Abstract
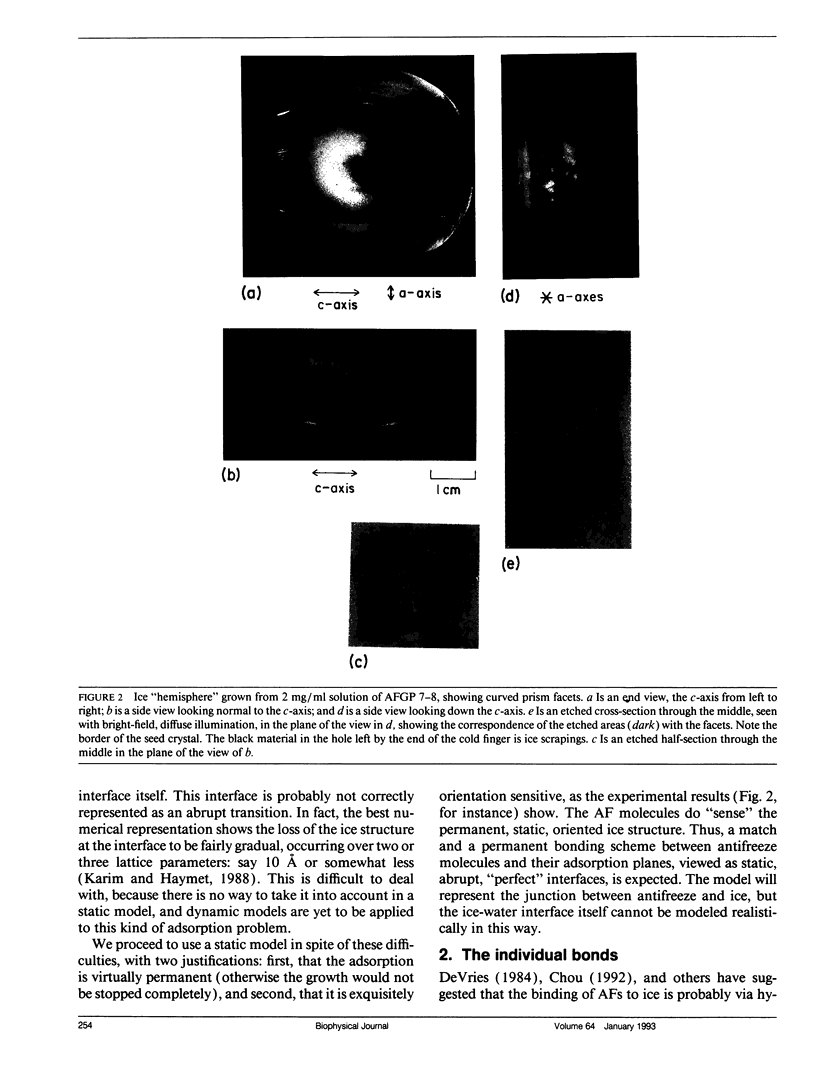
Experimental results show that fish antifreeze glycopeptides (AFGPs) 8 and 7 (with 4 and 5 repeats respectively of the Ala-Ala-Thr backbone sequence) bond onto ice prism planes aligned along a-axes, and inhibit crystal growth on prism planes and on surfaces close to that orientation. The 9.31-A repeat spacing of the AFGP in the polyproline II helix configuration, deduced from NMR studies, matches twice the repeat spacing of ice in the deduced alignment direction, 9.038 A, within 3%. A specific binding model is proposed for the AFGP and for the alpha-helical antifreeze peptide of winter flounder. For AFGP 7-8, two hydroxyl groups of each disaccharide (one disaccharide is attached to each threonine) reside within the ice surface, so that they are shared between the ice crystal and the disaccharide. This provides 24 hydrogen bonds between AFGP 8 and the ice and 30 for AFGP 7, explaining why the chemical adsorption is virtually irreversible and the crystal growth can be stopped virtually completely. The same scheme of sharing polar groups with the ice works well with the alpha-helical antifreeze of winter flounder, for which an amide as well as several hydroxyls are shared. The sharing of polar groups with the ice crystal, rather than hydrogen-bonding to the ice surface, may be a general requirement for adsoprtion-inhibition of freezing.
Full text
PDF
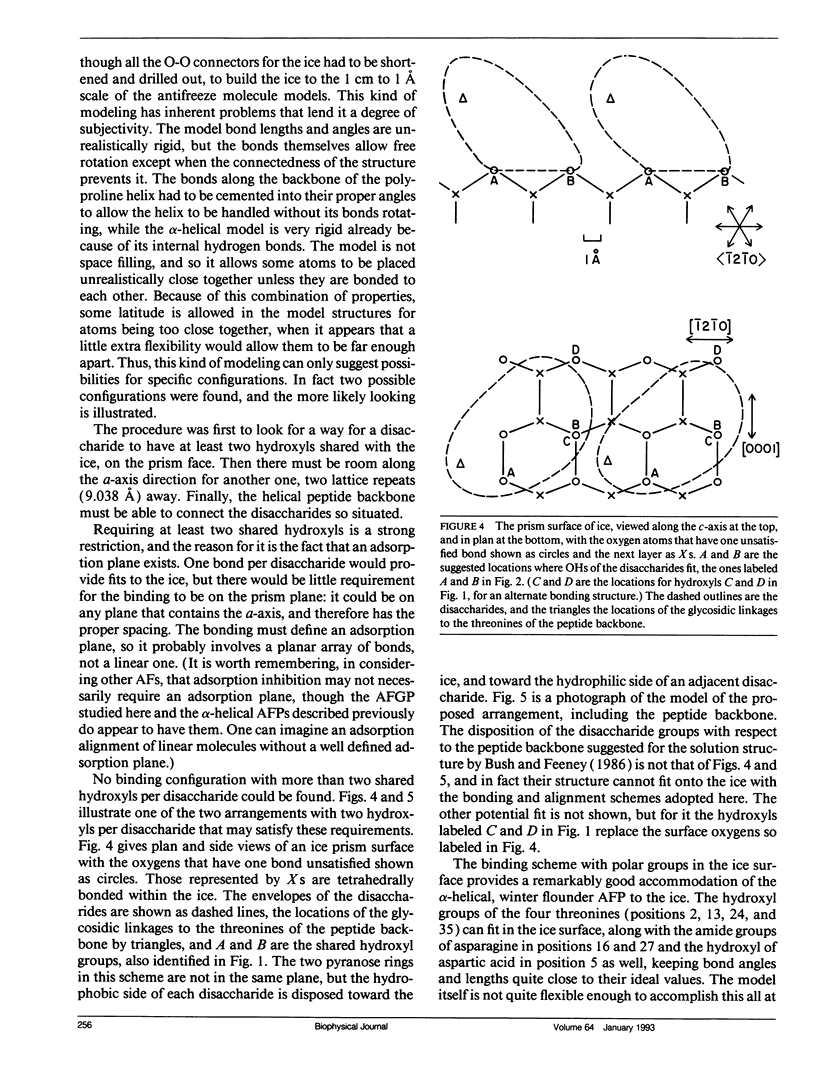
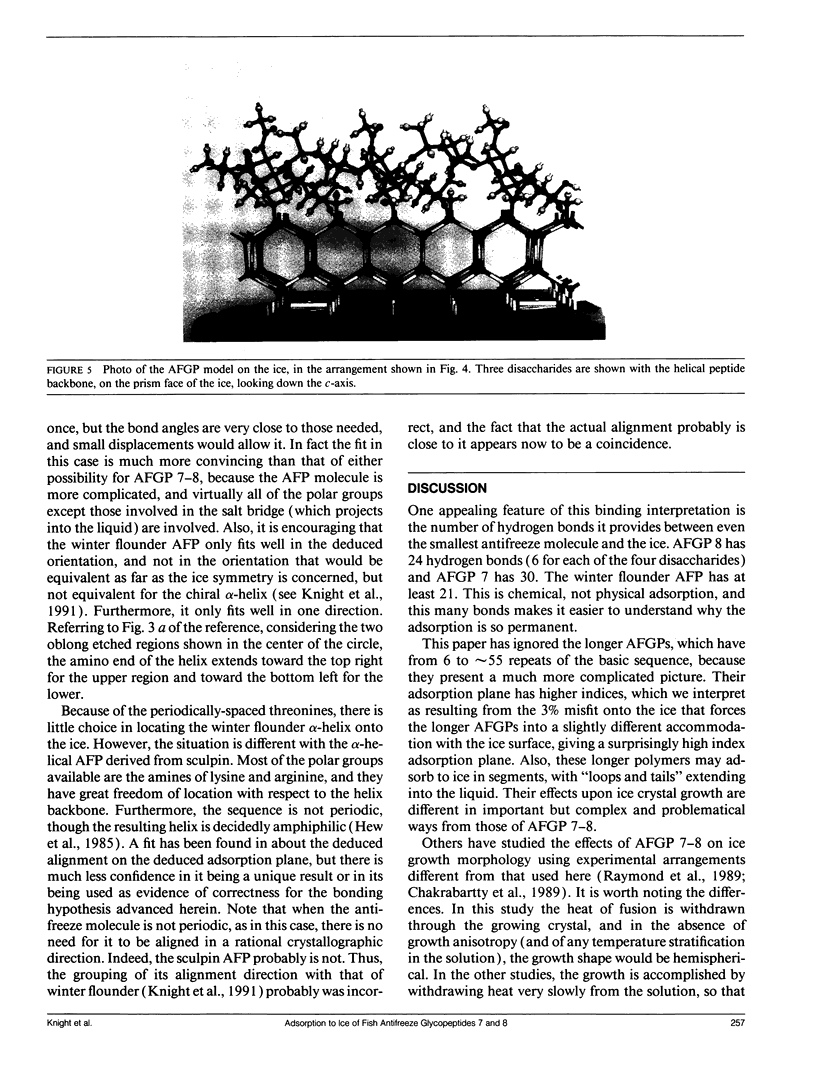
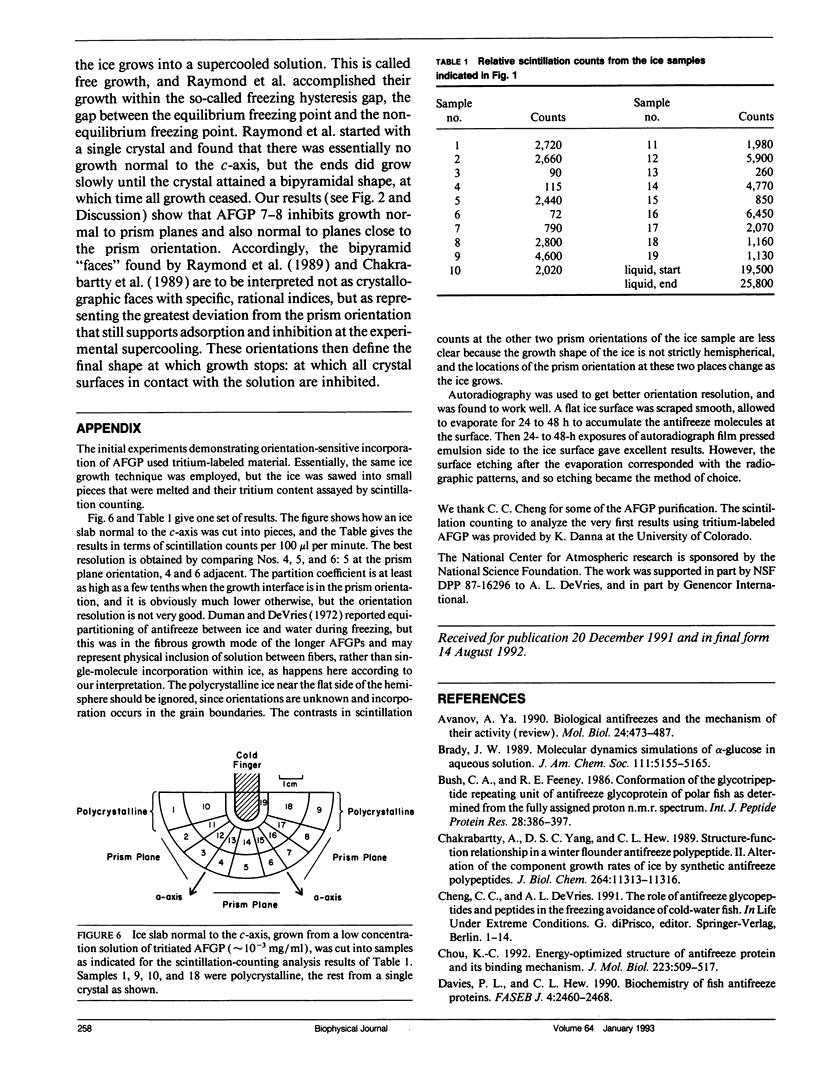

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bush C. A., Feeney R. E. Conformation of the glycotripeptide repeating unit of antifreeze glycoprotein of polar fish as determined from the fully assigned proton n.m.r. spectrum. Int J Pept Protein Res. 1986 Oct;28(4):386–397. doi: 10.1111/j.1399-3011.1986.tb03270.x. [DOI] [PubMed] [Google Scholar]
- Chakrabartty A., Yang D. S., Hew C. L. Structure-function relationship in a winter flounder antifreeze polypeptide. II. Alteration of the component growth rates of ice by synthetic antifreeze polypeptides. J Biol Chem. 1989 Jul 5;264(19):11313–11316. [PubMed] [Google Scholar]
- Chou K. C. Energy-optimized structure of antifreeze protein and its binding mechanism. J Mol Biol. 1992 Jan 20;223(2):509–517. doi: 10.1016/0022-2836(92)90666-8. [DOI] [PubMed] [Google Scholar]
- Davies P. L., Hew C. L. Biochemistry of fish antifreeze proteins. FASEB J. 1990 May;4(8):2460–2468. doi: 10.1096/fasebj.4.8.2185972. [DOI] [PubMed] [Google Scholar]
- DeVries A. L., Komatsu S. K., Feeney R. E. Chemical and physical properties of freezing point-depressing glycoproteins from Antarctic fishes. J Biol Chem. 1970 Jun 10;245(11):2901–2908. [PubMed] [Google Scholar]
- Duman J. G., DeVries A. L. Freezing behavior of aqueous solutions of glycoproteins from the blood of an Antarctic fish. Cryobiology. 1972 Oct;9(5):469–472. doi: 10.1016/0011-2240(72)90166-6. [DOI] [PubMed] [Google Scholar]
- Franks F., Morris E. R. Blood glycoprotein from antarctic fish. Possible conformational origin of antifreeze activity. Biochim Biophys Acta. 1978 May 3;540(2):346–356. doi: 10.1016/0304-4165(78)90148-4. [DOI] [PubMed] [Google Scholar]
- Hew C. L., Joshi S., Wang N. C., Kao M. H., Ananthanarayanan V. S. Structures of shorthorn sculpin antifreeze polypeptides. Eur J Biochem. 1985 Aug 15;151(1):167–172. doi: 10.1111/j.1432-1033.1985.tb09081.x. [DOI] [PubMed] [Google Scholar]
- Knight C. A., Cheng C. C., DeVries A. L. Adsorption of alpha-helical antifreeze peptides on specific ice crystal surface planes. Biophys J. 1991 Feb;59(2):409–418. doi: 10.1016/S0006-3495(91)82234-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao B. N., Bush C. A. Comparison by 1H-nmr spectroscopy of the conformation of the 2600 dalton antifreeze glycopeptide of polar cod with that of the high molecular weight antifreeze glycoprotein. Biopolymers. 1987 Aug;26(8):1227–1244. doi: 10.1002/bip.360260803. [DOI] [PubMed] [Google Scholar]
- Raymond J. A., Radding W., DeVries A. L. Circular dichroism of protein and glycoprotein fish antifreezes. Biopolymers. 1977 Nov;16(11):2575–2578. doi: 10.1002/bip.1977.360161119. [DOI] [PubMed] [Google Scholar]
- Raymond J. A., Wilson P., DeVries A. L. Inhibition of growth of nonbasal planes in ice by fish antifreezes. Proc Natl Acad Sci U S A. 1989 Feb;86(3):881–885. doi: 10.1073/pnas.86.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shier W. T., Lin Y., DeVries A. L. Structure of the carbohydrate of antifreeze glycoproteins from an antartic fish. FEBS Lett. 1975 Jun 15;54(2):135–138. doi: 10.1016/0014-5793(75)80060-3. [DOI] [PubMed] [Google Scholar]