Abstract
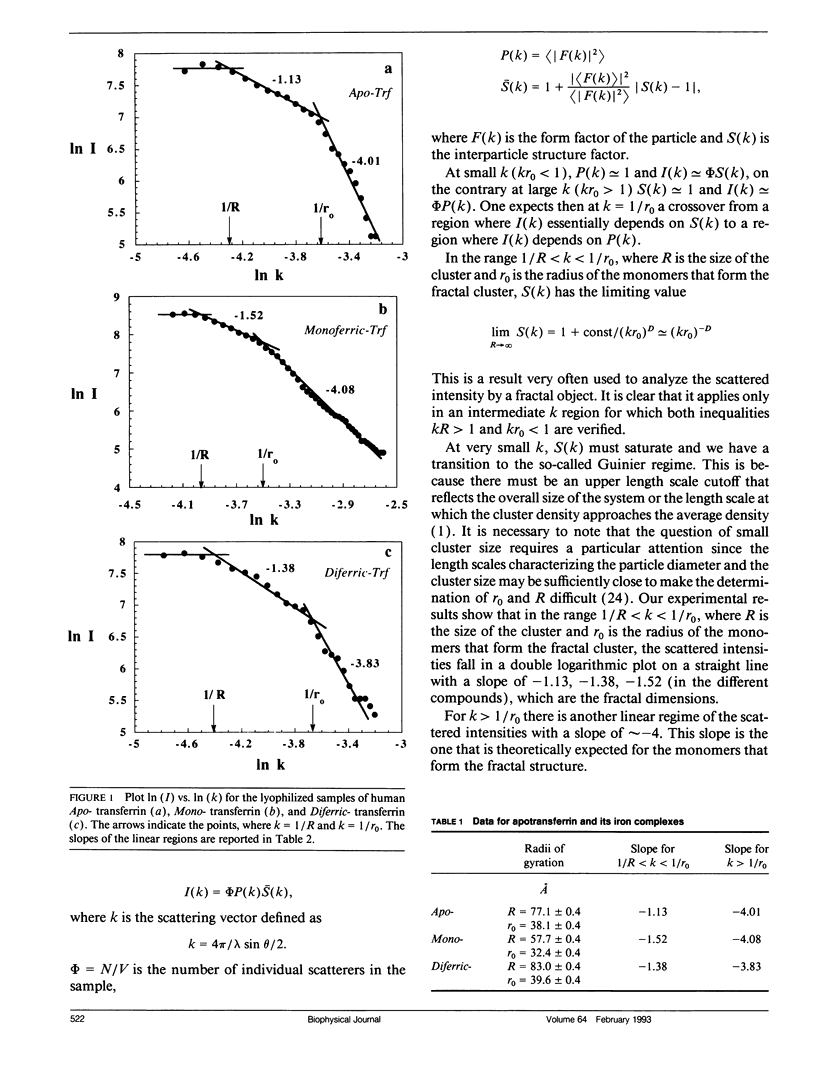
X-ray small angle scattering experiments, using a pin hole SAXS camera with Synchrotron radiation source, have been performed to study the conformational changes of lyophilized samples of Apo-, Mono-, and Diferric- human transferrin. We report the experimental evidence that the analysis of the scattered intensity through the fractal theory may give information on the particle size and its variation upon iron binding.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson B. F., Baker H. M., Norris G. E., Rumball S. V., Baker E. N. Apolactoferrin structure demonstrates ligand-induced conformational change in transferrins. Nature. 1990 Apr 19;344(6268):784–787. doi: 10.1038/344784a0. [DOI] [PubMed] [Google Scholar]
- Bailey S., Evans R. W., Garratt R. C., Gorinsky B., Hasnain S., Horsburgh C., Jhoti H., Lindley P. F., Mydin A., Sarra R. Molecular structure of serum transferrin at 3.3-A resolution. Biochemistry. 1988 Jul 26;27(15):5804–5812. doi: 10.1021/bi00415a061. [DOI] [PubMed] [Google Scholar]
- Kilár F., Simon I. The effect of iron binding on the conformation of transferrin. A small angle x-ray scattering study. Biophys J. 1985 Nov;48(5):799–802. doi: 10.1016/S0006-3495(85)83838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M., Rees D. C. Fractal surfaces of proteins. Science. 1985 Dec 6;230(4730):1163–1165. doi: 10.1126/science.4071040. [DOI] [PubMed] [Google Scholar]
- Martel P., Kim S. M., Powell B. M. Physical characteristics of human transferrin from small angle neutron scattering. Biophys J. 1980 Sep;31(3):371–380. doi: 10.1016/S0006-3495(80)85065-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskams A. J., Connor J. R. Aluminum access to the brain: a role for transferrin and its receptor. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9024–9027. doi: 10.1073/pnas.87.22.9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseneu-Motreff M. Y., Soetewey F., Lamote R., Peeters H. Size and shape determination of apotransferrin and transferrin monomers. Biopolymers. 1971 Jun;10(6):1039–1048. doi: 10.1002/bip.360100610. [DOI] [PubMed] [Google Scholar]
- Thompson C. P., Grady J. K., Chasteen N. D. The influence of uncoordinated histidines on iron release from transferrin. A chemical modification study. J Biol Chem. 1986 Oct 5;261(28):13128–13134. [PubMed] [Google Scholar]
- Trowbridge I. S., Lopez F. Monoclonal antibody to transferrin receptor blocks transferrin binding and inhibits human tumor cell growth in vitro. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1175–1179. doi: 10.1073/pnas.79.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]


