Abstract
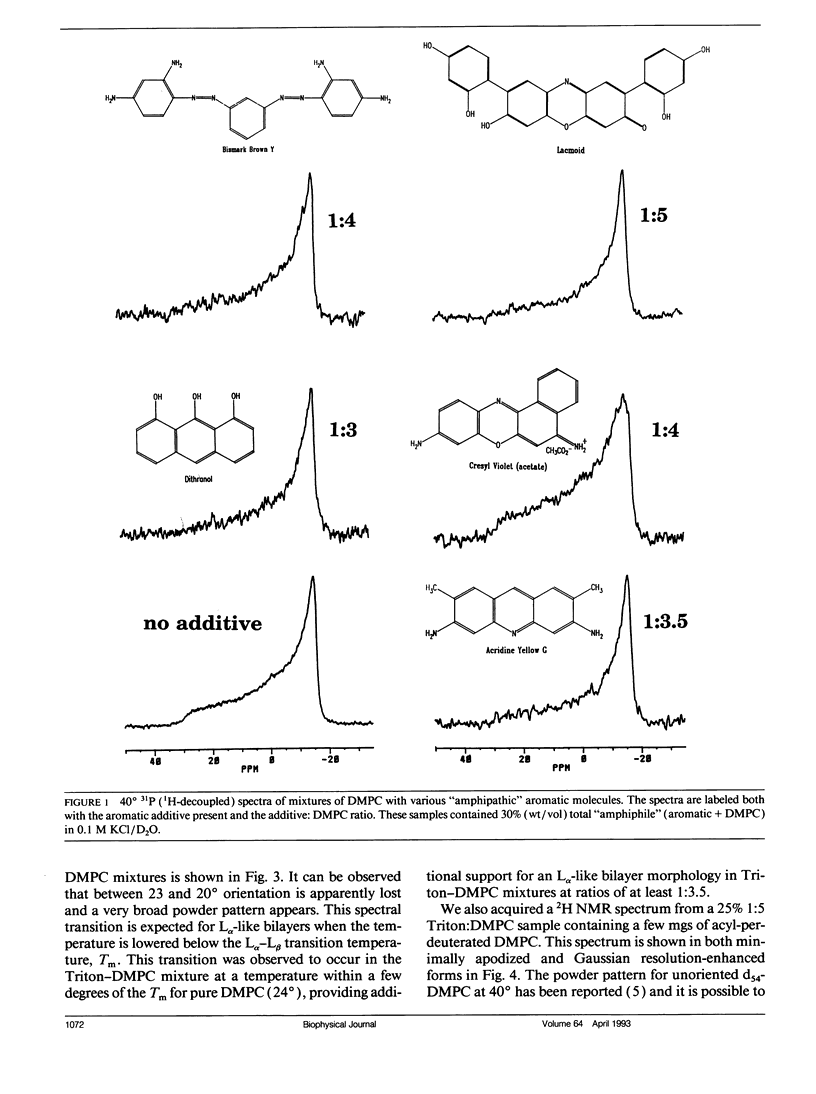
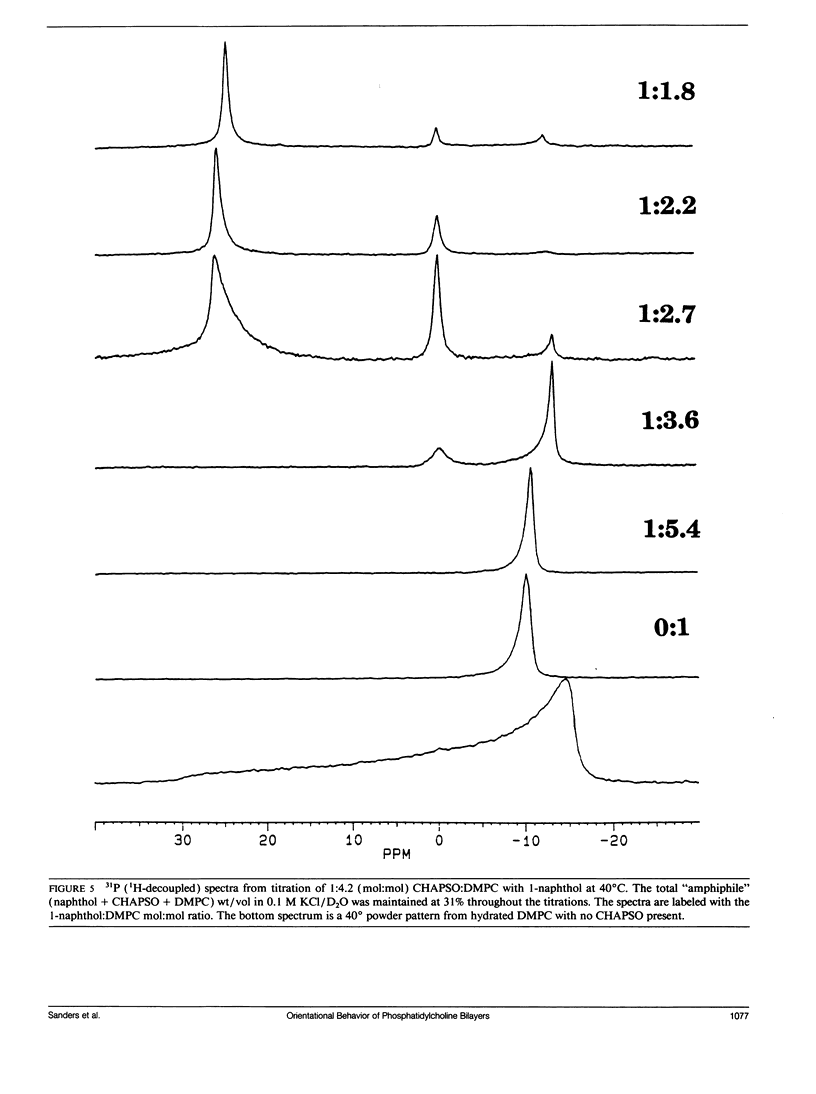
A number of aromatic-containing additives which can influence the orientation of fragments of lipid bilayer membranes by a magnetic field have been investigated. Two properties of these additives prove important: (1) sufficient detergency to facilitate reorganization of bilayer components and (2), sufficient anisotropy in magnetic susceptibility the preferred direction of fragment orientation. Triton X-100 is identified as effective in terms of facilitating magnetic field ordering of bilayer fragments but does not alter the preferred direction of orientation. A combination of the detergent CHAPSO (3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate) and the aromatic alcohol 1-naphthol facilitates both ordering and alters the preferred direction of bilayer orientation. As mixtures of dimyristoylphosphatidylcholine (DMPC) and CHAPSO, which orient with bilayer normals perpendicular to the magnetic field, were titrated with 1-naphthol, the assemblies underwent transitions, first to random orientation, and then to an orientation with bilayer normals parallel to the field. Based on temperature-induced phase transitions and the extent of motional averaging of the 31P shielding tensor of the DMPC headgroup, the DMPC in these oriented samples appears to maintain a bilayer morphology during transitions. The insight provided in this study regarding factors which influence fragment stability and orientation lays the groundwork for the design of improved field-oriented media for spectroscopic investigation of membrane components.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anantharamaiah G. M., Jones J. L., Brouillette C. G., Schmidt C. F., Chung B. H., Hughes T. A., Bhown A. S., Segrest J. P. Studies of synthetic peptide analogs of the amphipathic helix. Structure of complexes with dimyristoyl phosphatidylcholine. J Biol Chem. 1985 Aug 25;260(18):10248–10255. [PubMed] [Google Scholar]
- Anantharamaiah G. M. Synthetic peptide analogs of apolipoproteins. Methods Enzymol. 1986;128:627–647. doi: 10.1016/0076-6879(86)28096-9. [DOI] [PubMed] [Google Scholar]
- Boden N., Jones S. A., Sixl F. On the use of deuterium nuclear magnetic resonance as a probe of chain packing in lipid bilayers. Biochemistry. 1991 Feb 26;30(8):2146–2155. doi: 10.1021/bi00222a019. [DOI] [PubMed] [Google Scholar]
- Dempsey C. E., Sternberg B. Reversible disc-micellization of dimyristoylphosphatidylcholine bilayers induced by melittin and [Ala-14]melittin. Biochim Biophys Acta. 1991 Jan 30;1061(2):175–184. doi: 10.1016/0005-2736(91)90283-e. [DOI] [PubMed] [Google Scholar]
- Dempsey C. E. The actions of melittin on membranes. Biochim Biophys Acta. 1990 May 7;1031(2):143–161. doi: 10.1016/0304-4157(90)90006-x. [DOI] [PubMed] [Google Scholar]
- Dufourc E. J., Mayer C., Stohrer J., Althoff G., Kothe G. Dynamics of phosphate head groups in biomembranes. Comprehensive analysis using phosphorus-31 nuclear magnetic resonance lineshape and relaxation time measurements. Biophys J. 1992 Jan;61(1):42–57. doi: 10.1016/S0006-3495(92)81814-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geacintov N. E., Van Norstrand F., Becker J. F., Tinkel J. B. Magnetic field induced orientation of photosynthetic systems. Biochim Biophys Acta. 1972 Apr 20;267(1):65–79. doi: 10.1016/0005-2728(72)90138-7. [DOI] [PubMed] [Google Scholar]
- Goñi F. M., Urbaneja M. A., Arrondo J. L., Alonso A., Durrani A. A., Chapman D. The interaction of phosphatidylcholine bilayers with Triton X-100. Eur J Biochem. 1986 Nov 3;160(3):659–665. doi: 10.1111/j.1432-1033.1986.tb10088.x. [DOI] [PubMed] [Google Scholar]
- Jansson M., Thurmond R. L., Trouard T. P., Brown M. F. Magnetic alignment and orientational order of dipalmitoylphosphatidylcholine bilayers containing palmitoyllysophosphatidylcholine. Chem Phys Lipids. 1990 Jun;54(3-4):157–170. doi: 10.1016/0009-3084(90)90009-g. [DOI] [PubMed] [Google Scholar]
- Lichtenberg D., Robson R. J., Dennis E. A. Solubilization of phospholipids by detergents. Structural and kinetic aspects. Biochim Biophys Acta. 1983 May 24;737(2):285–304. doi: 10.1016/0304-4157(83)90004-7. [DOI] [PubMed] [Google Scholar]
- Neugebauer D. C., Blaurock A. E. Magnetic orientation of purple membranes demonstrated by optical measurements and neutron scattering. FEBS Lett. 1977;78(1):31–35. doi: 10.1016/0014-5793(77)80266-4. [DOI] [PubMed] [Google Scholar]
- Okahata Y., En-na G., Ebato H. Synthetic chemoreceptive membranes. Sensing bitter or odorous substances on a synthetic lipid multibilayer film by using quartz-crystal microbalances and electric responses. Anal Chem. 1990 Jul 15;62(14):1431–1438. doi: 10.1021/ac00213a017. [DOI] [PubMed] [Google Scholar]
- Ram P., Prestegard J. H. Magnetic field induced ordering of bile salt/phospholipid micelles: new media for NMR structural investigations. Biochim Biophys Acta. 1988 May 24;940(2):289–294. doi: 10.1016/0005-2736(88)90203-9. [DOI] [PubMed] [Google Scholar]
- Saibil H., Chabre M., Worcester D. Neutron diffraction studies of retinal rod outer segment membranes. Nature. 1976 Jul 22;262(5566):266–270. doi: 10.1038/262266a0. [DOI] [PubMed] [Google Scholar]
- Sakurai I., Kawamura Y., Ikegami A., Iwayanagi S. Magneto-orientation of lecithin crystals. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7232–7236. doi: 10.1073/pnas.77.12.7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. R., 2nd, Prestegard J. H. Magnetically orientable phospholipid bilayers containing small amounts of a bile salt analogue, CHAPSO. Biophys J. 1990 Aug;58(2):447–460. doi: 10.1016/S0006-3495(90)82390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz F., Boroske E., Helfrich W. Magnetic anisotropy of lecithin membranes. A new anisotropy susceptometer. Biophys J. 1984 Mar;45(3):589–592. doi: 10.1016/S0006-3495(84)84196-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig J. 31P nuclear magnetic resonance and the head group structure of phospholipids in membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):105–140. doi: 10.1016/0304-4157(78)90001-1. [DOI] [PubMed] [Google Scholar]
- Speyer J. B., Sripada P. K., Das Gupta S. K., Shipley G. G., Griffin R. G. Magnetic orientation of sphingomyelin-lecithin bilayers. Biophys J. 1987 Apr;51(4):687–691. doi: 10.1016/S0006-3495(87)83394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worcester D. L. Structural origins of diamagnetic anisotropy in proteins. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5475–5477. doi: 10.1073/pnas.75.11.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]


