Abstract
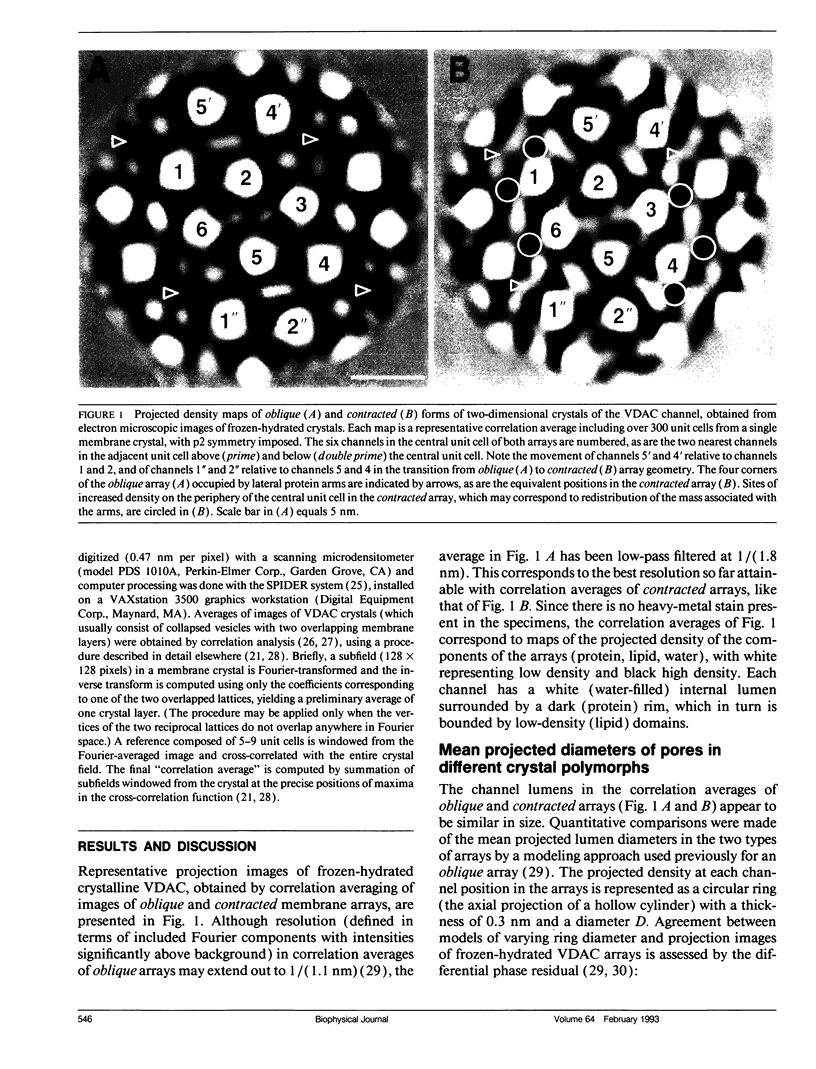
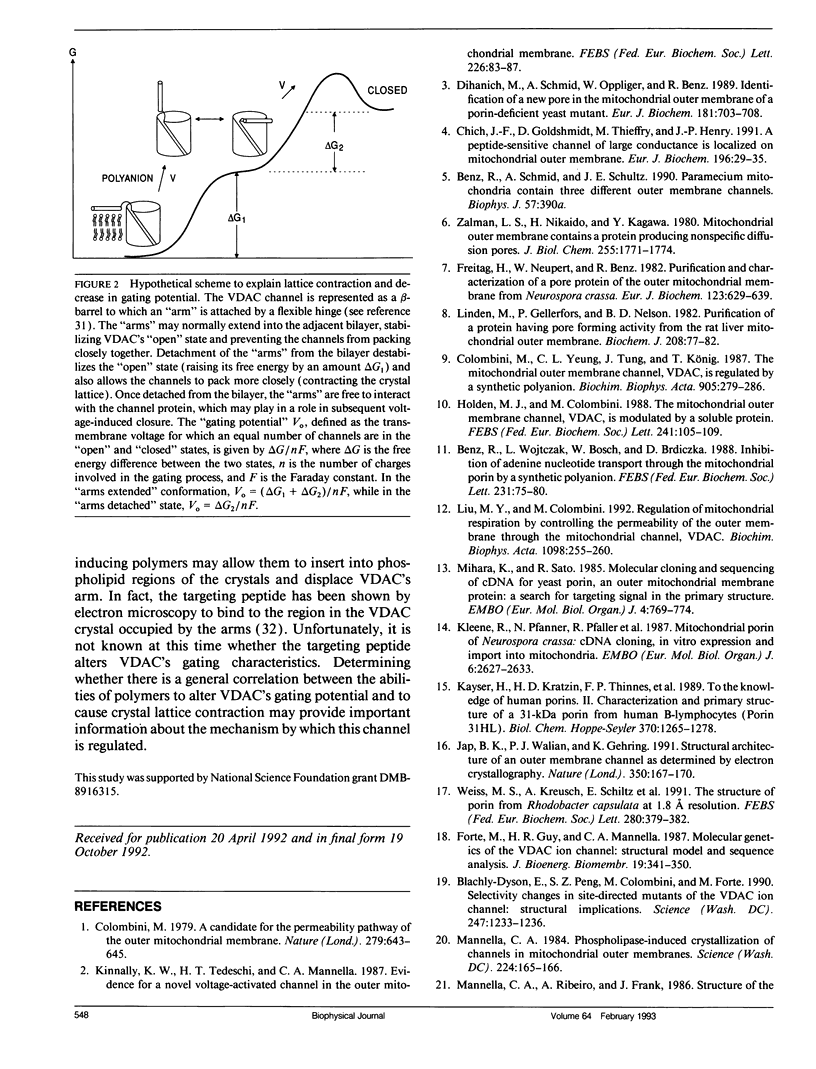
Crystalline arrays of the voltage-dependent channel, VDAC, can be produced by treatment of Neurospora mitochondrial outer membranes with phospholipase A2. The membrane crystals undergo a lateral phase transition (lattice contraction) that can be induced by an amphipathic polyanion, which also reduces the channel's gating potential. Electron cryo-microscopy of frozen-hydrated crystals indicates that the mean projected diameters of the channels do not decrease with lattice contraction. Instead, contraction is associated with the disappearance of lateral protein "arms" that normally extend between the channels. A model is presented that explains the changes in channel packing and gating potential in terms of a conformational change involving the movement of a protein "arm" between the bilayer and the channel.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Wojtczak L., Bosch W., Brdiczka D. Inhibition of adenine nucleotide transport through the mitochondrial porin by a synthetic polyanion. FEBS Lett. 1988 Apr 11;231(1):75–80. doi: 10.1016/0014-5793(88)80706-3. [DOI] [PubMed] [Google Scholar]
- Blachly-Dyson E., Peng S., Colombini M., Forte M. Selectivity changes in site-directed mutants of the VDAC ion channel: structural implications. Science. 1990 Mar 9;247(4947):1233–1236. doi: 10.1126/science.1690454. [DOI] [PubMed] [Google Scholar]
- Chich J. F., Goldschmidt D., Thieffry M., Henry J. P. A peptide-sensitive channel of large conductance is localized on mitochondrial outer membrane. Eur J Biochem. 1991 Feb 26;196(1):29–35. doi: 10.1111/j.1432-1033.1991.tb15781.x. [DOI] [PubMed] [Google Scholar]
- Colombini M. A candidate for the permeability pathway of the outer mitochondrial membrane. Nature. 1979 Jun 14;279(5714):643–645. doi: 10.1038/279643a0. [DOI] [PubMed] [Google Scholar]
- Colombini M., Yeung C. L., Tung J., König T. The mitochondrial outer membrane channel, VDAC, is regulated by a synthetic polyanion. Biochim Biophys Acta. 1987 Dec 11;905(2):279–286. doi: 10.1016/0005-2736(87)90456-1. [DOI] [PubMed] [Google Scholar]
- Dihanich M., Schmid A., Oppliger W., Benz R. Identification of a new pore in the mitochondrial outer membrane of a porin-deficient yeast mutant. Eur J Biochem. 1989 May 15;181(3):703–708. doi: 10.1111/j.1432-1033.1989.tb14780.x. [DOI] [PubMed] [Google Scholar]
- Forte M., Guy H. R., Mannella C. A. Molecular genetics of the VDAC ion channel: structural model and sequence analysis. J Bioenerg Biomembr. 1987 Aug;19(4):341–350. doi: 10.1007/BF00768537. [DOI] [PubMed] [Google Scholar]
- Frank J., Verschoor A., Boublik M. Computer averaging of electron micrographs of 40S ribosomal subunits. Science. 1981 Dec 18;214(4527):1353–1355. doi: 10.1126/science.7313694. [DOI] [PubMed] [Google Scholar]
- Freitag H., Neupert W., Benz R. Purification and characterisation of a pore protein of the outer mitochondrial membrane from Neurospora crassa. Eur J Biochem. 1982 Apr;123(3):629–636. doi: 10.1111/j.1432-1033.1982.tb06578.x. [DOI] [PubMed] [Google Scholar]
- Holden M. J., Colombini M. The mitochondrial outer membrane channel, VDAC, is modulated by a soluble protein. FEBS Lett. 1988 Dec 5;241(1-2):105–109. doi: 10.1016/0014-5793(88)81040-8. [DOI] [PubMed] [Google Scholar]
- Jap B. K., Walian P. J., Gehring K. Structural architecture of an outer membrane channel as determined by electron crystallography. Nature. 1991 Mar 14;350(6314):167–170. doi: 10.1038/350167a0. [DOI] [PubMed] [Google Scholar]
- Kayser H., Kratzin H. D., Thinnes F. P., Götz H., Schmidt W. E., Eckart K., Hilschmann N. Zur Kenntnis der Porine des Menschen. II. Charakterisierung und Primärstruktur eines 31-kDA-Porins aus menschlichen B-Lymphozyten (Porin 31HL). Biol Chem Hoppe Seyler. 1989 Dec;370(12):1265–1278. [PubMed] [Google Scholar]
- Kessel M., Radermacher M., Frank J. The structure of the stalk surface layer of a brine pond microorganism: correlation averaging applied to a double layered lattice structure. J Microsc. 1985 Jul;139(Pt 1):63–74. doi: 10.1111/j.1365-2818.1985.tb04662.x. [DOI] [PubMed] [Google Scholar]
- Kinnally K. W., Tedeschi H., Mannella C. A. Evidence for a novel voltage-activated channel in the outer mitochondrial membrane. FEBS Lett. 1987 Dec 21;226(1):83–87. doi: 10.1016/0014-5793(87)80555-0. [DOI] [PubMed] [Google Scholar]
- Kleene R., Pfanner N., Pfaller R., Link T. A., Sebald W., Neupert W., Tropschug M. Mitochondrial porin of Neurospora crassa: cDNA cloning, in vitro expression and import into mitochondria. EMBO J. 1987 Sep;6(9):2627–2633. doi: 10.1002/j.1460-2075.1987.tb02553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindén M., Gellerfors P., Nelson B. D. Purification of a protein having pore forming activity from the rat liver mitochondrial outer membrane. Biochem J. 1982 Oct 15;208(1):77–82. doi: 10.1042/bj2080077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. Y., Colombini M. Regulation of mitochondrial respiration by controlling the permeability of the outer membrane through the mitochondrial channel, VDAC. Biochim Biophys Acta. 1992 Jan 16;1098(2):255–260. doi: 10.1016/s0005-2728(05)80344-5. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Colombini M., Frank J. Structural and functional evidence for multiple channel complexes in the outer membrane of Neurospora crassa mitochondria. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2243–2247. doi: 10.1073/pnas.80.8.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella C. A., Guo X. W., Dias J. Binding of a synthetic targeting peptide to a mitochondrial channel protein. J Bioenerg Biomembr. 1992 Feb;24(1):55–61. doi: 10.1007/BF00769531. [DOI] [PubMed] [Google Scholar]
- Mannella C. A., Guo X. W. Interaction between the VDAC channel and a polyanionic effector. An electron microscopic study. Biophys J. 1990 Jan;57(1):23–31. doi: 10.1016/S0006-3495(90)82503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella C. A. Phospholipase-induced crystallization of channels in mitochondrial outer membranes. Science. 1984 Apr 13;224(4645):165–166. doi: 10.1126/science.6322311. [DOI] [PubMed] [Google Scholar]
- Mannella C. A. Structural analysis of mitochondrial pores. Experientia. 1990 Feb 15;46(2):137–145. doi: 10.1007/BF02027309. [DOI] [PubMed] [Google Scholar]
- Mihara K., Sato R. Molecular cloning and sequencing of cDNA for yeast porin, an outer mitochondrial membrane protein: a search for targeting signal in the primary structure. EMBO J. 1985 Mar;4(3):769–774. doi: 10.1002/j.1460-2075.1985.tb03695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. S., Kreusch A., Schiltz E., Nestel U., Welte W., Weckesser J., Schulz G. E. The structure of porin from Rhodobacter capsulatus at 1.8 A resolution. FEBS Lett. 1991 Mar 25;280(2):379–382. doi: 10.1016/0014-5793(91)80336-2. [DOI] [PubMed] [Google Scholar]
- Zalman L. S., Nikaido H., Kagawa Y. Mitochondrial outer membrane contains a protein producing nonspecific diffusion channels. J Biol Chem. 1980 Mar 10;255(5):1771–1774. [PubMed] [Google Scholar]



