Abstract
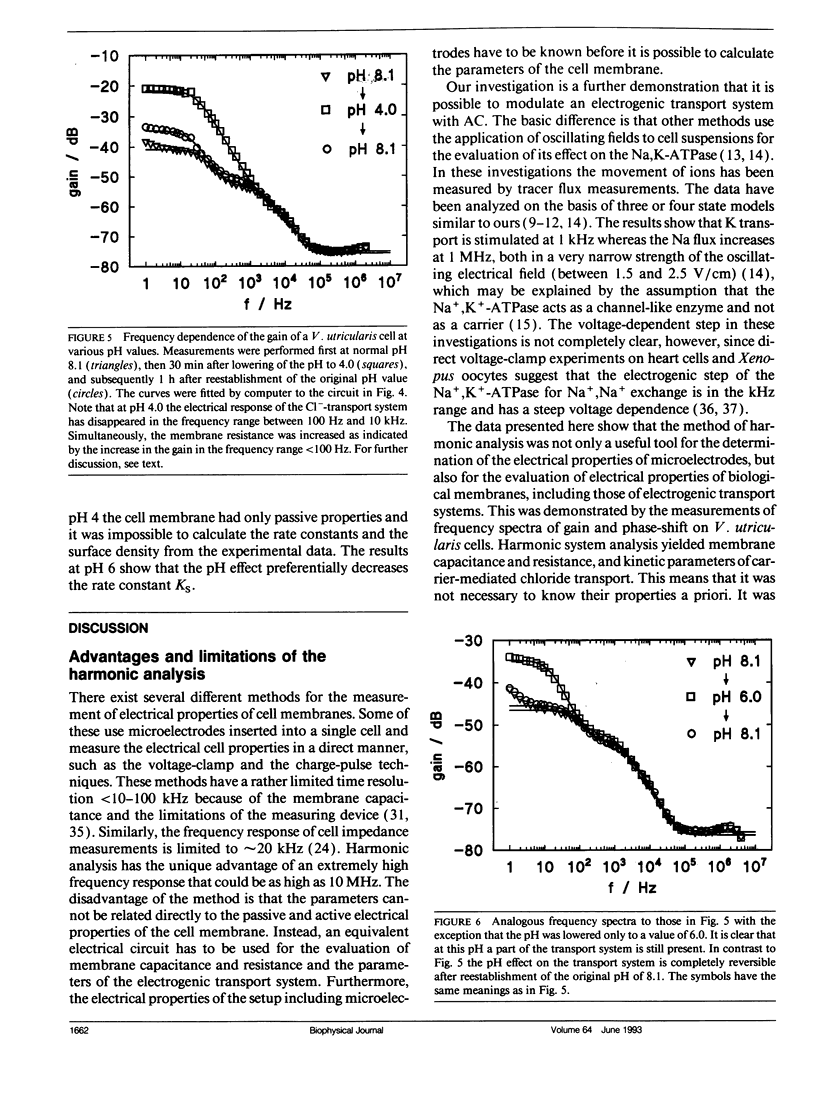
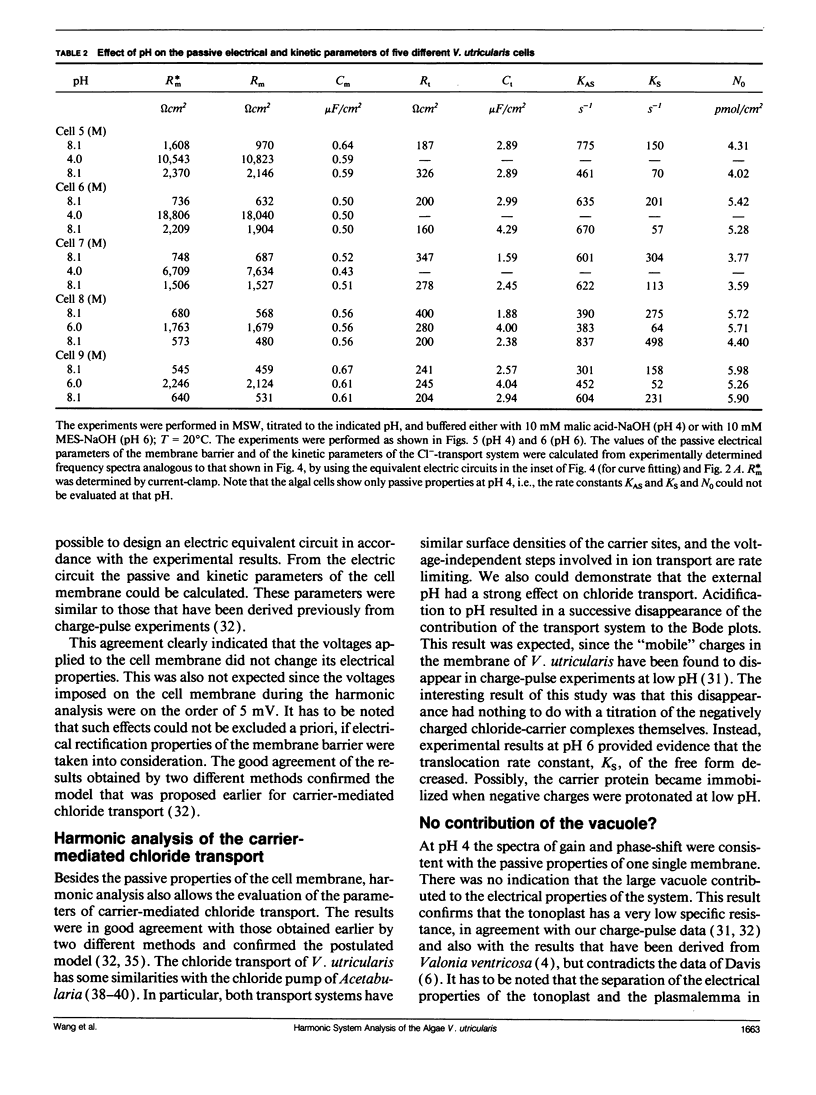
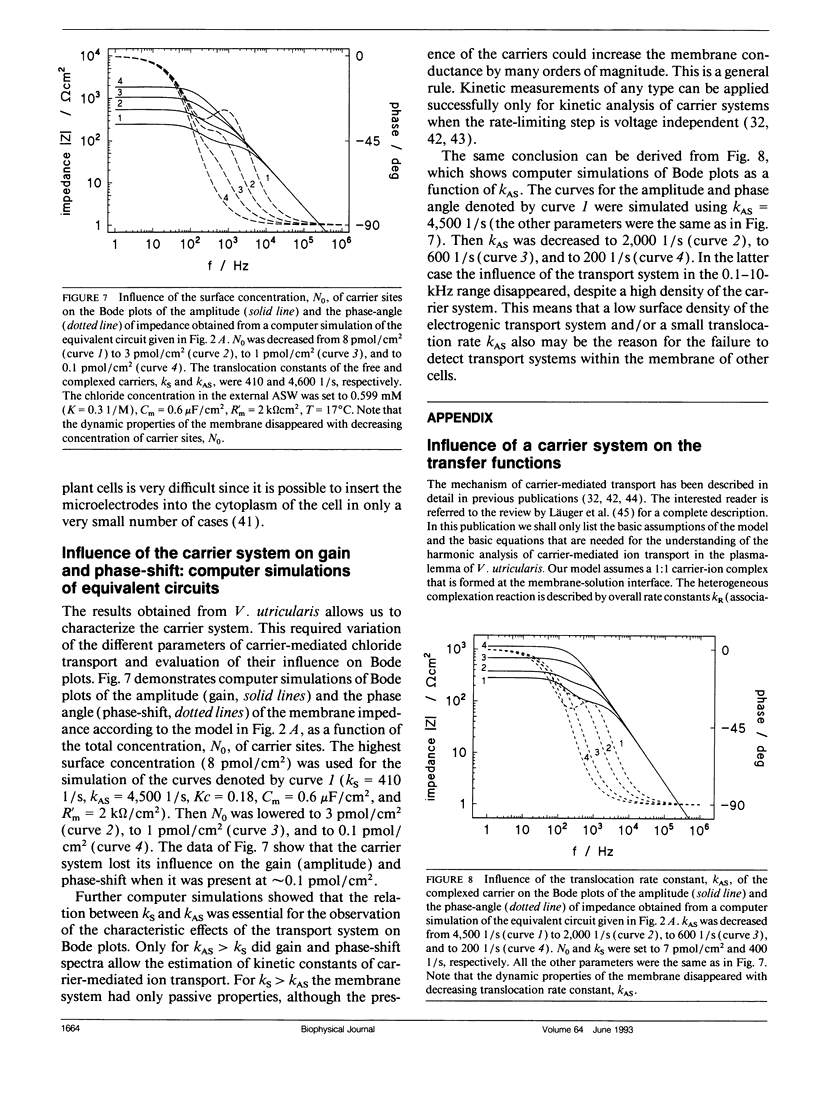
Cell membrane properties of the giant marine alga Valonia utricularis were measured in the frequency domain between 1 Hz and 10 MHz by harmonic system analysis. Harmonic analysis was performed by imposing a sinusoidal electrical voltage on the cell interior via an internal microelectrode. Gain and phase-shift of the resulting sinusoidal membrane voltage were measured over the whole frequency range with an internal voltage microelectrode. Bode plots of gain and phase-shift allowed the determination of the electrical parameters of the equivalent electronic circuits of the cell membrane of V. utricularis, which showed dynamic and passive properties dependent on the pH of the external aqueous solution. The dynamic components of the membrane impedance were caused by an electrogenic transport system for chloride described previously (Wang, J., G. Wehner, R. Benz, and U. Zimmermann. 1991. Biophys. J. 59:235-248). The kinetic and equilibrium parameters of the transport system could be evaluated from the fit of Bode plots of gain and phase-shift. The frequency domain technique revealed complete agreement of transport parameters with previously published results. The data demonstrate that an electrogenic transport system can be driven by an oscillating electric field.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benz R., Läuger P. Kinetic analysis of carrier-mediated ion transport by the charge-pulse technique. J Membr Biol. 1976 Jun 9;27(1-2):171–191. doi: 10.1007/BF01869135. [DOI] [PubMed] [Google Scholar]
- Benz R., McLaughlin S. The molecular mechanism of action of the proton ionophore FCCP (carbonylcyanide p-trifluoromethoxyphenylhydrazone). Biophys J. 1983 Mar;41(3):381–398. doi: 10.1016/S0006-3495(83)84449-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Zimmermann U. Evidence for the presence of mobile charges in the cell membrane of Valonia utricularis. Biophys J. 1983 Jul;43(1):13–26. doi: 10.1016/S0006-3495(83)84318-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia M. J., Christensen B. N., Moore L. E., Lang D. G. Studies of solitary semicircular canal hair cells in the adult pigeon. I. Frequency- and time-domain analysis of active and passive membrane properties. J Neurophysiol. 1989 Oct;62(4):924–934. doi: 10.1152/jn.1989.62.4.924. [DOI] [PubMed] [Google Scholar]
- Davis R. F. Electrical Properties of the Plasmalemma and Tonoplast in Valonia ventricosa. Plant Physiol. 1981 Apr;67(4):825–831. doi: 10.1104/pp.67.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman J. M., Hain T. C., Paige G. D. Central adaptation models of the vestibulo-ocular and optokinetic systems. Biol Cybern. 1989;61(4):255–264. doi: 10.1007/BF00203172. [DOI] [PubMed] [Google Scholar]
- Gerencser G. A., White J. F., Gradmann D., Bonting S. L. Is there a Cl- pump? Am J Physiol. 1988 Nov;255(5 Pt 2):R677–R692. doi: 10.1152/ajpregu.1988.255.5.R677. [DOI] [PubMed] [Google Scholar]
- Gradmann D. Analog circuit of the Acetabularia membrane. J Membr Biol. 1975 Dec 4;25(1-2):183–208. doi: 10.1007/BF01868574. [DOI] [PubMed] [Google Scholar]
- Lainson R., Field C. D. Electrical properties of Valonia ventricosa. J Membr Biol. 1976 Oct 20;29(1-2):81–94. doi: 10.1007/BF01868953. [DOI] [PubMed] [Google Scholar]
- Liu D. S., Astumian R. D., Tsong T. Y. Activation of Na+ and K+ pumping modes of (Na,K)-ATPase by an oscillating electric field. J Biol Chem. 1990 May 5;265(13):7260–7267. [PubMed] [Google Scholar]
- Läuger P., Benz R., Stark G., Bamberg E., Jordan P. C., Fahr A., Brock W. Relaxation studies of ion transport systems in lipid bilayer membranes. Q Rev Biophys. 1981 Nov;14(4):513–598. doi: 10.1017/s003358350000247x. [DOI] [PubMed] [Google Scholar]
- Markin V. S., Liu D., Gimsa J., Strobel R., Rosenberg M. D., Tsong T. Y. Ion channel enzyme in an oscillating electric field. J Membr Biol. 1992 Mar;126(2):137–145. doi: 10.1007/BF00231912. [DOI] [PubMed] [Google Scholar]
- Nakao M., Gadsby D. C. Voltage dependence of Na translocation by the Na/K pump. Nature. 1986 Oct 16;323(6089):628–630. doi: 10.1038/323628a0. [DOI] [PubMed] [Google Scholar]
- Onaral B., Sun H. H., Schwan H. P. Electrical properties of bioelectrodes. IEEE Trans Biomed Eng. 1984 Dec;31(12):827–832. doi: 10.1109/TBME.1984.325245. [DOI] [PubMed] [Google Scholar]
- Ragheb T., Geddes L. A. Electrical properties of metallic electrodes. Med Biol Eng Comput. 1990 Mar;28(2):182–186. doi: 10.1007/BF02441775. [DOI] [PubMed] [Google Scholar]
- Robertson B., Astumian R. D. Kinetics of a multistate enzyme in a large oscillating field. Biophys J. 1990 Apr;57(4):689–696. doi: 10.1016/S0006-3495(90)82590-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson B., Astumian R. D. Michaelis-Menten equation for an enzyme in an oscillating electric field. Biophys J. 1990 Oct;58(4):969–974. doi: 10.1016/S0006-3495(90)82441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan H. P. Altenating current electrode polarization. Biophysik. 1966;3(2):181–201. doi: 10.1007/BF01191612. [DOI] [PubMed] [Google Scholar]
- Serpersu E. H., Tsong T. Y. Activation of electrogenic Rb+ transport of (Na,K)-ATPase by an electric field. J Biol Chem. 1984 Jun 10;259(11):7155–7162. [PubMed] [Google Scholar]
- Sherman K. R., Keller E. L., Stark L. Simulations and sensitivity analysis of the vestibulo-ocular reflex. IEEE Trans Biomed Eng. 1987 Sep;34(9):749–752. doi: 10.1109/tbme.1987.326000. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y., Astumian R. D. Electroconformational coupling: how membrane-bound ATPase transduces energy from dynamic electric fields. Annu Rev Physiol. 1988;50:273–290. doi: 10.1146/annurev.ph.50.030188.001421. [DOI] [PubMed] [Google Scholar]
- Tsong T. Y. Molecular recognition and processing of periodic signals in cells: study of activation of membrane ATPases by alternating electric fields. Biochim Biophys Acta. 1992 Mar 26;1113(1):53–70. doi: 10.1016/0304-4157(92)90034-8. [DOI] [PubMed] [Google Scholar]
- Wang J., Wehner G., Benz R., Zimmermann U. Influence of external chloride concentration on the kinetics of mobile charges in the cell membrane of Valonia utricularis: Evidence for the existence of a chloride carrier. Biophys J. 1991 Jan;59(1):235–248. doi: 10.1016/S0006-3495(91)82214-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zimmermann U., Benz R. The voltage-dependent step of the chloride transporter of Valonia utricularis encounters a Nernst-Planck and not an Eyring type of potential energy barrier. Biophys J. 1993 Apr;64(4):1004–1016. doi: 10.1016/S0006-3495(93)81466-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


