Abstract
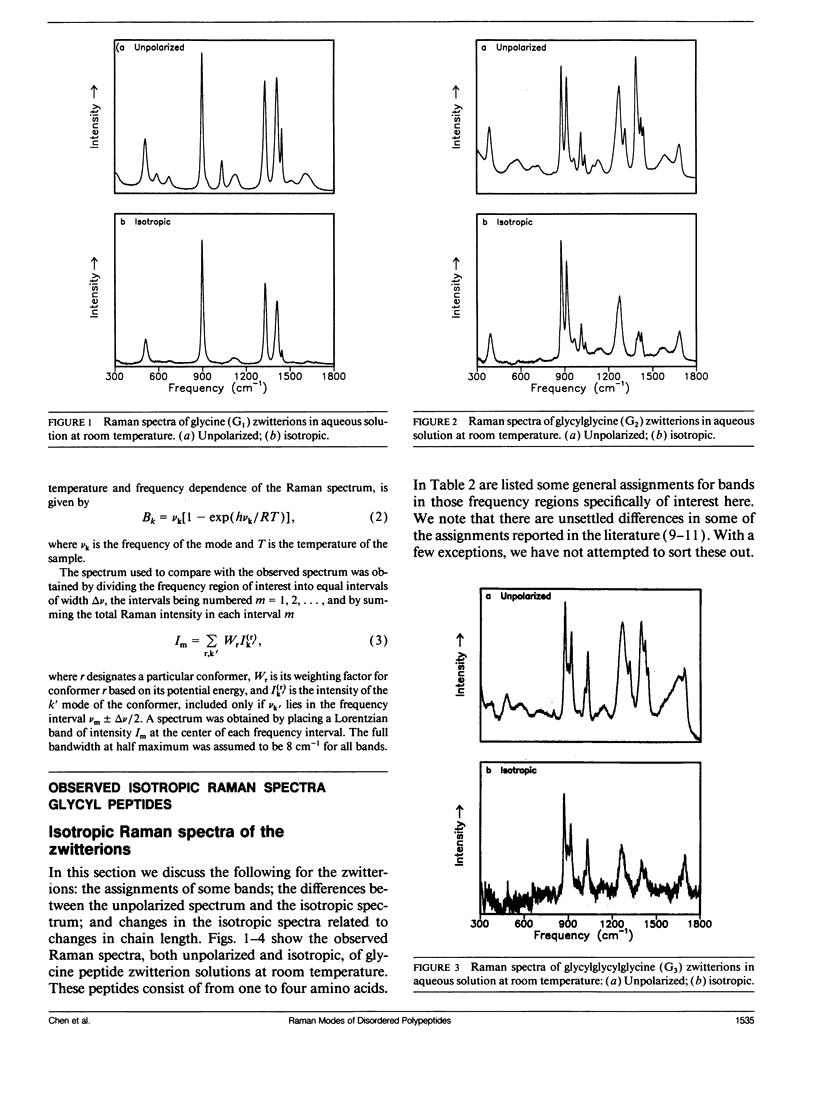
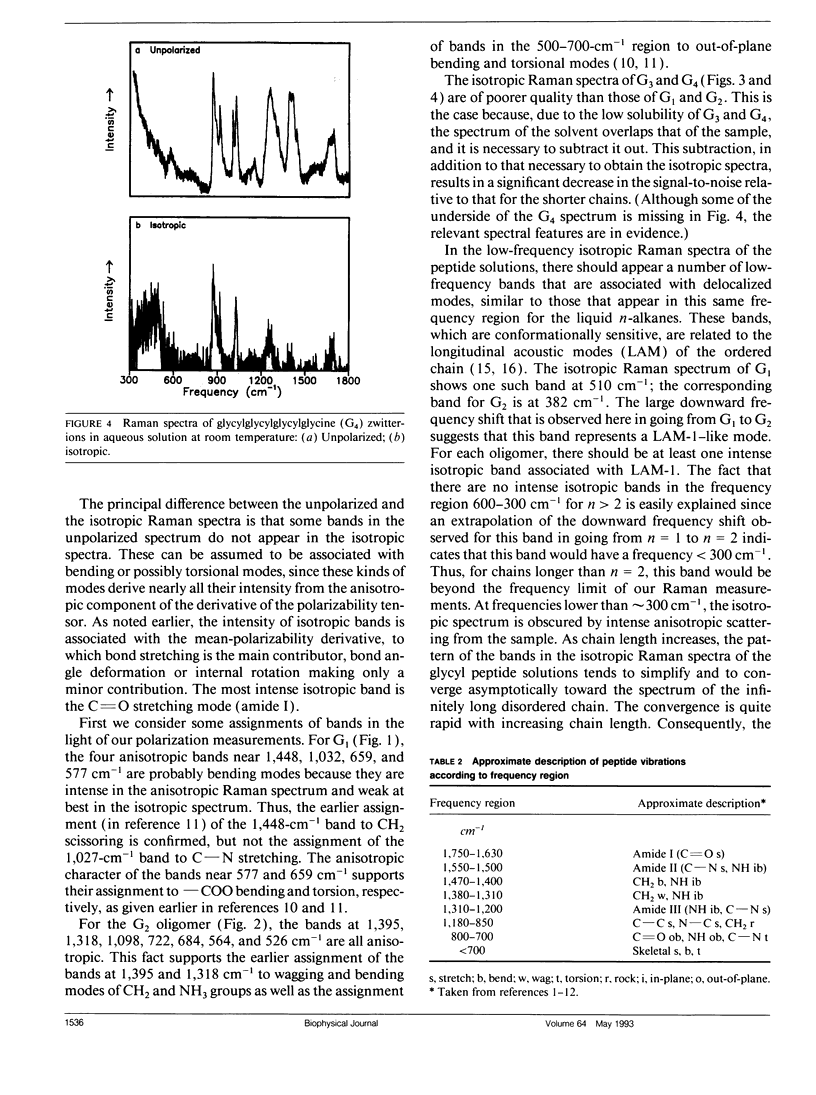
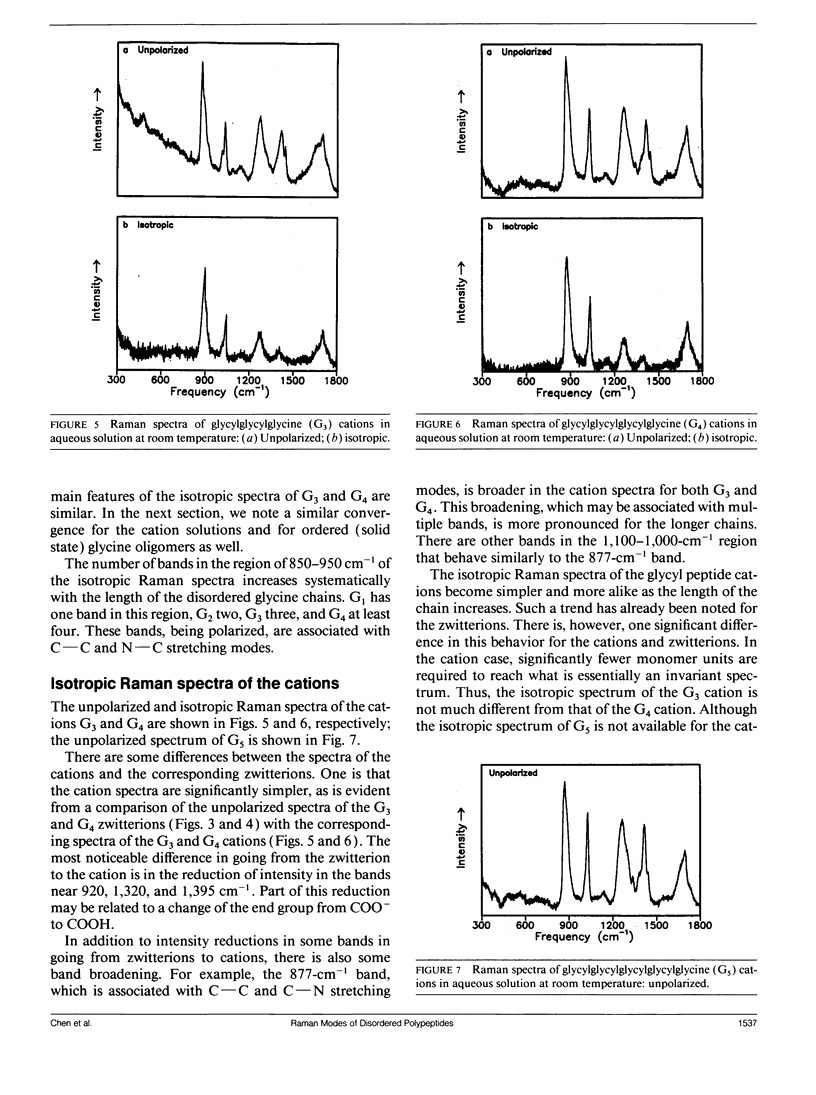
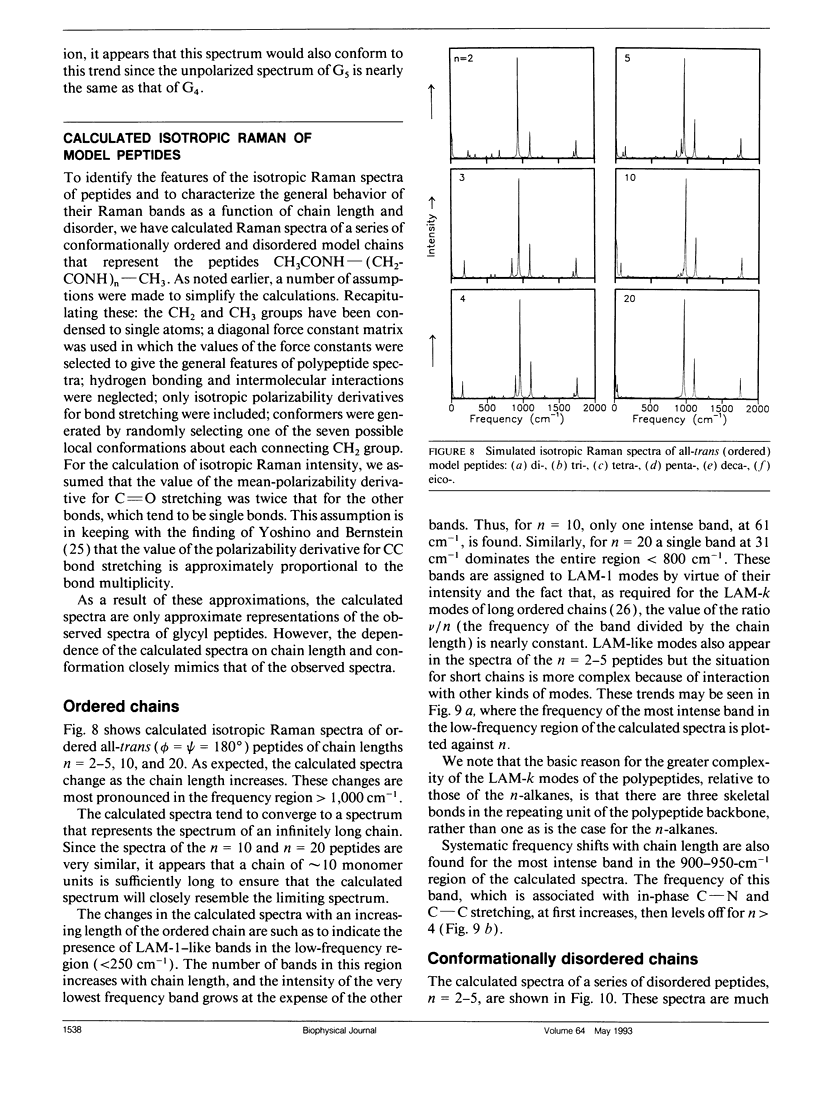
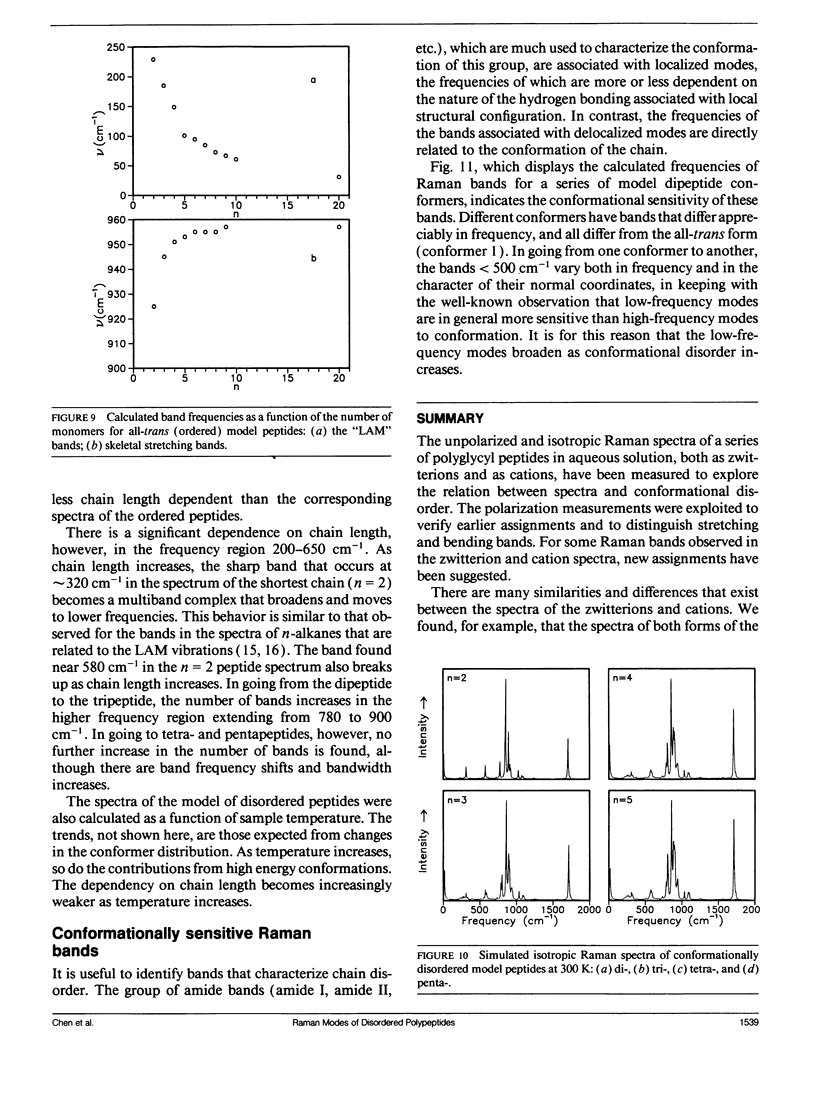
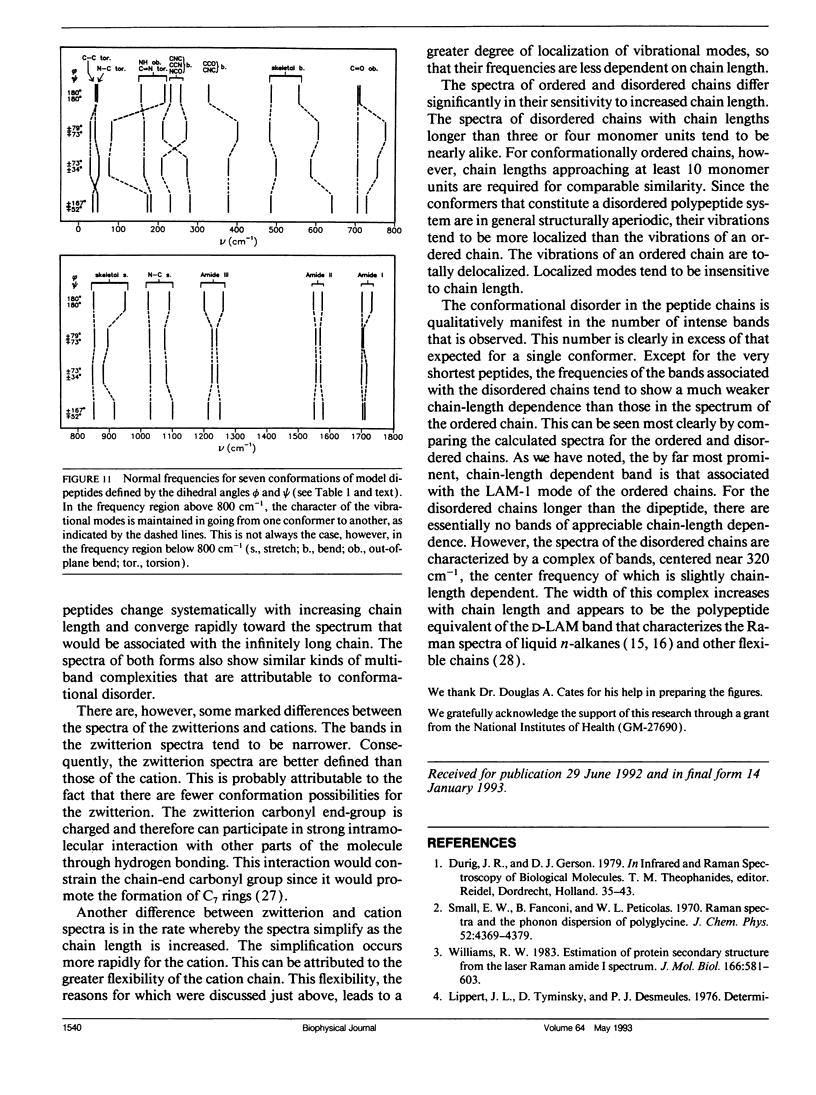
Bands associated with delocalized vibrational modes were identified in the isotropic Raman spectra of a series of polyglycine oligomers in aqueous solution as zwitterions and as cations. The dependence of these bands on conformational disorder and chain length was determined. The observed dependence is closely mimicked in spectra calculated for a series of corresponding model polypeptides. The simulated spectra were calculated in a skeletal approximation for ensembles of conformationally disordered chains. As the chain length of the conformationally disordered polypeptides increases, the observed isotropic spectra rapidly approach the spectrum of the infinitely long disordered chain. Convergence is nearly complete at the tripeptide for both the zwitterion and the cation. The stimulated spectra behave in essentially the same way. Convergence to the spectrum of the infinitely long chain is much more rapid for the conformationally disordered polyglycines than for the ordered polyglycines because of the mode localization that results from disorder. In the low-frequency region the bands in the calculated spectra have frequencies that are systematically dependent on chain length. These bands are related to the longitudinal acoustic modes of the ordered chain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avignon M., Garrigou-Lagrange C., Bothorel P. Conformational analysis of dipeptides in aqueous solution. II. Molecular structure of glycine and alanine dipeptides by depolarized Rayleigh scattering and laser Raman spectroscopy. Biopolymers. 1973;12(7):1651–1669. doi: 10.1002/bip.1973.360120716. [DOI] [PubMed] [Google Scholar]
- Brooks B., Karplus M. Harmonic dynamics of proteins: normal modes and fluctuations in bovine pancreatic trypsin inhibitor. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6571–6575. doi: 10.1073/pnas.80.21.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanconi B. Low-frequency vibrational spectra of some homopolypeptides in the solid state. Biopolymers. 1973 Dec;12(12):2759–2776. doi: 10.1002/bip.1973.360121210. [DOI] [PubMed] [Google Scholar]
- Go N., Noguti T., Nishikawa T. Dynamics of a small globular protein in terms of low-frequency vibrational modes. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3696–3700. doi: 10.1073/pnas.80.12.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimm S., Bandekar J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv Protein Chem. 1986;38:181–364. doi: 10.1016/s0065-3233(08)60528-8. [DOI] [PubMed] [Google Scholar]
- Lippert J. L., Tyminski D., Desmeules P. J. Determination of the secondary structure of proteins by laser Raman spectroscopy. J Am Chem Soc. 1976 Oct 27;98(22):7075–7080. doi: 10.1021/ja00438a057. [DOI] [PubMed] [Google Scholar]
- Peticolas W. L. Low frequency vibrations and the dynamics of proteins and polypeptides. Methods Enzymol. 1979;61:425–458. doi: 10.1016/0076-6879(79)61020-0. [DOI] [PubMed] [Google Scholar]
- Small E. W., Fanconi B., Peticolas W. L. Raman spectra and the phonon dispersion of polyglycine. J Chem Phys. 1970 May 1;52(9):4369–4379. doi: 10.1063/1.1673659. [DOI] [PubMed] [Google Scholar]
- Williams R. W. Estimation of protein secondary structure from the laser Raman amide I spectrum. J Mol Biol. 1983 Jun 5;166(4):581–603. doi: 10.1016/s0022-2836(83)80285-x. [DOI] [PubMed] [Google Scholar]


