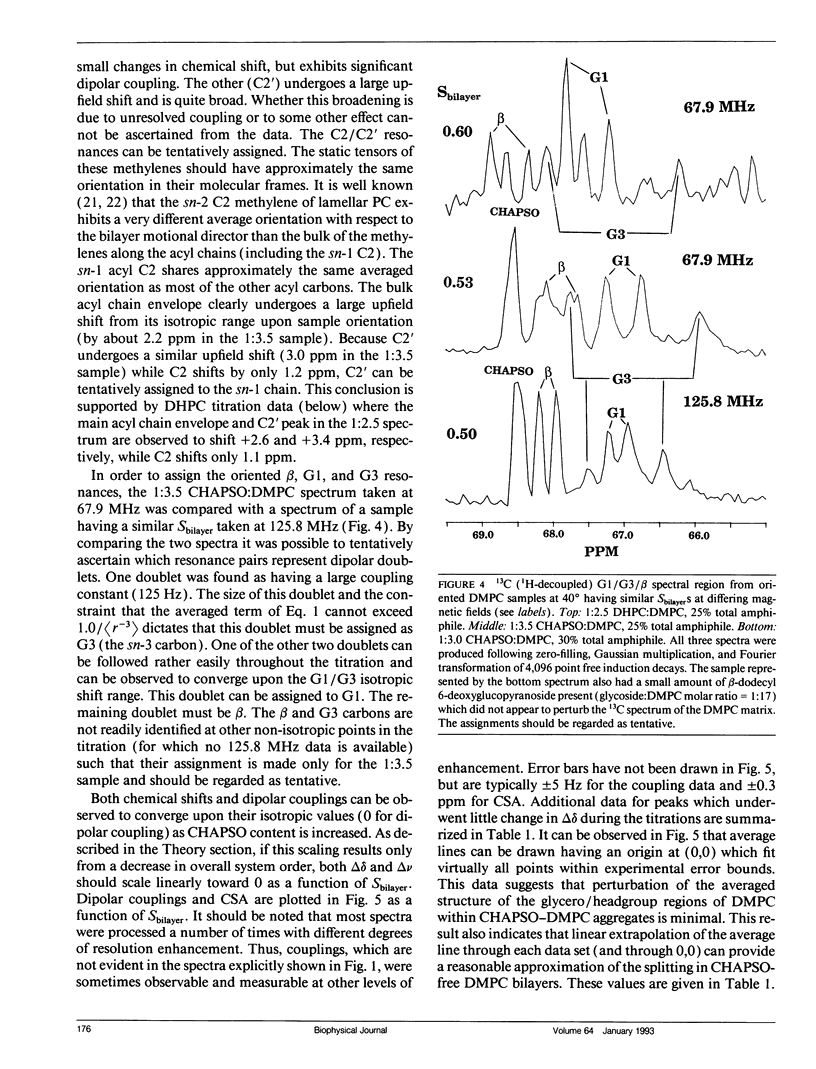
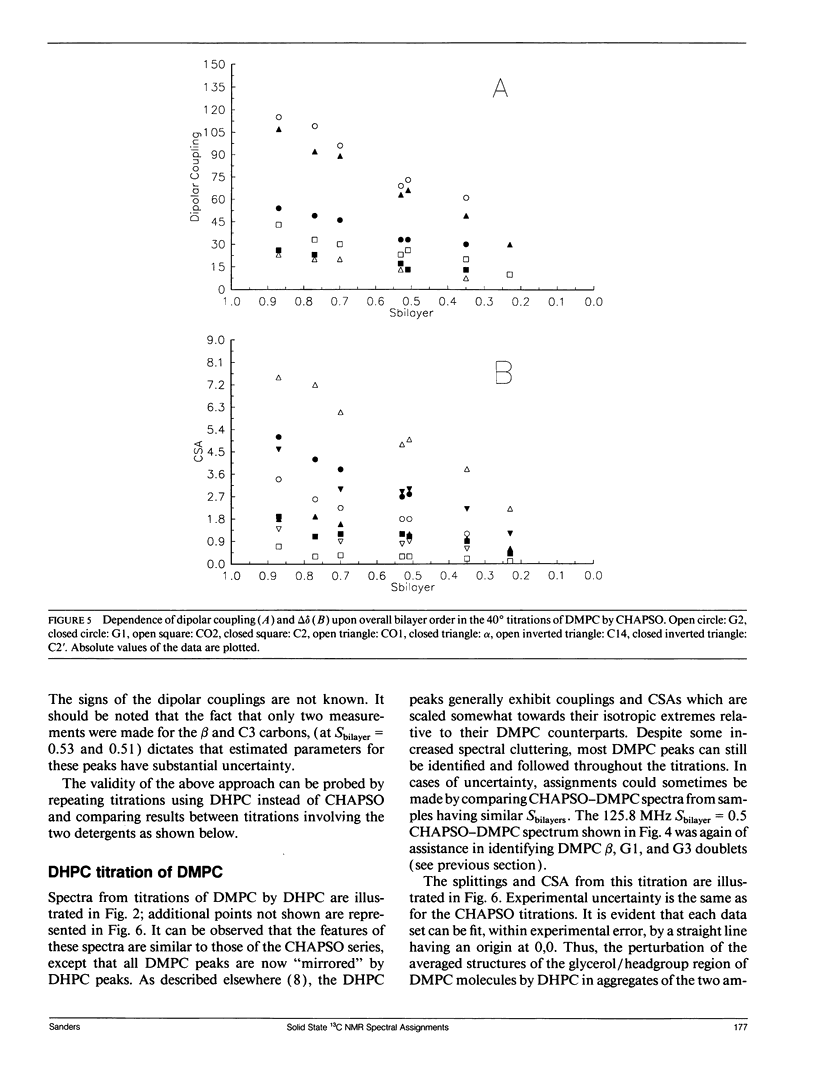
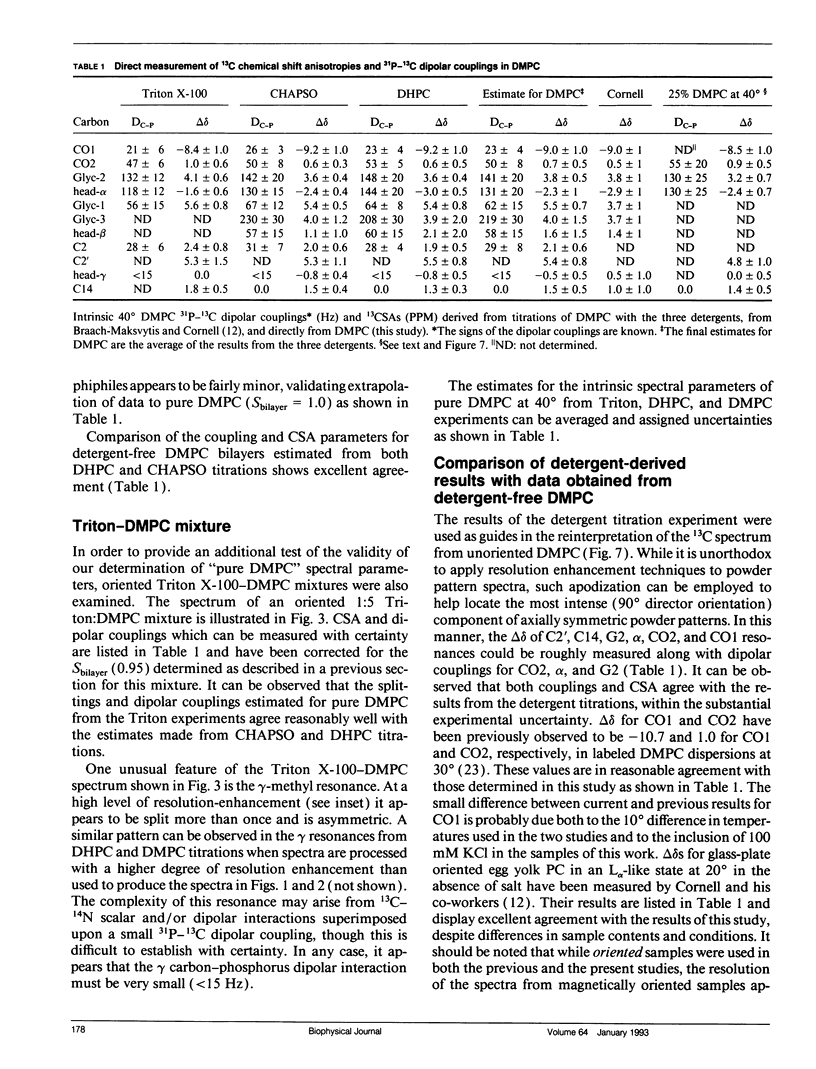
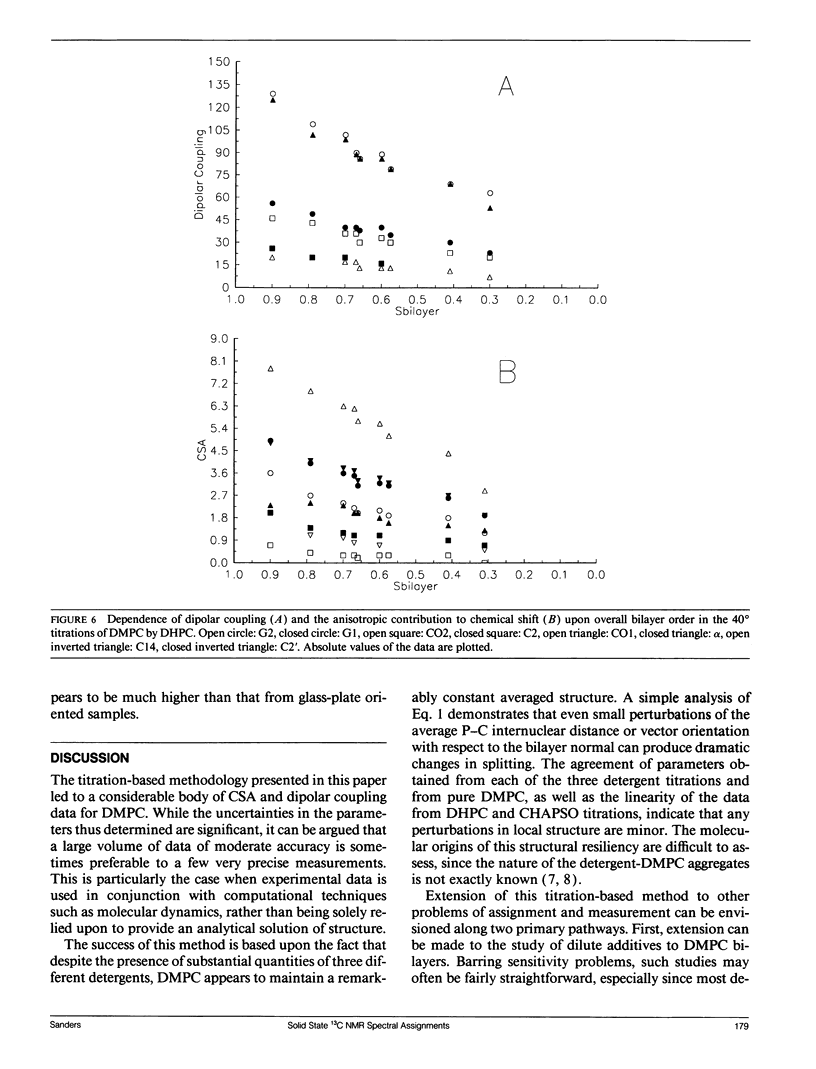
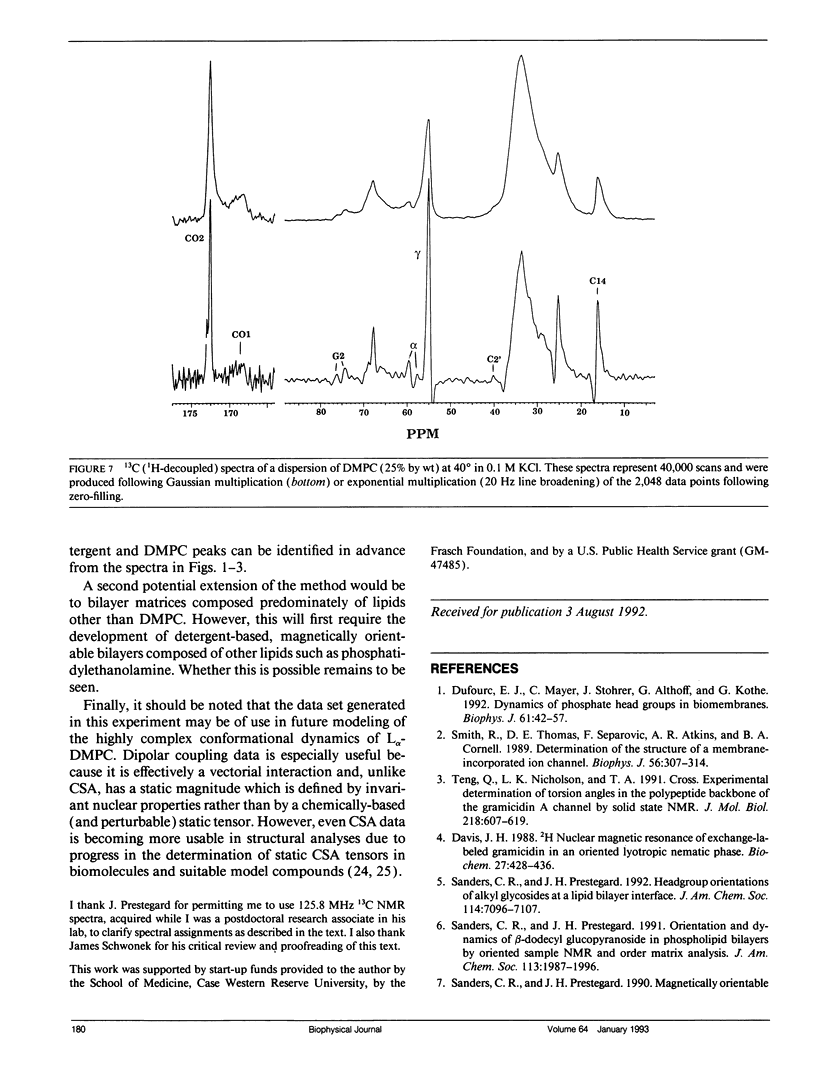
The direct measurement of 13C chemical shift anisotropies (CSA) and 31P-13C dipolar splitting in random dispersions of unlabeled L alpha-phase phosphatidylcholine (PC) has traditionally been difficult because of extreme spectral boradening due to anisotropy. In this study, mixtures of dimyristoyl phosphatidylcholine (DMPC) with three different detergents known to promote the magnetic orientation of DMPC were employed to eliminate the powder-pattern nature of signals without totally averaging out spectral anisotropy. The detergents utilized were CHAPSO, Triton X-100, and dihexanoylphosphatidylcholine (DHPC). Using such mixtures, many of the individual 13C resonances from DMPC were resolved and a number of 13C-31P dipolar couplings were evident. In addition, differing line widths were observed for the components of some dipolar doublets, suggestive of dipolar/chemical shift anisotropy (CSA) relaxation interference effects. Oriented sample resonance assignments were made by varying the CHAPSO or DHPC to DMPC ratio to systematically scale overall bilayer order towards the isotropic limit. In this manner, peaks could be identified based upon extrapolation to their isotropic positions, for which assignments have previously been made (Lee, C.W.B., and R.G. Griffin. 1989. Biophys. J. 55:355-358; Forbes, J., J. Bowers, X. Shan, L. Moran, E. Oldfield, and M.A. Moscarello. 1988. J. Chem. Soc., Faraday, Trans. 1 84:3821-3849). It was observed that the plots of CSA or dipolar coupling versus overall bilayer order obtained from DHPC and CHAPSO titrations were linear. Estimates of the intrinsic dipolar couplings and chemical shift anisotropies for pure DMPC bilayers were made by extrapolating shifts and couplings from the detergent titrations to zero detergent. Both detergent titrations led to similar "intrinsic" CSAs and dipolar couplings. Results extracted from an oriented Triton-DMPC mixture also led to similar estimates for the detergent-free DMPC shifts and couplings. The results from these experiments were found to compare favorably with limited measurements made from pure L alpha PC. This detergent-based method for assigning spectra and for determining dipolar couplings and CSA in detergent-free systems should be extendable to other lipid systems. The resulting data set from this study may prove useful in future modeling of the structure and dynamics of DMPC bilayers. In addition, the fact that experiments utilizing each of the three detergents led to similar estimates for the spectral parameters of pure DMPC, and the fact that spectral parameter versus bilayer order plots were linear, indicate that the averaged conformation and dynamics of DMPC in the presence of the three detergents are very similar to those of pure L alpha DMPC.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braach-Maksvytis V. L., Cornell B. A. Chemical shift anisotropies obtained from aligned egg yolk phosphatidylcholine by solid-state 13C nuclear magnetic resonance. Biophys J. 1988 May;53(5):839–843. doi: 10.1016/S0006-3495(88)83163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J. H. 2H nuclear magnetic resonance of exchange-labeled gramicidin in an oriented lyotropic nematic phase. Biochemistry. 1988 Jan 12;27(1):428–436. doi: 10.1021/bi00401a064. [DOI] [PubMed] [Google Scholar]
- Dufourc E. J., Mayer C., Stohrer J., Althoff G., Kothe G. Dynamics of phosphate head groups in biomembranes. Comprehensive analysis using phosphorus-31 nuclear magnetic resonance lineshape and relaxation time measurements. Biophys J. 1992 Jan;61(1):42–57. doi: 10.1016/S0006-3495(92)81814-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigenberg K. E., Chan S. I. The effect of surface curvature on the head-group structure and phase transition properties of phospholipid bilayer vesicles. Biochim Biophys Acta. 1980 Jun 20;599(1):330–335. doi: 10.1016/0005-2736(80)90079-6. [DOI] [PubMed] [Google Scholar]
- Hauser H., Pascher I., Pearson R. H., Sundell S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim Biophys Acta. 1981 Jun 16;650(1):21–51. doi: 10.1016/0304-4157(81)90007-1. [DOI] [PubMed] [Google Scholar]
- Lee C. W., Griffin R. G. Two-dimensional 1H/13C heteronuclear chemical shift correlation spectroscopy of lipid bilayers. Biophys J. 1989 Feb;55(2):355–358. doi: 10.1016/S0006-3495(89)82812-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield E., Adebodun F., Chung J., Montez B., Park K. D., Le H. B., Phillips B. Carbon-13 nuclear magnetic resonance spectroscopy of lipids: differential line broadening due to cross-correlation effects as a probe of membrane structure. Biochemistry. 1991 Nov 19;30(46):11025–11028. doi: 10.1021/bi00110a003. [DOI] [PubMed] [Google Scholar]
- Sanders C. R., 2nd, Prestegard J. H. Magnetically orientable phospholipid bilayers containing small amounts of a bile salt analogue, CHAPSO. Biophys J. 1990 Aug;58(2):447–460. doi: 10.1016/S0006-3495(90)82390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. R., 2nd, Schwonek J. P. Characterization of magnetically orientable bilayers in mixtures of dihexanoylphosphatidylcholine and dimyristoylphosphatidylcholine by solid-state NMR. Biochemistry. 1992 Sep 22;31(37):8898–8905. doi: 10.1021/bi00152a029. [DOI] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Seelig J., Seelig A. Lipid conformation in model membranes and biological membranes. Q Rev Biophys. 1980 Feb;13(1):19–61. doi: 10.1017/s0033583500000305. [DOI] [PubMed] [Google Scholar]
- Smith R., Thomas D. E., Separovic F., Atkins A. R., Cornell B. A. Determination of the structure of a membrane-incorporated ion channel. Solid-state nuclear magnetic resonance studies of gramicidin A. Biophys J. 1989 Aug;56(2):307–314. doi: 10.1016/S0006-3495(89)82677-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng Q., Nicholson L. K., Cross T. A. Experimental determination of torsion angles in the polypeptide backbone of the gramicidin A channel by solid state nuclear magnetic resonance. J Mol Biol. 1991 Apr 5;218(3):607–619. doi: 10.1016/0022-2836(91)90705-b. [DOI] [PubMed] [Google Scholar]
- Wittebort R. J., Blume A., Huang T. H., Das Gupta S. K., Griffin R. G. Carbon-13 nuclear magnetic resonance investigations of phase transitions and phase equilibria in pure and mixed phospholipid bilayers. Biochemistry. 1982 Jul 6;21(14):3487–3502. doi: 10.1021/bi00257a036. [DOI] [PubMed] [Google Scholar]


