Abstract
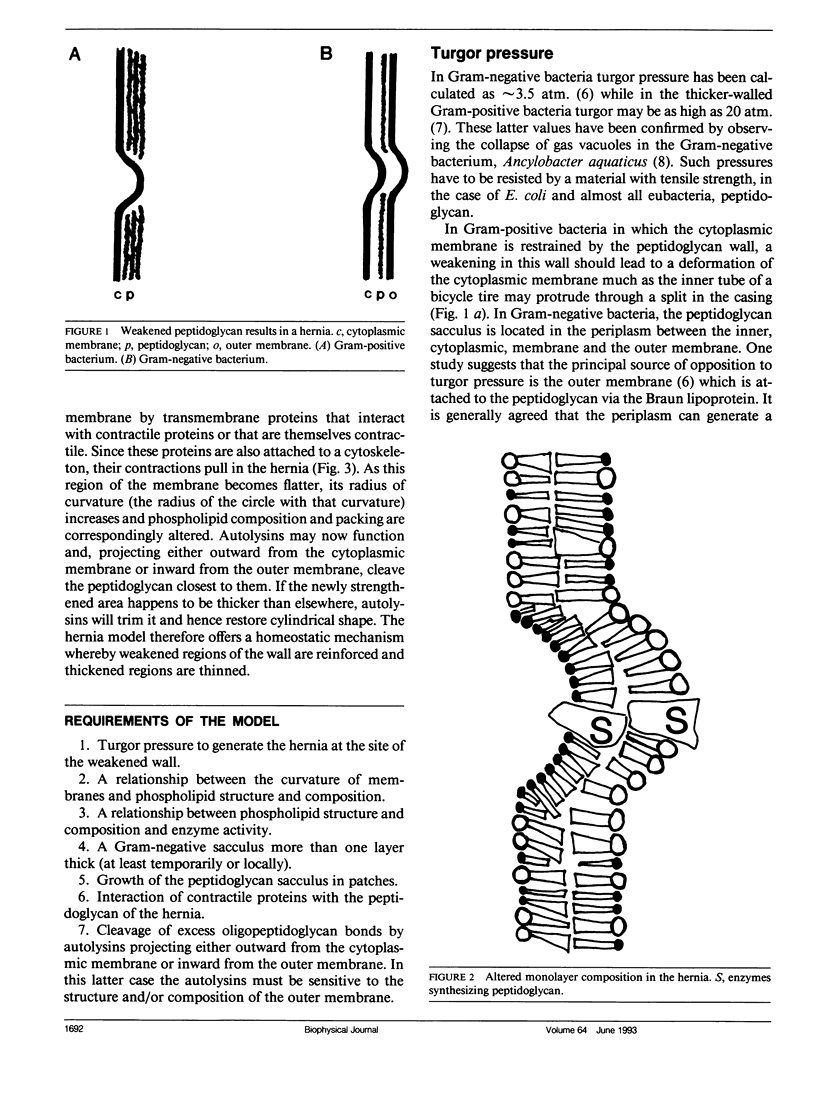
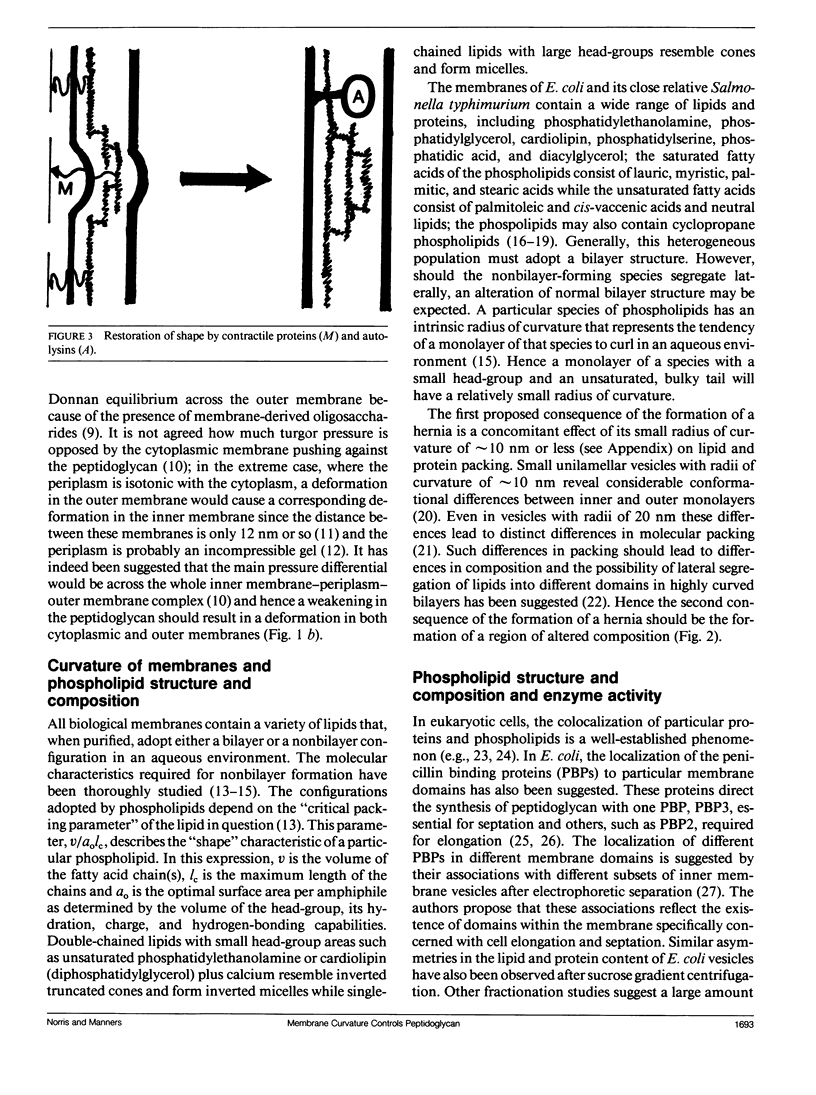
To explain the growth of the Gram-negative envelope and in particular how it could be strengthened where it is weakest, we propose in the hernia model that local weakening of the peptidoglycan sacculus allows turgor pressure to cause the envelope to bulge outwards in a hernia; the consequent local alteration in the radius of curvature of the cytoplasmic membrane causes local alterations in phospholipid structure and composition that determine both the synthesis and hydrolysis of peptidoglycan. This proposal is supported by evidence that phospholipid composition determines the activity of phospho-N-acetylmuramic acid pentapeptide translocase, UDP-N-acetylglucosamine:N-acetylmuramic acid-(pentapeptide)-P-P-bactoprenyl-N-acetylglucosamine transferase, bactoprenyl phosphate phosphokinase, and N-acetylmuramyl-L-alanine amidase. We also propose that the shape of Escherichia coli is maintained by contractile proteins acting at the hernia. Given the universal importance of membranes, these proposals have implications for the determination of shape in eukaryotic cells.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amako K., Murata K., Umeda A. Structure of the envelope of Escherichia coli observed by the rapid-freezing and substitution fixation method. Microbiol Immunol. 1983;27(1):95–99. doi: 10.1111/j.1348-0421.1983.tb03571.x. [DOI] [PubMed] [Google Scholar]
- Ames G. F. Lipids of Salmonella typhimurium and Escherichia coli: structure and metabolism. J Bacteriol. 1968 Mar;95(3):833–843. doi: 10.1128/jb.95.3.833-843.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas J. A., Díaz J., Rodríguez-Tébar A., Vázquez D. Specific location of penicillin-binding proteins within the cell envelope of Escherichia coli. J Bacteriol. 1986 Jan;165(1):269–275. doi: 10.1128/jb.165.1.269-275.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. H., Costello G. P., Bayer M. E. Isolation and partial characterization of membrane vesicles carrying markers of the membrane adhesion sites. J Bacteriol. 1982 Feb;149(2):758–767. doi: 10.1128/jb.149.2.758-767.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. H., Keck W., Bayer M. E. Localization of penicillin-binding protein 1b in Escherichia coli: immunoelectron microscopy and immunotransfer studies. J Bacteriol. 1990 Jan;172(1):125–135. doi: 10.1128/jb.172.1.125-135.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck B. D., Arscott P. G., Jacobson A. Novel properties of bacterial elongation factor Tu. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1250–1254. doi: 10.1073/pnas.75.3.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M., Evans E., Mouritsen O. G. Physical properties of the fluid lipid-bilayer component of cell membranes: a perspective. Q Rev Biophys. 1991 Aug;24(3):293–397. doi: 10.1017/s0033583500003735. [DOI] [PubMed] [Google Scholar]
- Bramhall J. Phospholipid packing asymmetry in curved membranes detected by fluorescence spectroscopy. Biochemistry. 1986 Jun 3;25(11):3479–3486. doi: 10.1021/bi00359a057. [DOI] [PubMed] [Google Scholar]
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Electron microscope study of septum formation in Escherichia coli strains B and B-r during synchronous growth. J Bacteriol. 1974 Sep;119(3):1039–1056. doi: 10.1128/jb.119.3.1039-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burman L. G., Park J. T. Molecular model for elongation of the murein sacculus of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1844–1848. doi: 10.1073/pnas.81.6.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casaregola S., Norris V., Goldberg M., Holland I. B. Identification of a 180 kD protein in Escherichia coli related to a yeast heavy-chain myosin. Mol Microbiol. 1990 Mar;4(3):505–511. doi: 10.1111/j.1365-2958.1990.tb00617.x. [DOI] [PubMed] [Google Scholar]
- Cooper S., Hsieh M. L., Guenther B. Mode of peptidoglycan synthesis in Salmonella typhimurium: single-strand insertion. J Bacteriol. 1988 Aug;170(8):3509–3512. doi: 10.1128/jb.170.8.3509-3512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S. Synthesis of the cell surface during the division cycle of rod-shaped, gram-negative bacteria. Microbiol Rev. 1991 Dec;55(4):649–674. doi: 10.1128/mr.55.4.649-674.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Csonka L. N., Hanson A. D. Prokaryotic osmoregulation: genetics and physiology. Annu Rev Microbiol. 1991;45:569–606. doi: 10.1146/annurev.mi.45.100191.003033. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., de Kruijff B. Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- Dubochet J., McDowall A. W., Menge B., Schmid E. N., Lickfeld K. G. Electron microscopy of frozen-hydrated bacteria. J Bacteriol. 1983 Jul;155(1):381–390. doi: 10.1128/jb.155.1.381-390.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geis A., Plapp R. Phospho-N-acetylmuramoyl-pentapeptide-transferase of Escherichia coli K12. Properties of the membrane-bound and the extracted and partially purified enzyme. Biochim Biophys Acta. 1978 Dec 8;527(2):414–424. doi: 10.1016/0005-2744(78)90355-8. [DOI] [PubMed] [Google Scholar]
- Gennis R. B., Strominger J. L. Activation of C55-isoprenoid alcohol phosphokinase from Staphylococcus aureus. I. Activation by phospholipids and fatty acids. J Biol Chem. 1976 Mar 10;251(5):1264–1269. [PubMed] [Google Scholar]
- Glauner B., Höltje J. V., Schwarz U. The composition of the murein of Escherichia coli. J Biol Chem. 1988 Jul 25;263(21):10088–10095. [PubMed] [Google Scholar]
- Goodell E. W., Schwarz U. Release of cell wall peptides into culture medium by exponentially growing Escherichia coli. J Bacteriol. 1985 Apr;162(1):391–397. doi: 10.1128/jb.162.1.391-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner S. M. Intrinsic curvature hypothesis for biomembrane lipid composition: a role for nonbilayer lipids. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3665–3669. doi: 10.1073/pnas.82.11.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harz H., Burgdorf K., Höltje J. V. Isolation and separation of the glycan strands from murein of Escherichia coli by reversed-phase high-performance liquid chromatography. Anal Biochem. 1990 Oct;190(1):120–128. doi: 10.1016/0003-2697(90)90144-x. [DOI] [PubMed] [Google Scholar]
- Haverstick D. M., Glaser M. Influence of proteins on the reorganization of phospholipid bilayers into large domains. Biophys J. 1989 Apr;55(4):677–682. doi: 10.1016/S0006-3495(89)82866-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XX. Identification of phosphatidylglycerol and cardiolipin as cofactors for isoprenoid alcohol phosphokinase. J Biol Chem. 1970 Jul 25;245(14):3691–3696. [PubMed] [Google Scholar]
- Hobot J. A., Carlemalm E., Villiger W., Kellenberger E. Periplasmic gel: new concept resulting from the reinvestigation of bacterial cell envelope ultrastructure by new methods. J Bacteriol. 1984 Oct;160(1):143–152. doi: 10.1128/jb.160.1.143-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Mason J. T. Geometric packing constraints in egg phosphatidylcholine vesicles. Proc Natl Acad Sci U S A. 1978 Jan;75(1):308–310. doi: 10.1073/pnas.75.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Glauner B. Structure and metabolism of the murein sacculus. Res Microbiol. 1990 Jan;141(1):75–89. doi: 10.1016/0923-2508(90)90100-5. [DOI] [PubMed] [Google Scholar]
- Israelachvili J. N., Marcelja S., Horn R. G. Physical principles of membrane organization. Q Rev Biophys. 1980 May;13(2):121–200. doi: 10.1017/s0033583500001645. [DOI] [PubMed] [Google Scholar]
- Kellenberger E. The 'Bayer bridges' confronted with results from improved electron microscopy methods. Mol Microbiol. 1990 May;4(5):697–705. doi: 10.1111/j.1365-2958.1990.tb00640.x. [DOI] [PubMed] [Google Scholar]
- Kennedy E. P. Osmotic regulation and the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1092–1095. doi: 10.1073/pnas.79.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. L. Additional arguments for the key role of "smart" autolysins in the enlargement of the wall of gram-negative bacteria. Res Microbiol. 1990 Jun;141(5):529–541. doi: 10.1016/0923-2508(90)90017-k. [DOI] [PubMed] [Google Scholar]
- Koch A. L. The surface stress theory of microbial morphogenesis. Adv Microb Physiol. 1983;24:301–366. doi: 10.1016/s0065-2911(08)60388-4. [DOI] [PubMed] [Google Scholar]
- Labischinski H., Goodell E. W., Goodell A., Hochberg M. L. Direct proof of a "more-than-single-layered" peptidoglycan architecture of Escherichia coli W7: a neutron small-angle scattering study. J Bacteriol. 1991 Jan;173(2):751–756. doi: 10.1128/jb.173.2.751-756.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc M., Frehel C., van Heijenoort J. Correlation between degradation and ultrastructure of peptidoglycan during autolysis of Escherichia coli. J Bacteriol. 1985 Feb;161(2):627–635. doi: 10.1128/jb.161.2.627-635.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. P., Weppner W. A., Neuhaus F. C. Initial membrane reaction in peptidoglycan synthesis: perturbation of lipid-phospho-N-acetylmuramyl-pentapeptide translocase interactions by n-butanol. Biochim Biophys Acta. 1980 Apr 24;597(3):603–613. doi: 10.1016/0005-2736(80)90231-x. [DOI] [PubMed] [Google Scholar]
- Leidenix M. J., Jacoby G. H., Henderson T. A., Young K. D. Separation of Escherichia coli penicillin-binding proteins into different membrane vesicles by agarose electrophoresis and sizing chromatography. J Bacteriol. 1989 Oct;171(10):5680–5686. doi: 10.1128/jb.171.10.5680-5686.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz B. R., Barenholz Y., Thompson T. E. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 2 Two-component phosphatidylcholine liposomes. Biochemistry. 1976 Oct 5;15(20):4529–4537. doi: 10.1021/bi00665a030. [DOI] [PubMed] [Google Scholar]
- Matsuhashi M., Wachi M., Ishino F. Machinery for cell growth and division: penicillin-binding proteins and other proteins. Res Microbiol. 1990 Jan;141(1):89–103. doi: 10.1016/0923-2508(90)90101-u. [DOI] [PubMed] [Google Scholar]
- Meyer H. W., Richter W., Gumpert J. Periodically curved bilayer structures observed in hyphal cells or stable L-form cells of a Streptomyces strain, and in liposomes formed by the extracted lipids. Biochim Biophys Acta. 1990 Jul 24;1026(2):171–178. doi: 10.1016/0005-2736(90)90061-r. [DOI] [PubMed] [Google Scholar]
- Minkoff L., Damadian R. Actin-like properties from Escherichia coli: concept of cytotonus as the missing link between cell metabolism and the biological ion-exchange resin. J Bacteriol. 1976 Jan;125(1):353–365. doi: 10.1128/jb.125.1.353-365.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Takahashi K., Watanabe S. Myosin and actin from Escherichia coli K12 C600. J Biochem. 1978 Dec;84(6):1453–1458. doi: 10.1093/oxfordjournals.jbchem.a132268. [DOI] [PubMed] [Google Scholar]
- Neimark H. C. Extraction of an actin-like protein from the prokaryote Mycoplasma pneumoniae. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4041–4045. doi: 10.1073/pnas.74.9.4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niggli V., Burger M. M. Interaction of the cytoskeleton with the plasma membrane. J Membr Biol. 1987;100(2):97–121. doi: 10.1007/BF02209144. [DOI] [PubMed] [Google Scholar]
- Niki H., Jaffé A., Imamura R., Ogura T., Hiraga S. The new gene mukB codes for a 177 kd protein with coiled-coil domains involved in chromosome partitioning of E. coli. EMBO J. 1991 Jan;10(1):183–193. doi: 10.1002/j.1460-2075.1991.tb07935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris V. Phospholipid domains determine the spatial organization of the Escherichia coli cell cycle: the membrane tectonics model. J Theor Biol. 1992 Jan 7;154(1):91–107. doi: 10.1016/s0022-5193(05)80190-0. [DOI] [PubMed] [Google Scholar]
- Pinette M. F., Koch A. L. Variability of the turgor pressure of individual cells of the gram-negative heterotroph Ancylobacter aquaticus. J Bacteriol. 1987 Oct;169(10):4737–4742. doi: 10.1128/jb.169.10.4737-4742.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R., Dowhan W. Biosynthesis and function of phospholipids in Escherichia coli. J Biol Chem. 1990 Jan 25;265(3):1235–1238. [PubMed] [Google Scholar]
- Rodgers W., Glaser M. Characterization of lipid domains in erythrocyte membranes. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1364–1368. doi: 10.1073/pnas.88.4.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. R., Paterson B. M. Yeast myosin heavy chain mutant: maintenance of the cell type specific budding pattern and the normal deposition of chitin and cell wall components requires an intact myosin heavy chain gene. Cell Motil Cytoskeleton. 1990;17(4):301–308. doi: 10.1002/cm.970170405. [DOI] [PubMed] [Google Scholar]
- Shpetner H. S., Vallee R. B. Identification of dynamin, a novel mechanochemical enzyme that mediates interactions between microtubules. Cell. 1989 Nov 3;59(3):421–432. doi: 10.1016/0092-8674(89)90027-5. [DOI] [PubMed] [Google Scholar]
- Sioud M., Baldacci G., Forterre P., de Recondo A. M. Antitumor drugs inhibit the growth of halophilic archaebacteria. Eur J Biochem. 1987 Dec 1;169(2):231–236. doi: 10.1111/j.1432-1033.1987.tb13602.x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A. In pursuit of myosin function. Cell Regul. 1989 Nov;1(1):1–11. doi: 10.1091/mbc.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Rauch B., Roseman S. Periplasmic space in Salmonella typhimurium and Escherichia coli. J Biol Chem. 1977 Nov 10;252(21):7850–7861. [PubMed] [Google Scholar]
- Taku A., Fan D. P. Identification of an isolated protein essential for peptidoglycan synthesis as the N-acetylglucosaminyltransferase. J Biol Chem. 1976 Oct 10;251(19):6154–6156. [PubMed] [Google Scholar]
- Umbreit J. N., Stone K. J., Strominger J. L. Isolation of polyisoprenyl alcohols from Streptococcus faecalis. J Bacteriol. 1972 Dec;112(3):1302–1305. doi: 10.1128/jb.112.3.1302-1305.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umbreit J. N., Strominger J. L. Complex lipid requirements for detergent-solubilized phosphoacetylmuramyl-pentapeptide translocase from Micrococcus luteus. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1972–1974. doi: 10.1073/pnas.69.7.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwinkel E., De Vlieghere M. Modulation of Escherichia coli N-acetylmuramoyl-L-alanine amidase activity by phosphatidylglycerol. Biochim Biophys Acta. 1985 Jan 28;838(1):54–59. doi: 10.1016/0304-4165(85)90249-1. [DOI] [PubMed] [Google Scholar]
- Walker J. M., Homan E. C., Sando J. J. Differential activation of protein kinase C isozymes by short chain phosphatidylserines and phosphatidylcholines. J Biol Chem. 1990 May 15;265(14):8016–8021. [PubMed] [Google Scholar]
- Watts F. Z., Shiels G., Orr E. The yeast MYO1 gene encoding a myosin-like protein required for cell division. EMBO J. 1987 Nov;6(11):3499–3505. doi: 10.1002/j.1460-2075.1987.tb02675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weppner W. A., Neuhaus F. C. Biosynthesis of peptidoglycan. Definition of the microenvironment of undecaprenyl diphosphate-N-acetylmuramyl-(5-dimethylaminonaphthalene-1-sulfonyl) pentapeptide by fluorescence spectroscopy. J Biol Chem. 1978 Jan 25;253(2):472–478. [PubMed] [Google Scholar]
- Weppner W. A., Neuhaus F. C. Initial membrane reaction in peptidoglycan synthesis. Interaction of lipid with phospho-N-acetylmuramyl-pentapeptide translocase. Biochim Biophys Acta. 1979 Apr 19;552(3):418–427. doi: 10.1016/0005-2736(79)90186-x. [DOI] [PubMed] [Google Scholar]
- de Jonge B. L., Wientjes F. B., Jurida I., Driehuis F., Wouters J. T., Nanninga N. Peptidoglycan synthesis during the cell cycle of Escherichia coli: composition and mode of insertion. J Bacteriol. 1989 Nov;171(11):5783–5794. doi: 10.1128/jb.171.11.5783-5794.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Bliek A. M., Meyerowitz E. M. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991 May 30;351(6325):411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]


