Abstract
Borna disease virus (BDV) frequently causes meningoencephalitis and fatal neurological disease in young but not old mice of strain MRL. Disease does not result from the virus-induced destruction of infected neurons. Rather, it is mediated by H-2k-restricted antiviral CD8 T cells that recognize a peptide derived from the BDV nucleoprotein N. Persistent BDV infection in mice is not spontaneously cleared. We report here that N-specific vaccination can protect wild-type MRL mice but not mutant MRL mice lacking gamma interferon (IFN-γ) from persistent infection with BDV. Furthermore, we observed a significant degree of resistance of old MRL mice to persistent BDV infection that depended on the presence of CD8 T cells. We found that virus initially infected hippocampal neurons around 2 weeks after intracerebral infection but was eventually cleared in most wild-type MRL mice. Unexpectedly, young as well as old IFN-γ-deficient MRL mice were completely susceptible to infection with BDV. Moreover, neurons in the CA1 region of the hippocampus were severely damaged in most diseased IFN-γ-deficient mice but not in wild-type mice. Furthermore, large numbers of eosinophils were present in the inflamed brains of IFN-γ-deficient mice but not in those of wild-type mice, presumably because of increased intracerebral synthesis of interleukin-13 and the chemokines CCL1 and CCL11, which can attract eosinophils. These results demonstrate that IFN-γ plays a central role in host resistance against infection of the central nervous system with BDV and in clearance of BDV from neurons. They further indicate that IFN-γ may function as a neuroprotective factor that can limit the loss of neurons in the course of antiviral immune responses in the brain.
The control of persistent virus infections is strongly dependent on CD8 T-cell responses. Upon recognition of their cognate peptide antigen presented on major histocompatibility complex class I (MHC-I) molecules, CD8 T cells can exert antiviral activity by at least two distinct pathways. They can either eliminate infected cells by releasing the contents of cytotoxic granula, which ultimately leads to the apoptosis of the target cell, or secrete antivirally active cytokines (67). The killing of infected cells is an inadequate mechanism in tissues where the regenerative capacity of essential cell populations is limited, such as the central nervous system (CNS). Interferon-γ (IFN-γ) secreted by antigen-specific CD8 T cells was shown to mediate noncytolytic clearance from neurons of a number of viral pathogens, including measles virus (49), Sindbis virus (8), herpes simplex virus type 1 (40) and lymphocytic choriomeningitis virus (65). IFN-γ is produced by CD4 Th1 cells, CD8 T cells, and NK cells. It has direct antiviral activity and exhibits important immunoregulatory functions, such as the stimulation of the Th1 subpopulation of T cells; the activation of CD8 T cells, NK cells, and macrophages; and the stimulation of MHC expression (52, 71). Various effects of IFN-γ on brain cells have been reported. The treatment of multiple sclerosis with IFN-γ-exacerbated disease (47) and the transgenic expression of IFN-γ in brain cells was detrimental for the CNS (33, 53). On the other hand, the lack of IFN-γ resulted in more-severe neurological disease in experimental allergic encephalomyelitis, the mouse model of the human disease multiple sclerosis (16, 70). IFN-γ was shown to prevent the apoptosis of neurons deprived of nerve growth factor (11). Nitric oxide produced by IFN-γ-induced NO synthase 2 (NOS-2) can have both neuroprotective and proapoptotic effects on neurons (3, 13).
Borna disease virus (BDV) is a highly neurotropic virus that can infect a broad range of warm-blooded animals and possibly also humans (58, 63). It has a strong tropism for CNS neurons, in which it replicates without inducing cell damage (23). BDV is the causative agent of severe meningoencephalitis and fatal neurological disease in horses and sheep. BDV infection of rodents is used as a unique model to study immune responses against a persisting virus in the CNS. When MRL mice are infected as newborns with mouse-adapted variants of BDV, they develop severe neurological disease at a frequency of more than 80% and are thus highly susceptible to BDV-induced neurological disease (25). Like MRL mice, other mouse strains, such as CBA, C3H, BALB/c, and C57BL/6, also can be persistently infected with BDV as newborns with 100% efficiency. In contrast to what is seen in MRL mice, however, BDV-induced neurological disease occurs with significantly lower frequencies in these mouse strains (25). The H-2k haplotype appears to be associated with severity of disease (25). Disease in susceptible MRL mice is mediated by CD8 T cells that recognize the immunodominant epitope TELEISSI derived from the viral nucleoprotein N (BDV-N) (60). Epitopes in other haplotypes have not been defined. IFN-γ was not required for CD8 T-cell-mediated neurological disease in MRL mice infected with BDV (28).
Clearance of BDV is generally not observed after experimental infection of various animal species, including the well-studied rat model (44, 64). Due to the insufficiency of available intravitam diagnostic methods, it is unclear whether some infected animals are temporarily replicating the virus before clearing it again (63). Infected animals either develop a lifelong persistent BDV infection of the brain or succumb to the disease due to immunopathology; these outcomes also apply to the mouse model (25). The only clear description of the spontaneous clearance of BDV was based on the particularly artificial condition of intracerebral inoculation with very large amounts of virus. This type of inoculation led to an early activation of immune responses, possibly via immediate leakage of antigen from the inoculation site into peripheral lymphoid organs (20). Therefore, immune control of BDV infection could generally be achieved only by antigen-specific immune priming (26, 31, 38) or by adoptive transfer of BDV-specific T cells (54).
We have previously demonstrated that CD8 T cells are essential for immune control of BDV and that perforin is not an essential effector mechanism of BDV-specific CD8 T cells in this process (26). A role for IFN-γ in the immune control of BDV in the CNS was suggested by recent experiments with transgenic mice expressing interleukin-12 (IL-12) in cerebellar astrocytes (32). Further, the multiplication of BDV was suppressed by IFN-γ in organotypic slice cultures from mouse brains (18). We now demonstrate that IFN-γ is essential for the in vivo control of BDV in mice and for the efficient elimination of the virus from neurons by CD8 T cells. Our data further show that IFN-γ greatly limits the neurodestructive effects of the antiviral immune response on hippocampal structures in the CNS.
MATERIALS AND METHODS
Mice.
MRL/MpJ and MRL/MpJ-β2m0/0 mice were originally purchased from The Jackson Laboratory (Bar Harbor, Maine). Breeding colonies were maintained in our local animal facility. IFN-γ-deficient MRL mice (MRL-GKO) were generated by backcrossing a defective IFN-γ allele (12) from C57BL/6 mice for five generations into MRL mice. All animal experiments were approved by the local authorities.
Viruses and animal infections.
Mice were infected intracerebrally into the thalamic region of the left brain hemisphere by injecting 10-μl samples of a 10% brain homogenate containing 300 focus-forming units of mouse-adapted BDV (32) by using Hamilton syringes. Construction and growth of recombinant parapoxvirus ovis, Orf virus (ORFV) D1701-VrV-p40 expressing BDV-N, and parental strain D1701-VrV expressing β-galactosidase (β-Gal) were previously described (31). For immune priming, 3-week-old mice were injected intramuscularly with 107 PFU of ORFV. N-specific immunity was boosted 1 week later by infecting animals intraperitoneally with 5 × 106 PFU of recombinant vaccinia virus expressing a FLAG-tagged version of BDV-N (60).
Scoring of neurological disease and CNS viral load.
The severities of neurological disease in BDV-infected mice were scored on a scale ranging from 0 to 3. A score of 0 indicated no symptoms; 1 indicated a low degree of ataxia and increased anxiety; 2 indicated clear ataxia, torticollis, unphysiological and uncontrolled movements of extremities when the animal was held up by the tail, and rough fur or hunched posture when animal was lifted by the tail; and 3 indicated pronounced weight loss, severe ataxia, inward folding of hind limbs and torticollis, paraparesis, apathy, and moribundity. Viral load in the CNS was assessed by immunohistological staining of BDV-infected neurons and astrocytes with monoclonal antibody (MAb) Bo18 directed against the viral nucleoprotein. BDV antigen-specific immunohistological staining was quantitatively analyzed by two independent researchers according to an arbitrary scale with scores from 0 to 3 as follows: 0 indicated no virus antigen-positive cells detected in at least three brain sections, 1 indicated less than 100 antigen-positive cells per brain section, 2 indicated a large number of antigen-positive cells in certain brain regions only, and 3 indicated a large number of antigen-positive cells in most brain regions.
In vitro restimulation of splenocytes.
Splenic lymphocytes were obtained by gently pressing the organ through a metal grid (60 mesh; Sigma). Splenocytes were seeded into 24-well plates at 3 × 106 cells/well and mixed with 6 × 105 mitomycin C-treated naive splenocytes pulsed with 10−5 M TELEISSI peptide, which represents the immunodominant CD8 T-cell epitope in H-2k mice (60). Half of the medium was replaced after 7 days. Four days later, restimulated cultures were analyzed by flow cytometry and in vitro cytotoxicity assays.
Peptides, MHC-I tetramer, and flow cytometry.
Peptides were purchased from Neosystem (Strasbourg, France) at purities of >65% (immunograde). TELEISSI-H-2Kk tetrameric complexes labeled with phycoerythrin were kindly provided by the NIAID tetramer facility (Bethesda, MD). Tetramers (5 μg/ml for 105 to 106 lymphocytes in 25 μl) were used together with allophycocyanin-conjugated anti-CD8α antibodies (1 μg/ml) (clone 53-6.7; BD PharMingen). Incubation was for 30 min at room temperature. Analysis of stained cells was performed on a FACSort flow cytometer (BD).
In vitro cytotoxicity assay.
The cytolytic activity of splenocytes was determined by a standard 51Cr release assay with slight modifications as described previously (60). As a control, target cells were pulsed with 10−4 M of the irrelevant H-2Kk-binding peptide FEANGNLI derived from the hemagglutinin protein of influenza virus A/PR/8/34 (H1N1) (24).
Histology, immunohistochemical analysis, and staining for eosinophil-specific peroxidase activity.
Treatment of brain sections and immunohistochemistry were done as described previously (15). For the specific detection of eosinophils, we took advantage of the fact that the peroxidase activity of eosinophils is highly resistant to cyanide treatment, whereas peroxidase of other granulocytes, mast cells, and endothelial cells can be poisoned by cyanide (72). Frozen sections of mouse brains were fixed with a 1:1 (vol/vol) mixture of methanol/acetone, air dried, and incubated for 20 min with cyanide-containing diaminobenzidine substrate solution (5 μl of 30% H2O2 and 5 drops of 100 mM KCN for 5 ml of diaminobenzidine substrate solution) at room temperature. After being washed, the sections were counterstained with Meyer's hematoxylin.
RNase protection assay.
RNase protection assays were performed as described previously (32). Five μg of total RNA was used for each sample and hybridized with the following probe sets: mCK5 (RiboQuant Multi-Probe template sets; BD Biosciences, Heidelberg, Germany), AP9, ML11, and ML26, which contained probes for CD3, IL-10, tumor necrosis factor alpha, NOS-1, NOS-2, NOS-3, IL-1α, IFN-γ, IL-12 p40, GFAP, IL-2, IL-4, IL-5, IL-6, IL-13, CCL5 (RANTES), CCL3 (MIP1α), CCL4 (MIP1β), CCL2 (MCP-1), CXCL10 (IP-10), CCL11 (eotaxin), CCL1 (TCA-3), and the RPL32-4A gene, which served as an internal loading control. Biomax films (Kodak) were exposed for various periods of time and scanned using a ScanJet 4C instrument (Hewlett Packard). NIH Image software, version 1.62, was used to quantify the autoradiographs.
RESULTS
Vaccination does not protect IFN-γ-deficient (GKO) mice from infection with BDV.
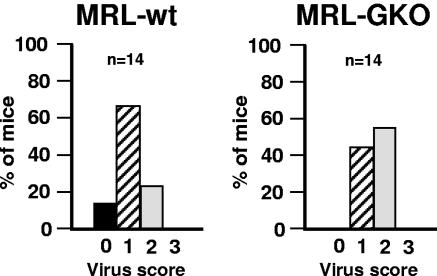
Since spontaneous clearance of BDV does not occur in infected mice, we employed a previously established heterologous prime-boost vaccination protocol (26) to determine the role of IFN-γ in vaccination-induced control of BDV spread in the CNS. Mice were first treated with recombinant parapoxvirus expressing BDV-N, followed by infection with a BDV-N-expressing recombinant vaccinia virus (Fig. 1A). As a control, mice were immunized with β-Gal-expressing poxvirus vectors. As reported previously (26), this immunization protocol induced the complete protection of wild-type MRL mice against subsequent intracerebral challenge with BDV (Fig. 1B, upper left panel). Under these conditions, about 50% of control-vaccinated wild-type MRL mice were susceptible to BDV and developed neurological disease (Fig. 1B, lower left panel). In contrast to the wild-type MRL mice, none of the 24 BDV-N-vaccinated MRL-GKO mice was protected against persistent infection with BDV (Fig. 1B, upper right panel), and neurological disease developed at similar frequencies in vaccinated and control MRL-GKO mice (Fig. 1B, lower right panel). Note that the number of BDV-infected cells in the CNS 5 weeks after intracerebral BDV challenge was slightly reduced in N-vaccinated versus controlvaccinated MRL-GKO mice, suggesting some residual antiviral activity of IFN-γ-deficient immune cells. Thus, N-specific preexposure vaccination effectively protected wild-type MRL mice against persistent infection after intracerebral challenge with BDV and against infection-associated neurological disease. In contrast, it completely failed to protect GKO mice against persistent infection and disease.
FIG. 1.
BDV-N-specific vaccination protects wild-type but not MRL-GKO mice against infection with BDV. (A) Heterologous prime-boost protocol. Numbers indicate ages (days) of animals when indicated actions were taken. ORFV, recombinant Orf virus D1701-VrV expressing either BDV-N or β-Gal; VV, recombinant vaccinia virus expressing either BDV-N or β-Gal. (B) Viral load and neurological disease of MRL wild-type (MRL-wt) and MRL-GKO mice. Scoring of neurological disease and viral load was done according to an arbitrary scale from 0 to 3 (see Materials and Methods). Each symbol represents an individual animal. The horizontal lines indicate the arithmetic means for each animal group. (C) Samples of restimulated splenocytes were analyzed for TELEISSI-specific cytolytic activity after N-specific immunization exactly following the protocol described for panel A. Lytic activity was determined in a 51Cr release assay using L929 cells pulsed with either TELEISSI (closed symbols) or control peptide FEANGNLI (open symbols) as target cells. Curves represent lytic activities of splenocytes from individual mice and are representative of at least four independent experiments.
To determine whether the vaccine was able to induce TELEISSI-specific CD8 T cells in MRL-GKO mice, splenocytes from immunized animals were restimulated in vitro with TELEISSI peptide. When tested for lytic activity towards L929 target cells pulsed with TELEISSI peptide, splenocytes from both wild-type and GKO mice showed high levels of TELEISSI-specific lytic activity (Fig. 1C). Restimulated splenocytes from naive mice did not exhibit TELEISSI-specific lytic activity in this assay. We do not know why in vitro-restimulated splenocyte cultures from poxvirus-vaccinated wild-type animals exhibited a relatively high level of background lysis of target cells coated with a nonrelated H-2Kk-binding control peptide. This background was also found with another irrelevant H-2Kk-binding peptide and was not dependent on restimulation time (data not shown).
Staining of restimulated cultures with a phycoerythrin- labeled TELEISSI/H-2Kk tetrameric MHC-I complex revealed that, on average, 12.4% of all CD8 T cells in splenocyte cultures from immunized GKO mice, compared to 15.9% of wild-type CD8 T cells, were TELEISSI specific (data not shown). Thus, the induction of a BDV-specific CD8 T-cell response by prime-boost vaccination in GKO mice was as efficient as it was in wild-type mice. Therefore, the high BDV susceptibility of immunized MRL-GKO mice could not be attributed to inefficient induction of TELEISSI-specific CD8 T cells in these animals but rather to a defect in a decisive antiviral effector mechanism.
Role of IFN-γ in BDV resistance of adult MRL mice.
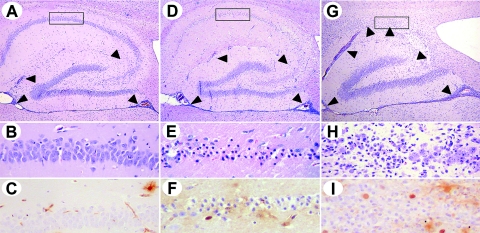
When MRL mice were inoculated between 10 and 21 days after birth, all animals became persistently infected and showed high viral antigen load levels and large numbers of infected cells in all parts of the brain 5 weeks after infection (Fig. 2). Consistent with our earlier observations (25), animals infected at this age were also highly susceptible to virus-induced neurological disease (Fig. 2). However, in our vaccination studies described here and in an earlier report (26), we consistently observed a certain proportion of about 20 to 25% of control-vaccinated animals that did not develop persistent infection after BDV challenge at an age of 42 days. We therefore analyzed the rate of persistently infected animals after the inoculation of naive mice at an age of 42 days. Infection of naive wild-type mice of this age group resulted in a decreased number of persistently infected animals upon analysis 5 weeks postinfection (p.i.). Five out of 21 naive mice infected at this age did not contain detectable viral antigen in the CNS (Fig. 2), and the rate of severe neurological disease decreased dramatically for this group of mice. The numbers of mice with established persistent infection and severe neurological disease are thus very similar to those of control-vaccinated animals that had been challenged at the same age (compare to Fig. 1). Most interestingly, the vast majority of 60- or 130-day-old MRL mice completely resisted infection with BDV when analyzed 5 weeks p.i. (Fig. 2). Of the only four animals from the latter groups that became persistently infected with BDV, three developed neurological disease. Animals inoculated at ages of 60 days or more are referred to as “old” mice below. In marked contrast to wild-type mice, IFN-γ-deficient MRL mice were all highly susceptible to persistent BDV infection, irrespective of age (Fig. 2), and showed high levels of susceptibility to severe neurological disease (scores 2 and 3) with mostly fatal outcome (Fig. 2). When the brains were analyzed by immunohistochemistry at very early times (16 to 17 days) postinfection, small numbers of virus-positive neurons were present in the hippocampal CA3 regions of most old MRL wild-type mice (Fig. 3). At this early time of infection, the levels of viral spread in the brains of old wild-type and GKO animals were comparable, indicating that BDV initially infected both types of mice but that the virus was later cleared from neurons of wild-type mice but not from those of GKO mice.
FIG. 2.
Age-related resistance toward BDV in MRL mice is dependent on IFN-γ. Mice of the indicated ages were infected with BDV and either sacrificed 5 weeks postinfection or killed prematurely in cases of severe neurological disease. BDV antigen loads in the CNS were determined by immunohistochemical staining of paraffin-embedded brain sections with BDV-N-specific MAb Bo18. Scoring of neurological disease and viral load levels was done according to an arbitrary scale from 0 to 3 (see Materials and Methods). Each symbol represents an individual animal. The horizontal lines indicate the arithmetic means for each animal group.
FIG. 3.
Hippocampus infection of old wild-type MRL but not of GKO mice is transient. MRL wild-type and MRL-GKO mice were infected with BDV at ages of 90 days and sacrificed for histological analysis of brains at days 16 and 17 (wild-type mice) or 15, 16, and 18 (GKO mice) postinfection. Viral load was assessed by immunohistochemical staining of brain sections with BDV-N-specific MAb Bo18. Scoring was performed as described in Materials and Methods.
We previously showed that brain-derived mononuclear cell preparations isolated from wild-type MRL mice infected as newborns contain around 30% CD8 T cells and around 10 to 15% CD4 T cells at the peak of neurological disease (27). We also showed previously that around 30% of the CD8 T cells were TELEISSI specific when labeled with a TELEISSI-H-2Kk tetramer directly after isolation (14). In brain mononuclear cell preparations of MRL-GKO mice (n = 19) infected at day 77, we found 38% CD8 T cells, of which 25% were TELEISSI specific, as judged by ex vivo tetramer staining (data not shown). The percentage of CD4 T cells was 20.5% on average (n = 19). Thus, the proportion of BDV-specific CD8 T cells and the originally described CD8-to-CD4 ratio of approximately 2:1 in inflamed MRL wild-type brains are also found in brain mononuclear cell preparations from MRL-GKO mice. Moreover, in MRL-GKO mice infected at 130 days of age, the distribution and number of T cells in the brain tissue were similar to those seen in the corresponding wild-type animals, as determined by immunohistological staining of brain sections with appropriate antibodies. We observed that CD4 T cells resided mainly in perivascular cuffs, whereas CD8 T cells were found mainly in the brain parenchyma (data not shown).
If resistance to BDV infection of adult mice depended on antigen-specific IFN-γ-secreting CD8 T cells, then antibody-mediated depletion of these cells from the circulation should abolish virus resistance. This was indeed the case: all animals in which CD8 T cells were temporarily depleted during the establishment of persistent infection showed persistent infection with a high level of viral spread (Table 1). Furthermore, 90-day-old MRL-β2m0/0 mice lacking mature CD8 T cells were also highly susceptible to BDV infection (Table 1), confirming a critical role for CD8 T cells in the control and elimination of BDV from the CNS of MRL mice infected at 42 days of age or later.
TABLE 1.
Resistance to BDV infection in old wild-type MRL mice is CD8 T-cell dependenta
| Mouse type, treatment | No. of mice positive for virus/total (%) | CNS viral load (average score ± SD) |
|---|---|---|
| Wild-type MRL, no treatment | 3/14 (21) | 0.3 ± 0.64 |
| Wild-type MRL, CD8 T-cell depletion | 5/5 (100) [P = 0.005*] | 2.4 ± 0.82 [P = 0.0007**] |
| MRL-β2m0/0, no treatment | 11/11 (100) [P < 0.001*] | 2.9 ± 0.15 [P < 0.0001**] |
MRL wild-type mice were infected with BDV at an age of 100 days and MRL-β2m0/0 mice were infected at ages of 90 (nine animals) and 180 (two animals) days. CD8 T cells from wild-type animals were depleted by intravenous administration of the CD8-specific monoclonal rat antibody YTS 169 on days 11 and 15 postinfection as previously described (27). Depletion efficiency was determined by CD4 and CD8 staining and subsequent flow cytometry analyses of peripheral blood leukocytes. It was >99% for CD8 T cells and >97% for CD4 T cells. Animals were observed for clinical symptoms and sacrificed when severe neurological disease occurred. Nonsymptomatic mice were sacrificed at day 28 (MRL wild type) or day 35 (MRL-β2m0/0) postinfection. All MRL-β2m0/0 mice were nonsymptomatic. Viral load was determined by scoring the numbers of BDV-N antigen-positive neurons and astrocytes in the CNS, which were detected by immunohistochemical staining with MAb Bo18. Scoring was done as described in Materials and Methods. *, Fisher's exact test; **, Mann-Whitney U test.
IFN-γ-dependent resistance to BDV of adults is not unique to MRL mice.
To demonstrate that IFN-γ is essential for the control of virus spread not only in highly disease-susceptible MRL mice of the H-2k haplotype but also in other strains, we determined whether age-dependent resistance to BDV also occurs in BALB/c mice (H-2d). We have previously demonstrated that such mice are susceptible to persistent BDV infection when inoculated as newborns (25). However, like old MRL mice, only about 50% of old BALB/c mice developed persistent BDV infection (Fig. 4). Moreover, viral spread through the CNS was limited in virus-positive BALB/c mice (average virus score, 0.7) (Fig. 4). In contrast, 100% of old IFN-γ-deficient BALB/c mice (BALB/c-GKO) showed persistent BDV infection, and the average virus score was very high (2.9), indicating unhindered viral spread in BALB/c-GKO mice. Thus, IFN-γ-dependent resistance in adult mice is not restricted to the MRL strain or the H-2k haplotype. Consistent with the low level of viral replication in brains of wild-type BALB/c mice, no neurological disease was detectable in these animals. By contrast, neurological disease was observed in the BALB/c-GKO mice (57%) (Fig. 4), although the severity of disease was generally lower than in MRL-GKO mice (Fig. 2). Thus, the lack of IFN-γ leads to decreased immune-mediated control of virus spread, which in turn strongly increases disease susceptibility, probably because higher numbers of immune cells enter the CNS of such mice.
FIG. 4.
Old BALB/c mice show IFN-γ-dependent resistance to BDV. Wild-type BALB/c mice (90 days old) and IFN-γ-deficient BALB/c-GKO mice (180 days old) were intracerebrally infected with BDV and either sacrificed 5 weeks postinfection or killed prematurely in cases of severe neurological disease. BDV antigen load in the CNS was determined by immunohistochemical staining of paraffin-embedded brain sections with BDV-N-specific MAb Bo18. Scoring of neurological disease and viral load levels was done according to an arbitrary scale from 0 to 3 (see Materials and Methods). Each symbol represents an individual animal. The horizontal lines indicate the arithmetic means for each animal group.
Neuroprotective activity of IFN-γ in the BDV-infected CNS.
In agreement with earlier reports using young MRL mice (25), neuronal damage was also minimal in brains of old wild-type MRL mice with BDV-induced disease (Fig. 5A and B), although diseased old MRL mice showed significant infiltration by mononuclear cells (Fig. 5A) that was comparable to the inflammation seen in diseased young MRL mice. In contrast, histological analysis of brains from persistently infected old MRL-GKO mice revealed severe and widespread neuronal damage in the CA1 region of the hippocampus (Fig. 5D, E, G, and H). These neurodegenerative alterations were found in the majority (∼70%) of the more than 50 GKO animals of ages between 42 and 180 days analyzed in the course of this study. Hippocampal damage occurred at a similar frequency (78%; n = 18) in MRL-GKO mice infected at ages of 14 to 20 days (data not shown). The pyramidal neurons of the CA1 region often appeared shrunken and showed eosinophilia of the cytoplasm and nuclear condensation (Fig. 5D and E). Some damaged hippocampi were massively infiltrated by mononuclear cells (Fig. 5G and H). We observed that the degrees of neuronal damage in the CA1 regions of individual animals varied. Most infected GKO mice had completely destroyed CA1 regions, whereas others presented with only partly damaged CA1 structures. In a number of cases, neuronal damage in the CA1 region was accompanied by CA3 region damage. It should be noted that neuronal damage was never observed in the hippocampi of uninfected MRL-GKO mice (data not shown), ruling out a direct effect of the IFN-γ deficiency. CA1 neurons are usually not infected with BDV in either wild-type or GKO mice (Fig. 5C, F, and I). Therefore, it is unlikely that the widespread degeneration of CA1 neurons in GKO mice is a direct consequence of virus replication in these cells.
FIG. 5.
Hippocampus damage in old BDV-infected mice lacking IFN-γ. Histological analysis of brains of diseased MRL wild-type (A through C) and diseased MRL-GKO (D through I) mice infected at 60 to 130 days of age. Panels A, B, D, E, G, and H show hematoxylin/eosin-stained 8-μm sections of hippocampi. Larger magnifications (B, E, and H) show sections of the same hippocampal CA1 regions (boxed in panels A, D, and G) that were specifically damaged in brains of GKO mice but not of wild-type mice. Panels C, F, and I show sections of the respective CA1 regions immunohistologically stained with BDV-N-specific MAb Bo18. Brown stain indicates virus-positive cells. Arrowheads indicate mononuclear infiltrates.
To determine whether neurodegeneration in BDV-infected GKO mice was T-cell dependent, either CD8 T cells alone or both CD8 and CD4 T cells were depleted with antibodies. Depletion was >99% efficient for CD8 T cells and >97% efficient for CD4 T cells. Whereas all 10 untreated MRL-GKO mice showed BDV-induced neurodegeneration in the hippocampus, only 1 out of 6 CD8 T-cell-depleted mice presented with neuronal damage in the hippocampus at day 28 postinfection (Table 2). Of the six animals with depletions of CD4 and CD8 T cells, none showed detectable neurodegeneration (Table 2). Thus, neuronal damage in BDV-infected GKO mice was clearly dependent on the presence of CD8 T cells.
TABLE 2.
T-cell dependence of eosinophil infiltration and neuronal damage in MRL-GKO micea
| Treatment | Eosinophil infiltration (score ± SEM) | No. of damaged hippocampi/total (%) |
|---|---|---|
| None | 3.3 ± 0.26 | 10/10 (100) |
| CD8 T-cell depletion | 1.83 ± 0.65 [P = 0.072**] | 1/6 (16) [P = 0.0014*] |
| CD4 and CD8 T-cell depletion | 0.83 ± 0.65 [P = 0.017**] | 0/6 (0) [P = 0.001*] |
Mice were infected with BDV at 100 days of age. The indicated T-cell subsets were depleted by intravenous administration of monoclonal antibodies to CD8 alone or to CD8 and CD4 on days 11 and 15 postinfection. Animals were observed for clinical symptoms and were sacrificed either when severe neurological disease occurred or else at 28 days postinfection. Scores are as follows: no eosinophils (score 0), single cells (score 1), few cells (score 2), many cells (score 3), and massive infiltration with eosinophils (score 4). *, Fisher's exact test; **, Mann-Whitney U test.
Most interestingly, clear neuronal damage in the CA1 region of the hippocampus was also observed in 6 out of 14 (43%) infected BALB/c-GKO mice. In contrast, none of 12 infected wild-type BALB/c mice showed hippocampal damage. Thus, the detrimental effect of IFN-γ deficiency is not specific for the MRL mouse but is also observed in mice of different genetic backgrounds.
Altered composition of immune cell infiltrates in BDV-infected GKO mice.
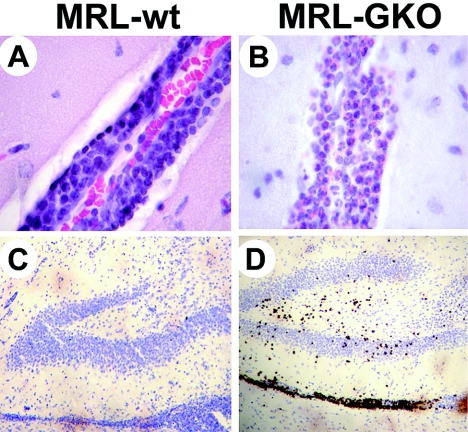
Infiltrating immune cells in brains of old wild-type animals had the regularly-shaped round nuclei typical of lymphocytes and macrophages (Fig. 6A), which has previously been described for MRL mice infected at young ages (25). In contrast, brains of infected MRL-GKO mice with severe meningoencephalitis contained high numbers of cells with polymorphic nuclei and strongly eosinophilic cytoplasms (Fig. 6B). Based on their characteristic morphologies, the polymorphonuclear leukocytes in brains of MRL-GKO mice appeared to represent eosinophilic granulocytes. Staining for cyanide-resistant peroxidase activity (which specifically detects eosinophilic granulocytes [72]) confirmed the identity of these cells (Fig. 6D). Eosinophils were found in the meninges and brain parenchyma as well as in perivascular cuffs. Eosinophil-specific peroxidase staining was never detected in inflamed brains of wild-type mice of any age (Fig. 6C). Eosinophil infiltrates were also observed in brains of MRL-GKO mice infected at young ages (data not shown). The infiltration of eosinophils into the infected brains of old GKO mice was clearly reduced when CD8 T cells were depleted (Table 2). If both CD8 and CD4 T cells were depleted, eosinophil infiltration was reduced even more strongly (Table 2), demonstrating a decisive role for T cells in determining the composition of immune cell infiltrates in the CNS.
FIG. 6.
BDV infection induces influx of eosinophilic granulocytes into brains of old MRL-GKO mice but not of wild-type mice. MRL wild-type and MRL-GKO mice infected at ages of 60 to 130 days with BDV were sacrificed at the peaks of neurological disease at days 25 to 30 p.i. Paraffin sections of brains were either stained with hematoxylin/eosin (A and B) or for cyanide-resistant peroxidase activity by brown stain which specifically labels eosinophilic granulocytes (C and D). Representative infiltrates around blood vessels in the hippocampus (panels A and B) and dentate gyrus regions at lower magnification (panels C and D) are shown.
Cytokine expression pattern in brains of infected MRL-GKO mice.
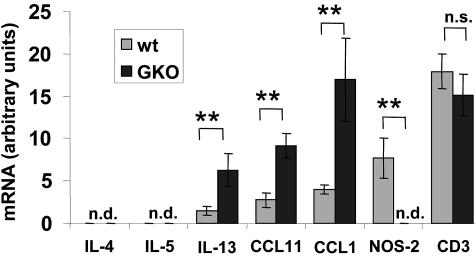
We analyzed the pattern of cytokine and chemokine gene expression in brains of old BDV-infected wild-type and GKO MRL mice using RNase protection assays. In brains of both wild-type and GKO animals, high numbers of infiltrating T cells were present, as judged from the strong signals for CD3ɛ mRNA (Fig. 7). We found no differences in the expression levels of IL-10, tumor necrosis factor alpha, NOS-1, NOS-3, IL-1α, IL-12 p40, GFAP, IL-2, IL-6, CCL5 (RANTES), CCL3 (MIP1α), CCL4 (MIP1β), CCL2 (MCP-1), and CXCL10 (IP-10) (data not shown). In contrast, we observed reduced expression of the NOS-2 gene in GKO mice (Fig. 7), which was expected because NOS-2 gene expression is strongly up-regulated by IFN-γ. Expression of the Th2 cytokines IL-4 and IL-5 was undetectable in either strain of mice, but IL-13 mRNA levels were enhanced at least threefold in GKO mice (Fig. 7). mRNA levels of the chemokines CCL-11 (eotaxin) and CCL-1 (TCA-3) were also enhanced about threefold in GKO mice. Thus, it is conceivable that the enhanced expression of these chemokine genes may account for the massive infiltration of eosinophilic granulocytes into the brains of BDV-infected GKO mice.
FIG. 7.
Altered cytokine and chemokine expression pattern in brains of BDV-infected GKO mice. MRL wild-type (gray bars; n = 6) and MRL-GKO (black bars, n = 6) mice were infected with BDV at ages of 90 days, and brains were collected when the animals developed disease (at day 25 p.i. at the earliest or at day 35 p.i. at the latest). Brain RNA was analyzed for transcripts of the genes corresponding to the indicated products by using RNase protection assays. Autoradiographs were scanned, and optical densities of the bands were measured and expressed as arbitrary units. Graphs show mean values from groups of six animals and statistical significances. Statistical analysis was performed with the Mann-Whitney U test. **, P < 0.005; n.s., not significant; n.d., not detectable.
DISCUSSION
We show here that the protective efficacy of recombinant poxvirus-mediated immunization against BDV in MRL mice was strongly dependent on IFN-γ, which is in agreement with our earlier finding that perforin, as a central molecule of the lytic effector function of CD8 T cells, is not essential for vaccination-induced immune control of BDV (26). The high degree of dependence of CD8 T cell-mediated immune control of BDV on a single secreted effector cytokine was unexpected, because the noncytolytic clearance of other viruses from neurons after immunization is dependent on more than one factor. Antibodies seem to play a prominent role in the case of neuronal infection with Theiler's murine encephalitis virus (19), the neurotropic JHM variant of mouse hepatitis virus (17), Sindbis virus (8, 37), and rabies virus (51). A contribution of IFN-γ in viral clearance from neurons has previously been noted in a number of neurotropic virus infections, including vaccinia virus and vesicular stomatitis virus (35, 36), measles virus (49, 69), neurotropic mouse hepatitis virus (7, 48, 50), Sindbis virus (8), and lymphocytic choriomeningitis virus (65). However, the strict and exclusive dependence of BDV resistance on IFN-γ during primary and secondary immune responses is unique. We previously found that the rat model may represent an exception in that IFN-γ could not prevent BDV infection of various rat cell lines, whereas BDV could be eliminated from infected human- and monkey-derived cell cultures by IFN-γ (59). More importantly, we recently were able to show that IFN-γ can clear BDV infection from organotypic slice cultures of murine cerebellum (18).
Our vaccination approach described here and elsewhere (26) as well as previous studies in other systems (68) demonstrated that antigen-specific CD8 T-cell responses can be efficiently primed after peripheral poxvirus immunization in IFN-γ-deficient mice. Moreover, we have shown here that comparable numbers of virus-specific CD8 T cells enter the brain of MRL-GKO mice and demonstrated previously that brain-derived mononuclear cell preparations from BDV-infected wild-type and GKO animals display similar in vitro cytolytic activity levels against target cells coated with the immunodominant TELEISSI epitope (28). Therefore, the lack of immune control of BDV in GKO mice is not due to an inefficient CD8 T-cell response but due to the inability of BDV-specific CD8 T cells to secrete antivirally active IFN-γ in the CNS, allowing the unhindered spread of BDV. We further showed in this report that the susceptibility of mice to persistent infection with BDV strongly depends on the age at the time of infection. Young MRL mice were uniformly susceptible to persistent CNS infection, whereas most old mice did not develop persistent infection. This age restriction was not observed if the mice lacked a functional IFN-γ gene. Interestingly, initial levels of virus spread in the CNS were similar in old wild-type and GKO mice, indicating that the former mice are not intrinsically resistant to BDV at higher age. Rather, the antiviral immune response in wild-type mice appears to clear the virus from infected neurons at an early stage of infection.
This finding suggests that unidentified components or mechanisms of the immune system that are essential for the control of BDV in the CNS undergo a maturation process leading to a more effective antiviral CD8 T-cell response that continues until the first weeks of adulthood. Major steps of the antiviral immune response against BDV involve the priming phase requiring transport of virus or viral antigen out of the CNS, which might be mediated by immune cells, the activation of antigen-presenting cells and antigen presentation in secondary lymphoid organs (most probably cervical lymph nodes [4]), the activation of T cells, the migration of effector cells into the CNS, and the initiation of effector mechanisms of CD8 T cells. It remains unknown which phase of the antiviral immune response is critically depending on maturation for an efficient CD8 T-cell response during persistent BDV infection of the CNS. However, priming of a CD8 T-cell response by a strictly neurotropic virus inoculated intracerebrally most probably follows rules that are different than those for priming of the CD8 T-cell response by poxviruses inoculated into the periphery. The sites of replication and antigen production, the amounts of antigen in lymphoid organs, the sites and modes of CD8 T-cell induction, the natures and amounts of cytokines induced in the priming environment, and the immunomodulators expressed by poxviruses but not by BDV differ substantially between these two settings. Therefore, it was possible to induce a potent antiviral CD8 T-cell response in 3- to 4-week-old wild-type animals, whereas priming of CD8 T cells by BDV itself was not efficient enough to substantially limit viral spread through the CNS. Rather, sufficient numbers of antiviral CD8 T cells appear too late in the CNS and cause immunopathology because BDV has already spread efficiently through the organ. Thus, these results demonstrate that the kinetics of immune cell priming and virus spread are very important for the two possible outcomes of BDV infection in susceptible MRL mice, i.e., elimination or immunopathology. This notion is underscored by our previous finding that postexposure vaccination of symptomless persistently infected mice cannot clear the infection but rather induces immunopathology (27). Similar conclusions with respect to the delicate balance between protective and immunopathological effects of the antiviral T-cell response were drawn from BDV infection experiments using rats (38). Future analysis of the maturation phenomena of the immune system in newborn and adult BDV-infected mice might provide important insights into organ-specific immune system maturation and immune surveillance of the CNS.
Our finding that age-related resistance to BDV infection is not a phenomenon of the specific genetic makeup of MRL mice but could also be observed in the genetically distinct mouse strain BALB/c (H-2d) indicates the general applicability of these findings. We have shown that in MRL mice, this phenomenon was dependent on antiviral CD8 T cells. Thus, antiviral CD8 T cells are most probably also responsible for the low susceptibility to persistent infection of aged BALB/c mice, suggesting the presence of effectively processed and presented CD8 T-cell epitopes in the H-2d haplotype. Indeed, computer algorithm-based prediction of CD8 T cells indicates the presence of two H-2d-restricted CD8 T-cell epitopes within the BDV nucleoprotein (data not shown). Thus, age-dependent resistance to persistent BDV infection might be correlated with the presence of potent CD8 T-cell epitopes. No CD8 T-cell epitopes could be identified in the C57BL/6 haplotype H-2b when the viral nucleoprotein or phosphoprotein was analyzed by two independent computer algorithms. This would predict a lack of immune-mediated resistance in the C57BL/6 strain of mice. Indeed, our preliminary data indicate that adult C57BL/6 mice of any age are susceptible to persistent BDV infection (unpublished result).
The mechanism of IFN-γ-mediated clearance of viruses from neurons is still largely unknown. IFN-γ may either act directly on neurons or induce the synthesis of antivirally active molecules in other cells, such as macrophages, astrocytes, and microglia. Receptors for IFN-γ are widely expressed on neurons, although at various densities (55). IFN-γ is able to induce the expression of hundreds of genes whose functions are mostly undefined (9). The induction of NO synthase in macrophages, microglia, or neurons (30) and the subsequent synthesis of nitric oxide could contribute to the antiviral activity of IFN-γ toward BDV. Nitric oxide is involved in the elimination of vesicular stomatitis virus from the CNS of mice (35). MRL mice with a defective NOS-2 gene showed a slightly enhanced rate of neurological disease after neonatal infection with BDV compared to NOS-2-expressing littermates (28), suggesting that IFN-γ-induced synthesis of nitric oxide could indeed play a role in the resistance of mice to BDV.
A surprising observation from our study was the massive infiltration of brains from BDV-infected GKO mice with eosinophilic granulocytes. Eosinophils are involved in the pathology of allergic asthma (62), but in the CNS they are usually not found except after infection with certain parasites (41). The entry of eosinophils into inflamed tissues is dependent on the cytokine-induced expression of adhesion molecules on endothelial cells and on the expression of chemokines such as CCL1 and CCL11 (10, 46). In experimental allergic encephalomyelitis, IFN-γ regulates the expression of chemokines during CNS inflammation, thereby shaping the composition of the immune cell infiltrates. Under such experimental conditions, high numbers of neutrophilic granulocytes were found in brains of mice with IFN-γ deficiency (66). In brains of BDV-infected MRL-GKO mice, the eosinophil-attracting chemokine CCL11 and the granulocyte-attracting chemokine CCL1 were clearly more abundant than in brains of their wild-type counterparts. Moreover, the Th2 cytokine IL-13, which can induce expression of CCL11 in a STAT-6-dependent manner (39, 42), was selectively up-regulated in BDV-infected MRL-GKO brains. This pathway is effectively blocked by IFN-γ (29), suggesting that IFN-γ might also be able to block putative CCL11 expression in brains of BDV-infected MRL mice. It should be noted that the IL-13-induced CCL1 expression observed in airway epithelial cells might not apply to CNS cells. Nevertheless, it is conceivable that IFN-γ plays an important role in orchestrating the cytokine and chemokine expression pattern during viral infections of the CNS and that a lack of IFN-γ can result in alterations of the composition of immune cell infiltrates and of eosinophil infiltration. Whether the infiltrating eosinophils contribute to neurological disease and brain pathology in BDV-infected GKO mice remains unknown. Since neurological disease in MRL-GKO mice was slightly more severe and showed progression faster than that in wild-type MRL mice (28), a contribution of eosinophils in the pathogenic process seems likely.
Our infection experiments suggested that IFN-γ plays a neuroprotective role during inflammatory processes of the CNS. The selective CA1 hippocampal neuron cell death in BDV-infected GKO mice resembles the lesions observed in human temporal lobe epilepsy. The administration of kainate to rodents leads to epileptiform seizures and patterns of neuronal damage similar to those observed in humans (5). Kainate administration is thought to suppress GABAergic neurotransmission in the hippocampus, which results in hyperexcitability states of CA1 and CA3 pyramidal neurons, ultimately leading to the deaths of these neuronal populations by excitotoxic mechanisms (6, 22, 57). Glutamate excitotoxicity and hippocampal neuronal degeneration were previously observed after infection of mouse brains with the HNT strain of measles virus (2) and were more recently observed with neuroadapted Sindbis virus (45). Neuronal damage in these infection models was reduced after administration of the glutamate receptor antagonist MK-801 (1, 45). It is thus conceivable that excitotoxic mechanisms are also responsible for the observed neurodegeneration in BDV-infected mouse brains. However, unlike in the other virus infection models, neuronal damage after BDV infection was observed only in animals devoid of IFN-γ. It has previously been shown that antigen-specific T cells protect neurons from secondary degeneration after primary injury of neural tissue (43). Recently, it could be demonstrated that one important effector mechanism of such neuroprotective T cells was the secretion of IFN-γ, which induced the uptake and removal of excess glutamate in neuronal tissue by activated microglia (61). Our data suggest that IFN-γ exerts a hitherto-unappreciated neuroprotective activity also in the course of virus-induced neuroinflammatory processes. Whether glutamate receptor antagonists can ameliorate or prevent BDV-induced neurodegeneration in GKO mice remains to be determined. Very recently, it was shown that the homeostatic effect of IFN-γ that dampens the inflammation in a model of rheumatoid arthritis is at least in part mediated by IFN-γ-induced down-regulation of the receptor for the key proinflammatory cytokine IL-1 (34).
Since CD8 T cells were required for the neurodegenerative effect of BDV in GKO mice, the selective loss of hippocampal neurons alternatively could result from CD8 T-cell-mediated axonal damage of infected mossy fibers and Schaffer collaterals that project to CA3 and CA1 region neurons, respectively. Damaged axons might release large amounts of glutamate at their synaptic connections, leading to excitotoxicity in connected neurons. The question of how IFN-γ could interfere with such a mechanism remains. IFN-γ was shown to exhibit neuroprotective activity in an in vitro model of neuronal cell death induced by nerve growth factor deprivation (11). In an adoptive T-cell transfer model, activated T cells with low capacity to secrete IFN-γ induced more axonal damage than T cells with high capacity to secrete IFN-γ (21). A neuroprotective effect of IFN-γ was also deduced from experiments with Theiler's murine encephalomyelitis virus DA strain in mouse brains (56). However, in this system, IFN-γ probably exerted a protective effect on infected spinal cord neurons simply by suppressing viral replication. Therefore, the protective effect of IFN-γ on neurons in Theiler's virus-infected animals probably differs mechanistically from the IFN-γ-mediated protection of uninfected hippocampal CA1 region neurons in BDV-infected mouse brains.
Acknowledgments
We thank Rosita Frank and Tina Hagena for excellent technical assistance, the NIAID Tetramer Facility (Atlanta, Georgia) and the NIH AIDS Research and Reference Reagent Program for producing H-2Kk-TELEISSI tetramer.
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 620).
REFERENCES
- 1.Andersson, T., M. Schultzberg, R. Schwarcz, A. Love, C. Wickman, and K. Kristensson. 1991. NMDA receptor antagonist prevents measles virus-induced neurodegeneration. Eur. J. Neurosci. 3:66-71. [DOI] [PubMed] [Google Scholar]
- 2.Andersson, T., R. Schwarcz, A. Love, and K. Kristensson. 1993. Measles virus-induced hippocampal neurodegeneration in the mouse: a novel, subacute model for testing neuroprotective agents. Neurosci. Lett. 154:109-112. [DOI] [PubMed] [Google Scholar]
- 3.Barish, M. E., N. B. Mansdorf, and S. S. Raissdana. 1991. Gamma-interferon promotes differentiation of cultured cortical and hippocampal neurons. Dev. Biol. 144:412-423. [DOI] [PubMed] [Google Scholar]
- 4.Batra, A., O. Planz, T. Bilzer, and L. Stitz. 2003. Precursors of Borna disease virus-specific T cells in secondary lymphatic tissue of experimentally infected rats. J. Neurovirol. 9:325-335. [DOI] [PubMed] [Google Scholar]
- 5.Ben-Ari, Y., and R. Cossart. 2000. Kainate, a double agent that generates seizures: two decades of progress. Trends Neurosci. 23:580-587. [DOI] [PubMed] [Google Scholar]
- 6.Benedikz, E., P. Casaccia-Bonnefil, A. Stelzer, and P. J. Bergold. 1993. Hyperexcitability and cell loss in kainate-treated hippocampal slice cultures. Neuroreport 5:90-92. [DOI] [PubMed] [Google Scholar]
- 7.Bergmann, C. C., B. Parra, D. R. Hinton, C. Ramakrishna, K. C. Dowdell, and S. A. Stohlman. 2004. Perforin and gamma interferon-mediated control of coronavirus central nervous system infection by CD8 T cells in the absence of CD4 T cells. J. Virol. 78:1739-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Binder, G. K., and D. E. Griffin. 2001. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science 293:303-306. [DOI] [PubMed] [Google Scholar]
- 9.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15:749-795. [DOI] [PubMed] [Google Scholar]
- 10.Broide, D., and P. Sriramarao. 2001. Eosinophil trafficking to sites of allergic inflammation. Immunol. Rev. 179:163-172. [DOI] [PubMed] [Google Scholar]
- 11.Chang, J. Y., D. P. Martin, and E. M. Johnson, Jr. 1990. Interferon suppresses sympathetic neuronal cell death caused by nerve growth factor deprivation. J. Neurochem. 55:436-445. [DOI] [PubMed] [Google Scholar]
- 12.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 259:1739-1742. [DOI] [PubMed] [Google Scholar]
- 13.Dawson, V. L., and T. M. Dawson. 1996. Nitric oxide neurotoxicity. J. Chem. Neuroanat. 10:179-190. [DOI] [PubMed] [Google Scholar]
- 14.Engelhardt, K. R., K. Richter, K. Baur, P. Staeheli, and J. Hausmann. 2005. The functional avidity of virus-specific CD8+ T cells is down-modulated in Borna disease virus-induced immunopathology of the central nervous system. Eur. J. Immunol. 35:487-497. [DOI] [PubMed] [Google Scholar]
- 15.Fassnacht, U., A. Ackermann, P. Staeheli, and J. Hausmann. 2004. Immunization with dendritic cells can break immunological ignorance toward a persisting virus in the central nervous system and induce partial protection against intracerebral viral challenge. J. Gen. Virol. 85:2379-2387. [DOI] [PubMed] [Google Scholar]
- 16.Ferber, I. A., S. Brocke, C. Taylor-Edwards, W. Ridgway, C. Dinisco, L. Steinman, D. Dalton, and C. G. Fathman. 1996. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J. Immunol. 156:5-7. [PubMed] [Google Scholar]
- 17.Fleming, J. O., R. A. Shubin, M. A. Sussman, N. Casteel, and S. A. Stohlman. 1989. Monoclonal antibodies to the matrix (E1) glycoprotein of mouse hepatitis virus protect mice from encephalitis. Virology 168:162-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedl, G., M. Hofer, B. Auber, C. Sauder, J. Hausmann, P. Staeheli, and A. Pagenstecher. 2004. Borna disease virus multiplication in mouse organotypic slice cultures is site-specifically inhibited by gamma interferon but not by interleukin-12. J. Virol. 78:1212-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujinami, R. S., A. Rosenthal, P. W. Lampert, A. Zurbriggen, and M. Yamada. 1989. Survival of athymic (nu/nu) mice after Theiler's murine encephalomyelitis virus infection by passive administration of neutralizing monoclonal antibody. J. Virol. 63:2081-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furrer, E., T. Bilzer, L. Stitz, and O. Planz. 2001. High-dose Borna disease virus infection induces a nucleoprotein-specific cytotoxic T-lymphocyte response and prevention of immunopathology. J. Virol. 75:11700-11708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gimsa, U., S. V. Peter, K. Lehmann, I. Bechmann, and R. Nitsch. 2000. Axonal damage induced by invading T cells in organotypic central nervous system tissue in vitro: involvement of microglial cells. Brain Pathol. 10:365-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goelz, M. F., J. Mahler, J. Harry, P. Myers, J. Clark, J. E. Thigpen, and D. B. Forsythe. 1998. Neuropathologic findings associated with seizures in FVB mice. Lab. Anim. Sci. 48:34-37. [PubMed] [Google Scholar]
- 23.Gosztonyi, G., and H. Ludwig. 1995. Borna disease—neuropathology and pathogenesis. Curr. Top. Microbiol. Immunol. 190:39-73. [PubMed] [Google Scholar]
- 24.Gould, K. G., H. Scotney, and G. G. Brownlee. 1991. Characterization of two distinct major histocompatibility complex class I Kk-restricted T-cell epitopes within the influenza A/PR/8/34 virus hemagglutinin. J. Virol. 65:5401-5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hallensleben, W., M. Schwemmle, J. Hausmann, L. Stitz, B. Volk, A. Pagenstecher, and P. Staeheli. 1998. Borna disease virus-induced neurological disorder in mice: infection of neonates results in immunopathology. J. Virol. 72:4379-4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hausmann, J., K. Baur, K. Engelhardt, T. Fischer, H. Rziha, and P. Staeheli. 2005. Vaccine-induced protection against Borna disease of wild-type and perforin-deficient mice. J. Gen. Virol. 86:399-403. [DOI] [PubMed] [Google Scholar]
- 27.Hausmann, J., W. Hallensleben, J. C. de la Torre, A. Pagenstecher, C. Zimmermann, H. Pircher, and P. Staeheli. 1999. T cell ignorance in mice to Borna disease virus can be overcome by peripheral expression of the viral nucleoprotein. Proc. Natl. Acad. Sci. USA 96:9769-9774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hausmann, J., C. Sauder, M. Wasmer, B. Lu, and P. Staeheli. 2004. Neurological disorder after Borna disease virus infection in the absence of either interferon-gamma, Fas, inducible NO synthase, or chemokine receptor CXCR3. Viral Immunol. 17:79-85. [DOI] [PubMed] [Google Scholar]
- 29.Heller, N. M., S. Matsukura, S. N. Georas, M. R. Boothby, P. B. Rothman, C. Stellato, and R. P. Schleimer. 2004. Interferon-gamma inhibits STAT6 signal transduction and gene expression in human airway epithelial cells. Am. J. Respir. Cell. Mol. Biol. 31:573-582. [DOI] [PubMed] [Google Scholar]
- 30.Heneka, M. T., and D. L. Feinstein. 2001. Expression and function of inducible nitric oxide synthase in neurons. J. Neuroimmunol. 114:8-18. [DOI] [PubMed] [Google Scholar]
- 31.Henkel, M., O. Planz, T. Fischer, L. Stitz, and H. J. Rziha. 2005. Prevention of virus persistence and protection against immunopathology after Borna disease virus infection of the brain by a novel Orf virus recombinant. J. Virol. 79:314-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofer, M., J. Hausmann, P. Staeheli, and A. Pagenstecher. 2004. Cerebral expression of interleukin-12 induces neurological disease via differential pathways and recruits antigen-specific T cells in virus-infected mice. Am. J. Pathol. 165:949-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horwitz, M. S., C. F. Evans, F. G. Klier, and M. B. Oldstone. 1999. Detailed in vivo analysis of interferon-gamma induced major histocompatibility complex expression in the central nervous system: astrocytes fail to express major histocompatibility complex class I and II molecules. Lab. Investig. 79:235-242. [PubMed] [Google Scholar]
- 34.Hu, X., H. Ho, O. Lou, C. Hidaka, and L. B. Ivashkiv. 2005. Homeostatic role of interferons conferred by inhibition of IL-1-mediated inflammation and tissue destruction. J. Immunol. 175:131-138. [DOI] [PubMed] [Google Scholar]
- 35.Komatsu, T., Z. Bi, and C. S. Reiss. 1996. Interferon-gamma induced type I nitric oxide synthase activity inhibits viral replication in neurons. J. Neuroimmunol. 68:101-108. [DOI] [PubMed] [Google Scholar]
- 36.Kundig, T. M., H. Hengartner, and R. M. Zinkernagel. 1993. T cell-dependent IFN-gamma exerts an antiviral effect in the central nervous system but not in peripheral solid organs. J. Immunol. 150:2316-2321. [PubMed] [Google Scholar]
- 37.Levine, B., J. M. Hardwick, B. D. Trapp, T. O. Crawford, R. C. Bollinger, and D. E. Griffin. 1991. Antibody-mediated clearance of alphavirus infection from neurons. Science 254:856-860. [DOI] [PubMed] [Google Scholar]
- 38.Lewis, A. J., J. L. Whitton, C. G. Hatalski, H. Weissenbock, and W. I. Lipkin. 1999. Effect of immune priming on Borna disease. J. Virol. 73:2541-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, L., Y. Xia, A. Nguyen, Y. H. Lai, L. Feng, T. R. Mosmann, and D. Lo. 1999. Effects of Th2 cytokines on chemokine expression in the lung: IL-13 potently induces eotaxin expression by airway epithelial cells. J. Immunol. 162:2477-2487. [PubMed] [Google Scholar]
- 40.Liu, T., K. M. Khanna, B. N. Carriere, and R. L. Hendricks. 2001. Gamma interferon can prevent herpes simplex virus type 1 reactivation from latency in sensory neurons. J. Virol. 75:11178-11184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lo Re, V., III, and S. J. Gluckman. 2003. Eosinophilic meningitis. Am. J. Med. 114:217-223. [DOI] [PubMed] [Google Scholar]
- 42.Matsukura, S., C. Stellato, S. N. Georas, V. Casolaro, J. R. Plitt, K. Miura, S. Kurosawa, U. Schindler, and R. P. Schleimer. 2001. Interleukin-13 upregulates eotaxin expression in airway epithelial cells by a STAT6-dependent mechanism. Am. J. Respir. Cell. Mol. Biol. 24:755-761. [DOI] [PubMed] [Google Scholar]
- 43.Moalem, G., R. Leibowitz-Amit, E. Yoles, F. Mor, I. R. Cohen, and M. Schwartz. 1999. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat. Med. 5:49-55. [DOI] [PubMed] [Google Scholar]
- 44.Narayan, O., S. Herzog, K. Frese, H. Scheefers, and R. Rott. 1983. Behavioral disease in rats caused by immunopathological responses to persistent Borna virus in the brain. Science 220:1401-1403. [DOI] [PubMed] [Google Scholar]
- 45.Nargi-Aizenman, J. L., M. B. Havert, M. Zhang, D. N. Irani, J. D. Rothstein, and D. E. Griffin. 2004. Glutamate receptor antagonists protect from virus-induced neural degeneration. Ann. Neurol. 55:541-549. [DOI] [PubMed] [Google Scholar]
- 46.Oliveira, S. H., S. Lira, A. C. Martinez, M. Wiekowski, L. Sullivan, and N. W. Lukacs. 2002. Increased responsiveness of murine eosinophils to MIP-1beta (CCL4) and TCA-3 (CCL1) is mediated by their specific receptors, CCR5 and CCR8. J. Leukoc. Biol. 71:1019-1025. [PubMed] [Google Scholar]
- 47.Panitch, H. S., R. L. Hirsch, A. S. Haley, and K. P. Johnson. 1987. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet i(8538):893-895. [DOI] [PubMed] [Google Scholar]
- 48.Parra, B., D. R. Hinton, N. W. Marten, C. C. Bergmann, M. T. Lin, C. S. Yang, and S. A. Stohlman. 1999. IFN-gamma is required for viral clearance from central nervous system oligodendroglia. J. Immunol. 162:1641-1647. [PubMed] [Google Scholar]
- 49.Patterson, C. E., D. M. Lawrence, L. A. Echols, and G. F. Rall. 2002. Immune-mediated protection from measles virus-induced central nervous system disease is noncytolytic and gamma interferon dependent. J. Virol. 76:4497-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pearce, B. D., M. V. Hobbs, T. S. McGraw, and M. J. Buchmeier. 1994. Cytokine induction during T-cell-mediated clearance of mouse hepatitis virus from neurons in vivo. J. Virol. 68:5483-5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perry, L. L., and D. L. Lodmell. 1991. Role of CD4+ and CD8+ T cells in murine resistance to street rabies virus. J. Virol. 65:3429-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Popko, B., J. G. Corbin, K. D. Baerwald, J. Dupree, and A. M. Garcia. 1997. The effects of interferon-gamma on the central nervous system. Mol. Neurobiol. 14:19-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Renno, T., V. Taupin, L. Bourbonniere, G. Verge, E. Tran, R. De Simone, M. Krakowski, M. Rodriguez, A. Peterson, and T. Owens. 1998. Interferon-gamma in progression to chronic demyelination and neurological deficit following acute EAE. Mol. Cell. Neurosci. 12:376-389. [DOI] [PubMed] [Google Scholar]
- 54.Richt, J. A., A. Schmeel, K. Frese, K. M. Carbone, O. Narayan, and R. Rott. 1994. Borna disease virus-specific T cells protect against or cause immunopathological Borna disease. J. Exp. Med. 179:1467-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robertson, B., G. Kong, Z. Peng, M. Bentivoglio, and K. Kristensson. 2000. Interferon-gamma-responsive neuronal sites in the normal rat brain: receptor protein distribution and cell activation revealed by Fos induction. Brain Res. Bull. 52:61-74. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez, M., L. J. Zoecklein, C. L. Howe, K. D. Pavelko, J. D. Gamez, S. Nakane, and L. M. Papke. 2003. Gamma interferon is critical for neuronal viral clearance and protection in a susceptible mouse strain following early intracranial Theiler's murine encephalomyelitis virus infection. J. Virol. 77:12252-12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez-Moreno, A., J. C. Lopez-Garcia, and J. Lerma. 2000. Two populations of kainate receptors with separate signaling mechanisms in hippocampal interneurons. Proc. Natl. Acad. Sci. USA 97:1293-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rott, R., and H. Becht. 1995. Natural and experimental Borna disease in animals. Curr. Top. Microbiol. Immunol. 190:17-30. [DOI] [PubMed] [Google Scholar]
- 59.Sauder, C., I. Herpfer, C. Hassler, and P. Staeheli. 2004. Susceptibility of Borna disease virus to the antiviral action of gamma-interferon: evidence for species-specific differences. Arch. Virol. 149:2171-2186. [DOI] [PubMed] [Google Scholar]
- 60.Schamel, K., P. Staeheli, and J. Hausmann. 2001. Identification of the immunodominant H-2Kk-restricted cytotoxic T-cell epitope in the Borna disease virus nucleoprotein. J. Virol. 75:8579-8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shaked, I., D. Tchoresh, R. Gersner, G. Meiri, S. Mordechai, X. Xiao, R. P. Hart, and M. Schwartz. 2005. Protective autoimmunity: interferon-gamma enables microglia to remove glutamate without evoking inflammatory mediators. J. Neurochem. 92:997-1009. [DOI] [PubMed] [Google Scholar]
- 62.Shen, H. H., S. I. Ochkur, M. P. McGarry, J. R. Crosby, E. M. Hines, M. T. Borchers, H. Wang, T. L. Biechelle, K. R. O'Neill, T. L. Ansay, D. C. Colbert, S. A. Cormier, J. P. Justice, N. A. Lee, and J. J. Lee. 2003. A causative relationship exists between eosinophils and the development of allergic pulmonary pathologies in the mouse. J. Immunol. 170:3296-3305. [DOI] [PubMed] [Google Scholar]
- 63.Staeheli, P., C. Sauder, J. Hausmann, F. Ehrensperger, and M. Schwemmle. 2000. Epidemiology of Borna disease virus. J. Gen. Virol. 81:2123-2135. [DOI] [PubMed] [Google Scholar]
- 64.Stitz, L., T. Bilzer, and O. Planz. 2002. The immunopathogenesis of Borna disease virus infection. Front. Biosci. 7:d541-d555. [DOI] [PubMed] [Google Scholar]
- 65.Tishon, A., H. Lewicki, G. Rall, M. Von Herrath, and M. B. Oldstone. 1995. An essential role for type 1 interferon-gamma in terminating persistent viral infection. Virology 212:244-250. [DOI] [PubMed] [Google Scholar]
- 66.Tran, E. H., E. N. Prince, and T. Owens. 2000. IFN-gamma shapes immune invasion of the central nervous system via regulation of chemokines. J. Immunol. 164:2759-2768. [DOI] [PubMed] [Google Scholar]
- 67.Trapani, J. A., and M. J. Smyth. 2002. Functional significance of the perforin/granzyme cell death pathway. Nat. Rev. Immunol. 2:735-747. [DOI] [PubMed] [Google Scholar]
- 68.van den Broek, M., M. F. Bachmann, G. Kohler, M. Barner, R. Escher, R. Zinkernagel, and M. Kopf. 2000. IL-4 and IL-10 antagonize IL-12-mediated protection against acute vaccinia virus infection with a limited role of IFN-gamma and nitric oxide synthetase 2. J. Immunol. 164:371-378. [DOI] [PubMed] [Google Scholar]
- 69.Weidinger, G., G. Henning, V. ter Meulen, and S. Niewiesk. 2001. Inhibition of major histocompatibility complex class II-dependent antigen presentation by neutralization of gamma interferon leads to breakdown of resistance against measles virus-induced encephalitis. J. Virol. 75:3059-3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Willenborg, D. O., S. Fordham, C. C. Bernard, W. B. Cowden, and I. A. Ramshaw. 1996. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J. Immunol. 157:3223-3227. [PubMed] [Google Scholar]
- 71.Young, H. A., and K. J. Hardy. 1995. Role of interferon-gamma in immune cell regulation. J. Leukoc. Biol. 58:373-381. [PubMed] [Google Scholar]
- 72.Zucker-Franklin, D., and G. Grusky. 1976. The identification of eosinophil colonies in soft-agar cultures by differential staining for peroxidase. J. Histochem. Cytochem. 24:1270-1272. [DOI] [PubMed] [Google Scholar]