Abstract
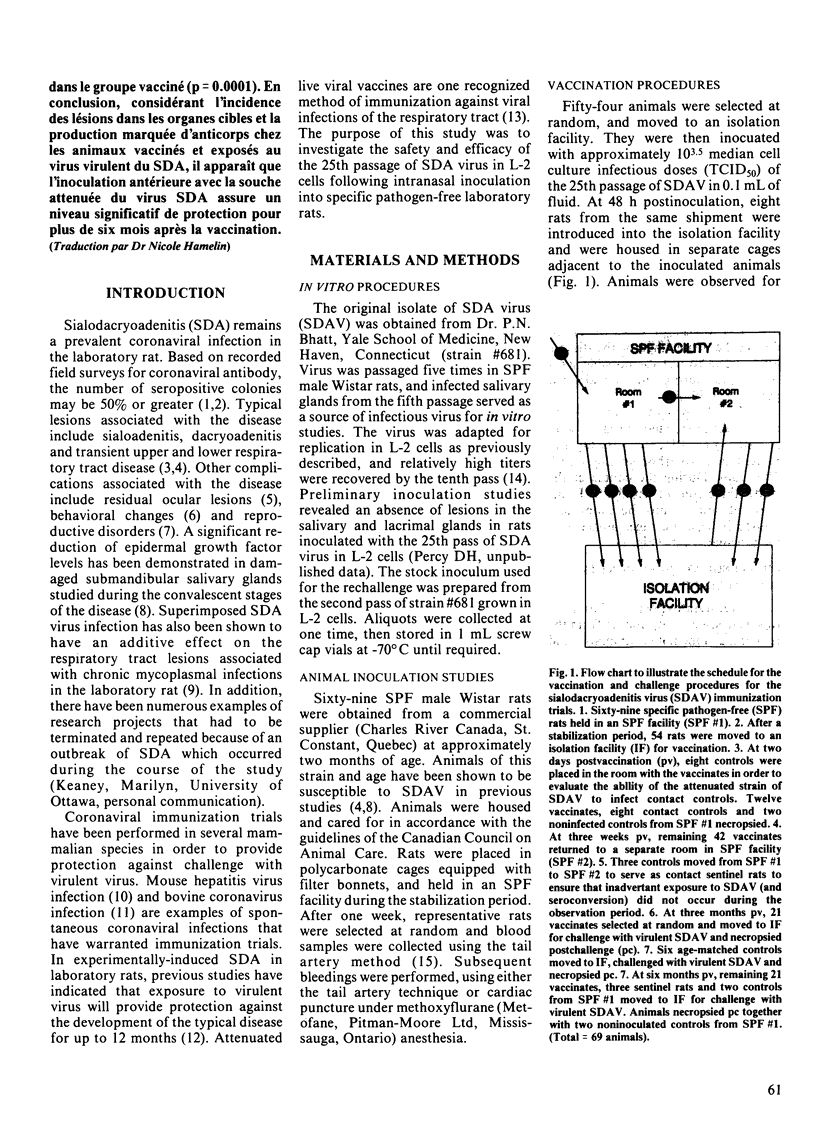
Sixty-nine specific pathogen-free male Wistar rats approximately eight weeks of age were used to evaluate the efficacy of an attentuated strain of sialodacryoadenitis (SDA) virus in providing protection against infection on subsequent challenge with virulent SDA virus. Fifty-four animals were inoculated intranasally with approximately 10(3.5) median cell culture infectious doses of the 25th passage of SDA virus in L-2 cells. Randomly-selected vaccinated animals were killed in order to evaluate the safety and efficacy of attenuated virus by histopathological examination of the salivary glands, lacrimal glands, and lower respiratory tract, and titration of sera for antibody to SDA virus. At three months and six months postvaccination (pv), animals were selected at random and challenged with virulent SDA virus. Seronegative, age-matched animals were also challenged, and served as controls. In animals examined at six to ten days pv, lesions were absent in submandibular and parotid salivary glands and lacrimal glands, but transient lesions were present in major airways of the lower respiratory tract. In a comparison of the incidence and extent of lesions, and antibody titers in challenged vaccinates and seronegative controls, lesions were minimal or absent in vaccinates compared to challenged naive rats, particularly in animals inoculated at three months pv. In addition, antibody titers in challenged vaccinates were much higher than were postinoculation titers in inoculated controls. In a comparison of lesions in salivary and lacrimal glands in vaccinated and control animals challenged at six months pv, there was a significant reduction in the number of animals without lesions in the vaccinated group (p = less than 0.0001).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

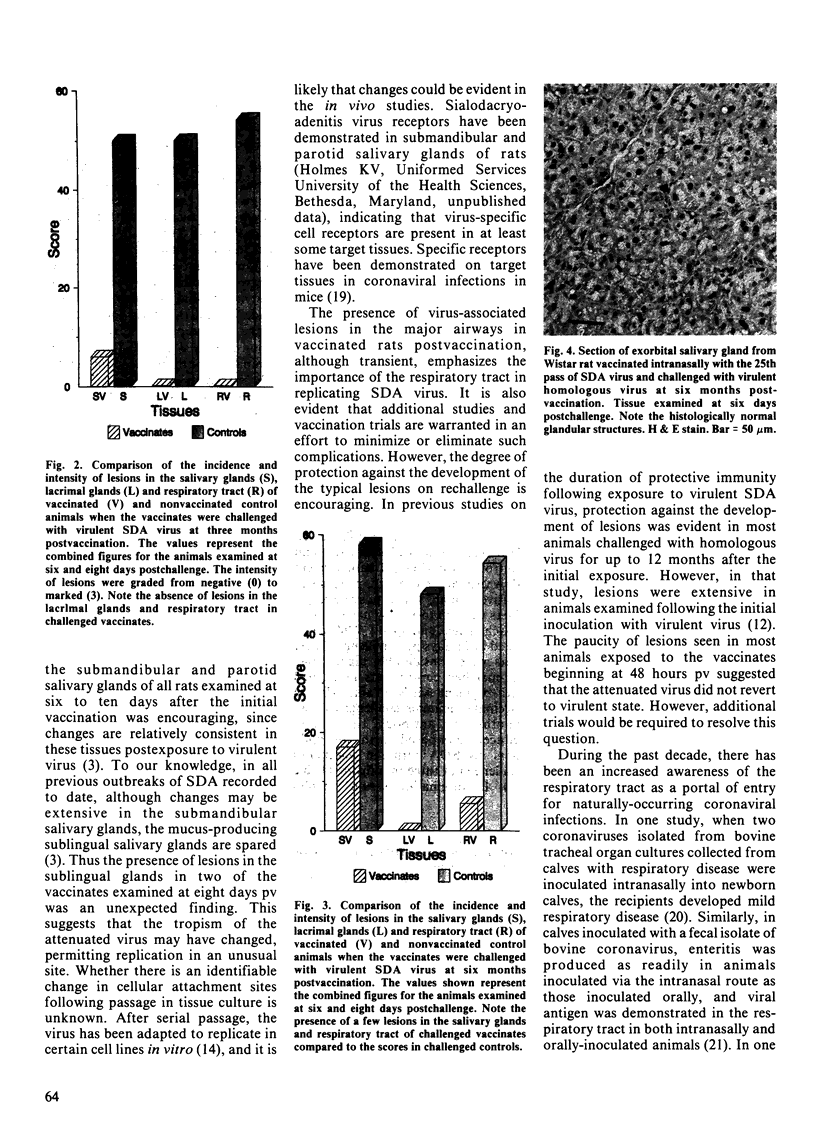


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barthold S. W., Smith A. L. Duration of challenge immunity to coronavirus JHM in mice. Arch Virol. 1989;107(3-4):171–177. doi: 10.1007/BF01317914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Smith A. L. Virus strain specificity of challenge immunity to coronavirus. Arch Virol. 1989;104(3-4):187–196. doi: 10.1007/BF01315542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle J. F., Weismiller D. G., Holmes K. V. Genetic resistance to mouse hepatitis virus correlates with absence of virus-binding activity on target tissues. J Virol. 1987 Jan;61(1):185–189. doi: 10.1128/jvi.61.1.185-189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein D. G., Barthold S. W. Mouse hepatitis virus immunofluorescence in formalin- or Bouin's-fixed tissues using trypsin digestion. Lab Anim Sci. 1982 Feb;32(1):37–39. [PubMed] [Google Scholar]
- Callow K. A. Effect of specific humoral immunity and some non-specific factors on resistance of volunteers to respiratory coronavirus infection. J Hyg (Lond) 1985 Aug;95(1):173–189. doi: 10.1017/s0022172400062410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casebolt D. B., Lindsey J. R., Cassell G. H. Prevalence rates of infectious agents among commercial breeding populations of rats and mice. Lab Anim Sci. 1988 Jun;38(3):327–329. [PubMed] [Google Scholar]
- Chanock R. M., Murphy B. R., Collins P. L., Coelingh K. V., Olmsted R. A., Snyder M. H., Spriggs M. K., Prince G. A., Moss B., Flores J. Live viral vaccines for respiratory and enteric tract diseases. Vaccine. 1988 Apr;6(2):129–133. doi: 10.1016/s0264-410x(88)80014-8. [DOI] [PubMed] [Google Scholar]
- Cutlip R. C., Mengeling W. L. Lesions induced by hemagglutinating encephalomyelitis virus strain 67N in pigs. Am J Vet Res. 1972 Oct;33(10):2003–2009. [PubMed] [Google Scholar]
- Daniel C., Talbot P. J. Protection from lethal coronavirus infection by affinity-purified spike glycoprotein of murine hepatitis virus, strain A59. Virology. 1990 Jan;174(1):87–94. doi: 10.1016/0042-6822(90)90057-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenbrandt D. L., Hubbard G. B., Schmidt R. E. A subclinical epizootic of sialodacryoadenitis in rats. Lab Anim Sci. 1982 Dec;32(6):655–659. [PubMed] [Google Scholar]
- Jacoby R. O., Bhatt P. N., Jonas A. M. Pathogenesis of sialodacryoadenitis in gnotobiotic rats. Vet Pathol. 1975;12(3):196–209. doi: 10.1177/030098587501200305. [DOI] [PubMed] [Google Scholar]
- Kemeny L. J., Wiltsey V. L., Riley J. L. Upper respiratory infection of lactating sows with transmissible gastroenteritis virus following contact exposure to infected piglets. Cornell Vet. 1975 Jul;65(3):352–362. [PubMed] [Google Scholar]
- Lussier G., Descôteaux J. P. Prevalence of natural virus infections in laboratory mice and rats used in Canada. Lab Anim Sci. 1986 Apr;36(2):145–148. [PubMed] [Google Scholar]
- McNulty M. S., Bryson D. G., Allan G. M., Logan E. F. Coronavirus infection of the bovine respiratory tract. Vet Microbiol. 1984 Sep;9(5):425–434. doi: 10.1016/0378-1135(84)90063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullaney T. P., Newman L. E., Whitehair C. K. Humoral immune response of the bovine fetus to in utero vaccination with attenuated bovine coronavirus. Am J Vet Res. 1988 Feb;49(2):156–159. [PubMed] [Google Scholar]
- Percy D. H., Bond S. J., Paturzo F. X., Bhatt P. N. Duration of protection from reinfection following exposure to sialodacryoadenitis virus in Wistar rats. Lab Anim Sci. 1990 Mar;40(2):144–149. [PubMed] [Google Scholar]
- Percy D. H., Hayes M. A., Kocal T. E., Wojcinski Z. W. Depletion of salivary gland epidermal growth factor by sialodacryoadenitis virus infection in the Wistar rat. Vet Pathol. 1988 May;25(3):183–192. doi: 10.1177/030098588802500301. [DOI] [PubMed] [Google Scholar]
- Percy D., Bond S., MacInnes J. Replication of sialodacryoadenitis virus in mouse L-2 cells. Arch Virol. 1989;104(3-4):323–333. doi: 10.1007/BF01315553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saif L. J., Redman D. R., Moorhead P. D., Theil K. W. Experimentally induced coronavirus infections in calves: viral replication in the respiratory and intestinal tracts. Am J Vet Res. 1986 Jul;47(7):1426–1432. [PubMed] [Google Scholar]
- Schoeb T. R., Lindsey J. R. Exacerbation of murine respiratory mycoplasmosis by sialodacryoadenitis virus infection in gnotobiotic F344 rats. Vet Pathol. 1987 Sep;24(5):392–399. doi: 10.1177/030098588702400505. [DOI] [PubMed] [Google Scholar]
- Smith A. L. An immunofluorescence test for detection of serum antibody to rodent coronaviruses. Lab Anim Sci. 1983 Apr;33(2):157–160. [PubMed] [Google Scholar]
- Thorsen J., Djurickovic S. Experimental immunization of sows with inactivated transmissible gastroenteritis (TGE) virus. Can J Comp Med. 1971 Apr;35(2):99–102. [PMC free article] [PubMed] [Google Scholar]
- Utsumi K., Ishikawa T., Maeda T., Shimizu S., Tatsumi H., Fujiwara K. Infectious sialodacryoadenitis and rat breeding. Lab Anim. 1980 Oct;14(4):303–307. doi: 10.1258/002367780781071229. [DOI] [PubMed] [Google Scholar]
- Voets M. T., Pensaert M., Rondhuis P. R. Vaccination of pregnant sows against transmissible gastroenteritis with two attenuated virus strains and different inoculation routes. Tijdschr Diergeneeskd. 1980 Oct 15;105(20):211–219. [PubMed] [Google Scholar]
- Weisbroth S. H., Peress N. Ophthalmic lesions and dacryoadenitis: a naturally occurring aspect of sialodacryoadenitis virus infection of the laboratory rat. Lab Anim Sci. 1977 Aug;27(4):466–473. [PubMed] [Google Scholar]
- Wojcinski Z. W., Percy D. H. Sialodacryoadenitis virus-associated lesions in the lower respiratory tract of rats. Vet Pathol. 1986 May;23(3):278–286. doi: 10.1177/030098588602300308. [DOI] [PubMed] [Google Scholar]






