Abstract
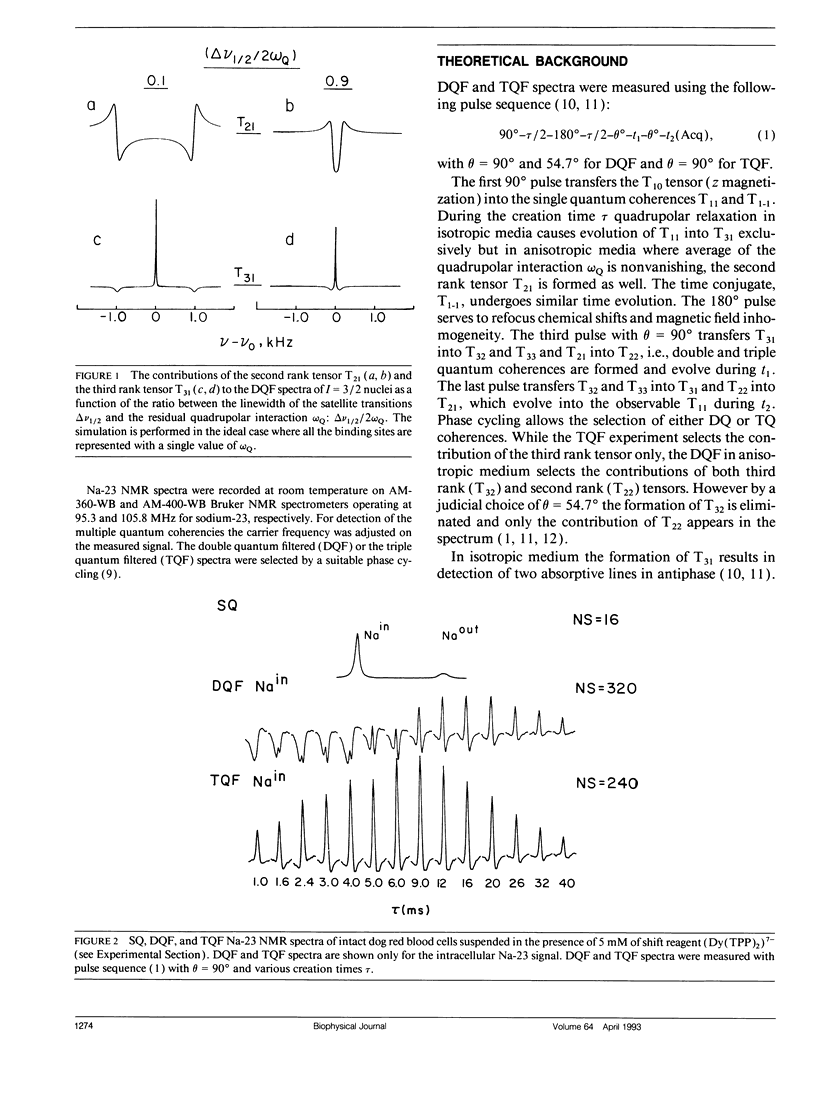
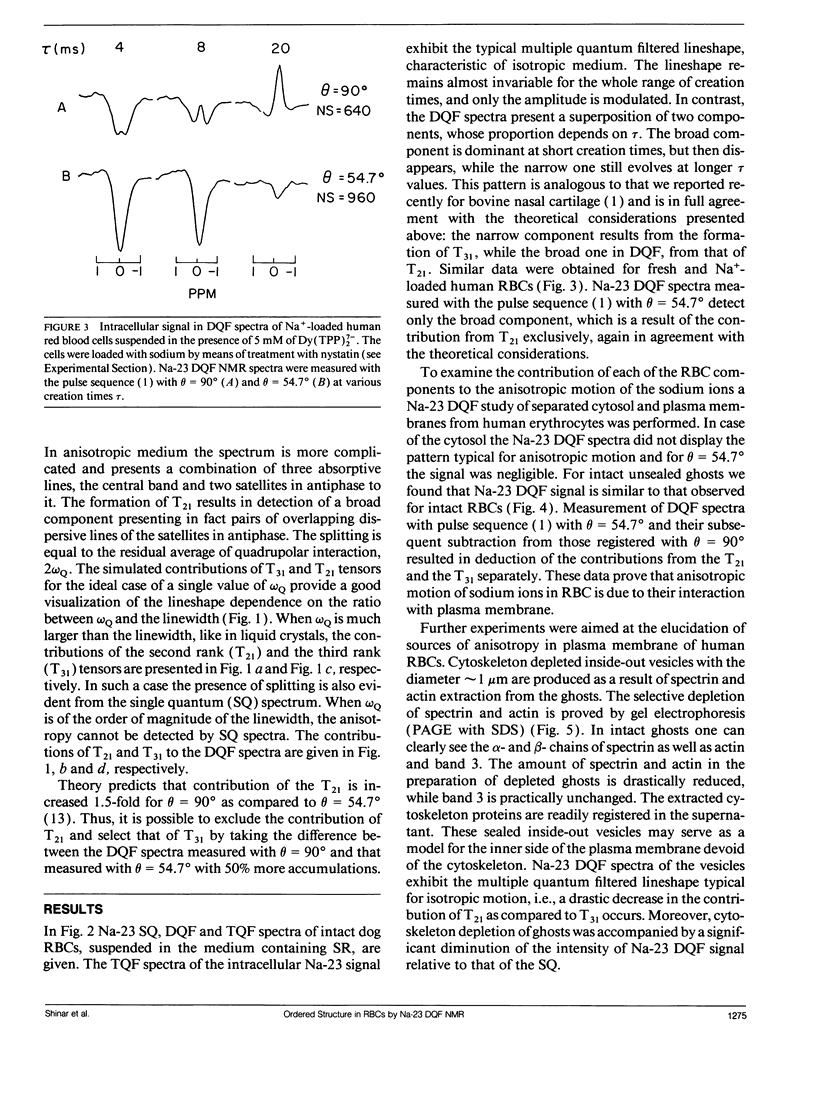
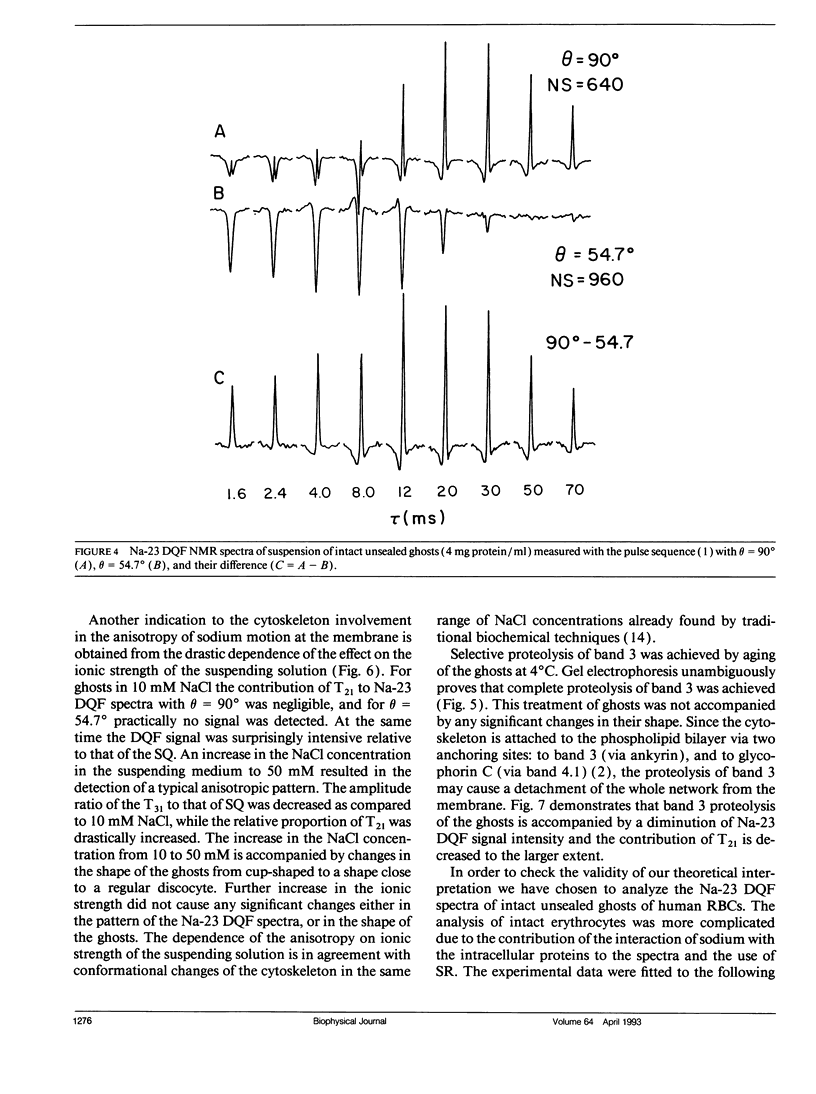
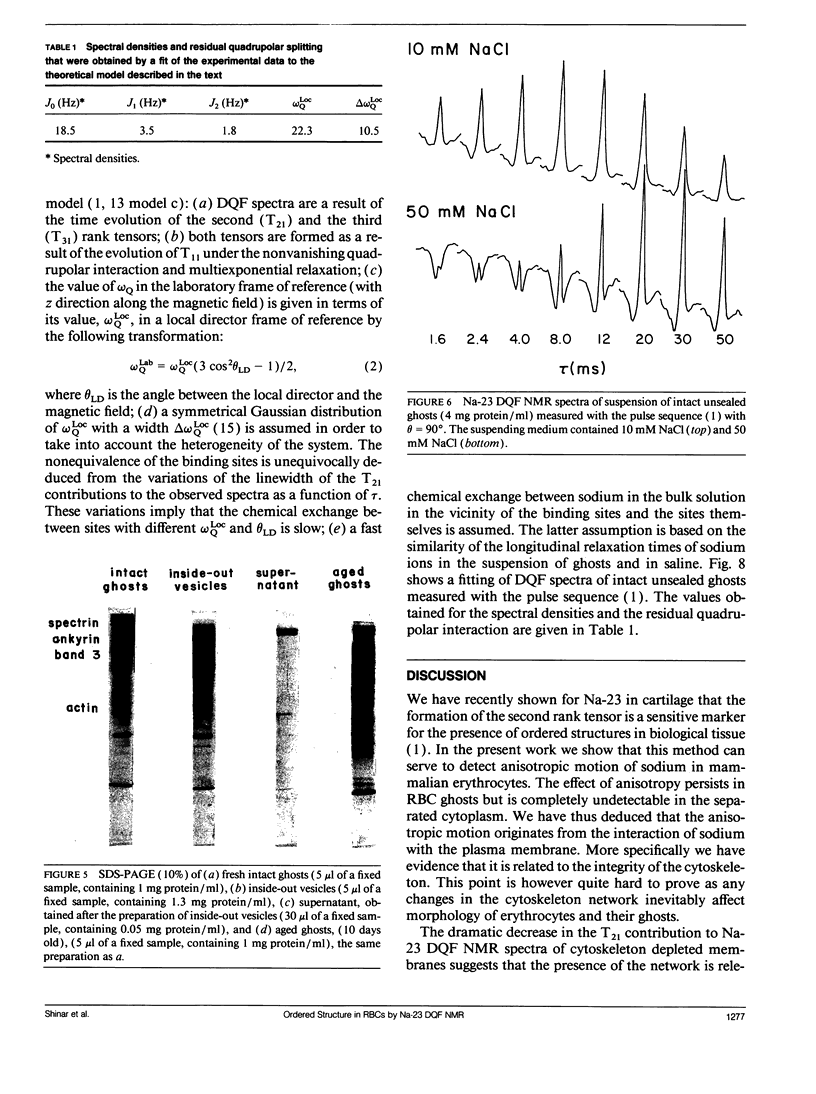
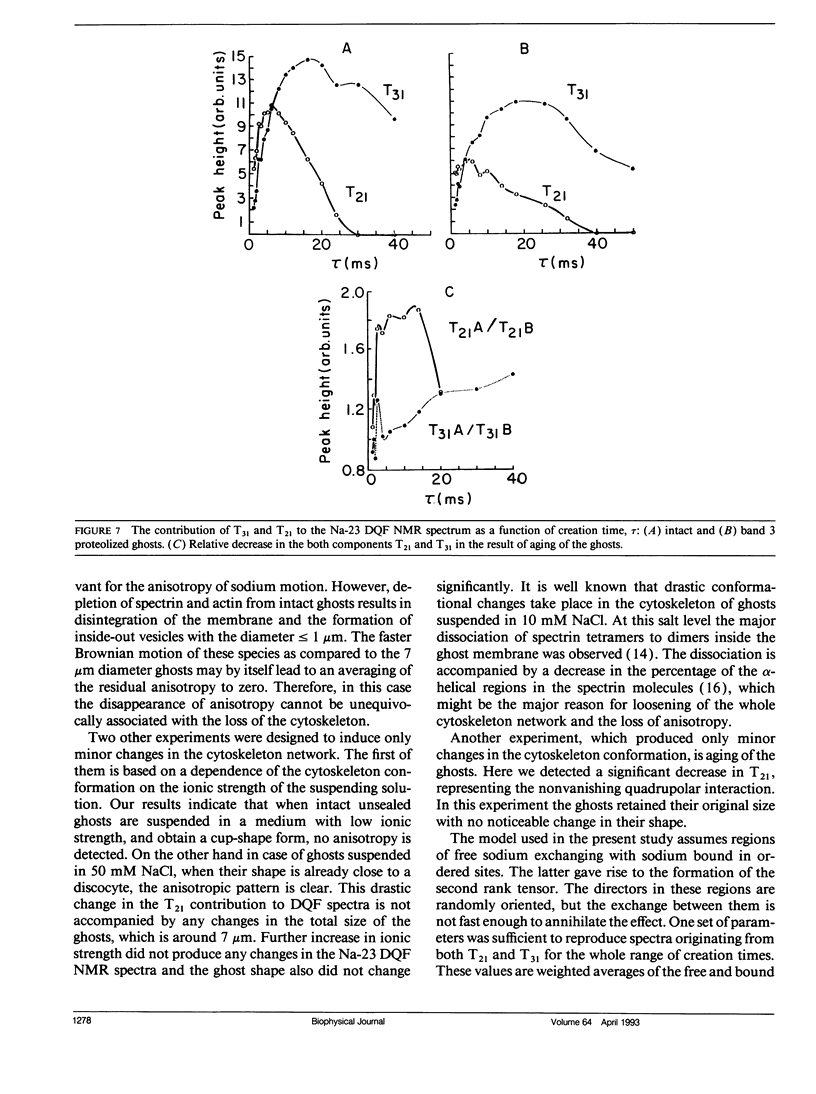
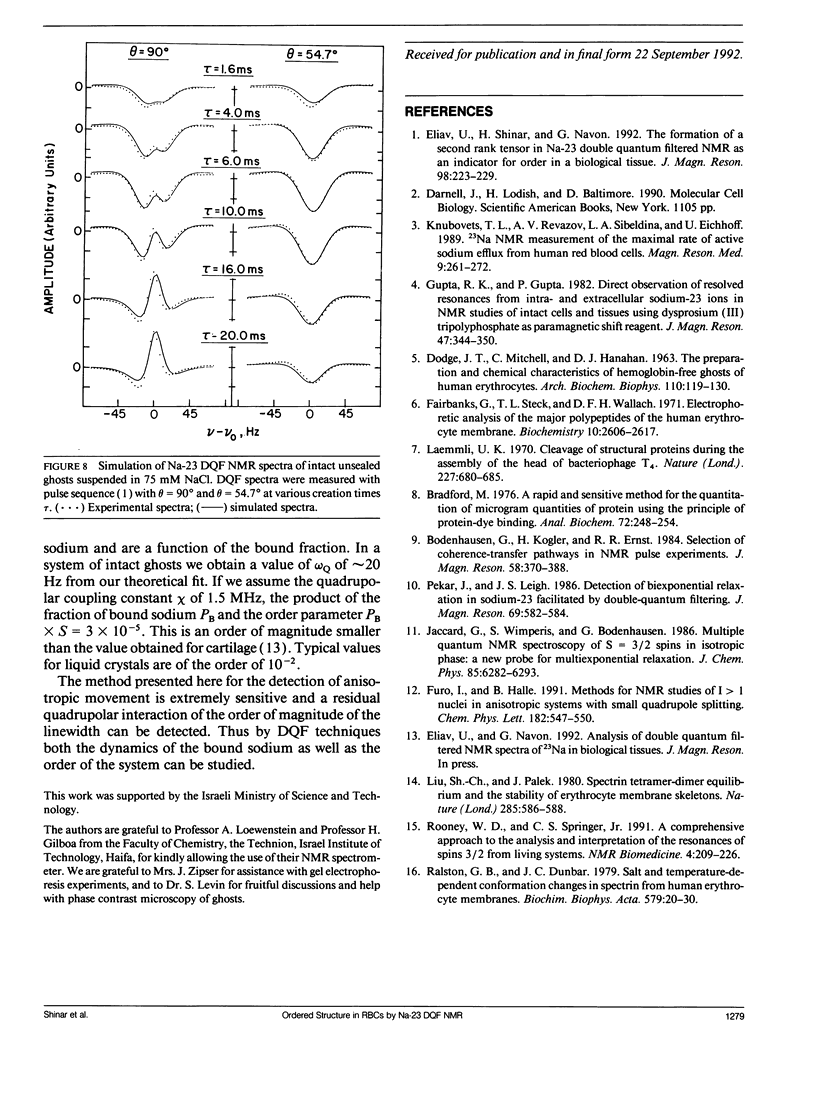
Na-23 double and triple quantum filtered NMR spectra of intact dog and human red blood cells were measured with the pulse sequence 90 degrees-tau/2-180 degrees-tau/2-theta degrees-t1-theta degrees-t2(Acq). For theta = 90 degrees the triple quantum filtered spectra exhibited the typical multiple quantum filtered lineshape, characteristic of isotropic media, while the double quantum filtered ones presented a superposition of two signals, whose proportion depended on the creation time tau. This effect is due to the formation of both second and third rank tensors. The formation of the second rank tensor, T21 results from non-zero residual quadrupolar interaction and is related to the anisotropic motion of sodium ions. Measurements of the double quantum filtered spectra with theta = 54.7 degrees enabled the detection of the contribution of T21 exclusively. No residual quadrupolar interaction was detected for sodium in the cytoplasm, while unsealed ghosts displayed the double quantum filtered spectral pattern, similar to that of intact cells. The anisotropy of motion of the sodium at the plasma membrane of mammalian erythrocytes depended on the integrity of the cytoskeleton network. Theoretical analysis of the double quantum filtered spectra gave a value of residual quadrupolar splitting of approximately 20 Hz for intact unsealed ghosts. The data presented prove that double quantum filtering is a sensitive technique for detection of motional anisotropies in biological systems.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
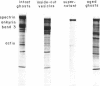
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Knubovets T. L., Revazov A. V., Sibeldina L. A., Eichhoff U. 23Na NMR measurement of the maximal rate of active sodium efflux from human red blood cells. Magn Reson Med. 1989 Feb;9(2):261–272. doi: 10.1002/mrm.1910090211. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu S. C., Palek J. Spectrin tetramer-dimer equilibrium and the stability of erythrocyte membrane skeletons. Nature. 1980 Jun 19;285(5766):586–588. doi: 10.1038/285586a0. [DOI] [PubMed] [Google Scholar]
- Ralston G. B., Dunbar J. C. Salt and temperature-dependent conformation changes in spectrin from human erythrocyte membranes. Biochim Biophys Acta. 1979 Jul 25;579(1):20–30. doi: 10.1016/0005-2795(79)90083-7. [DOI] [PubMed] [Google Scholar]
- Rooney W. D., Springer C. S., Jr A comprehensive approach to the analysis and interpretation of the resonances of spins 3/2 from living systems. NMR Biomed. 1991 Oct;4(5):209–226. doi: 10.1002/nbm.1940040502. [DOI] [PubMed] [Google Scholar]