Abstract
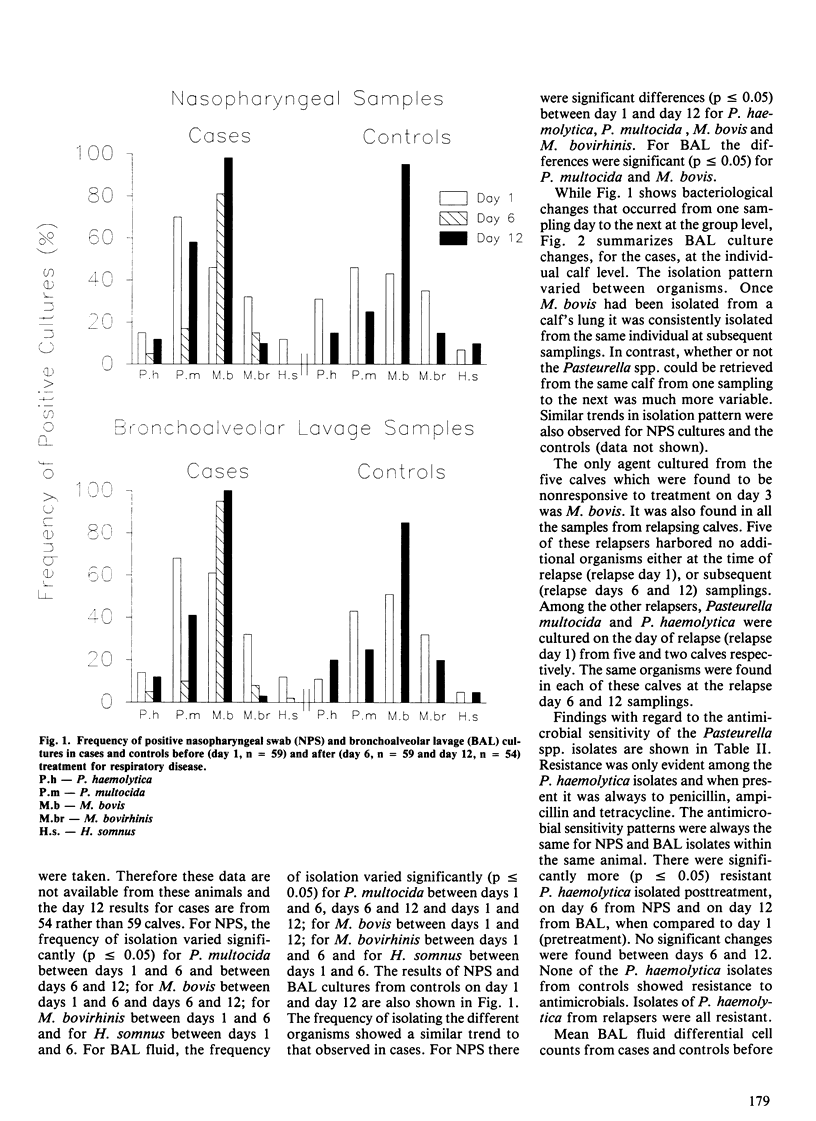
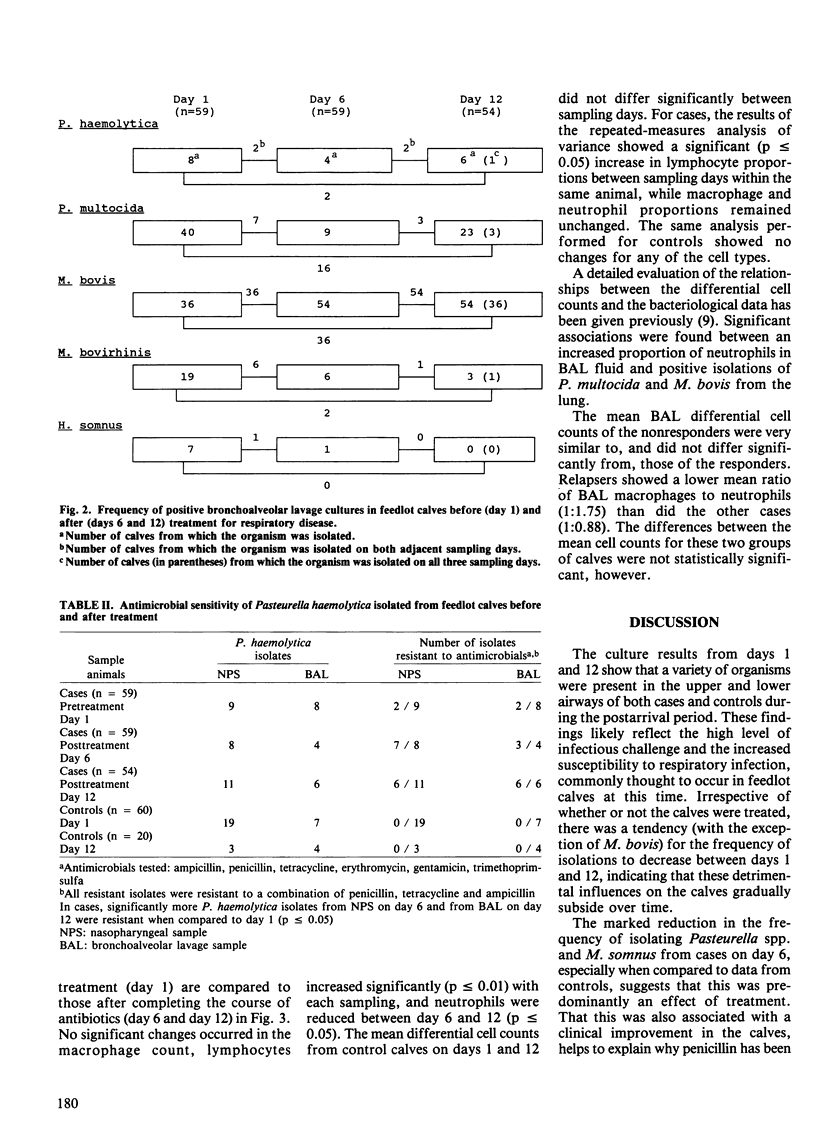
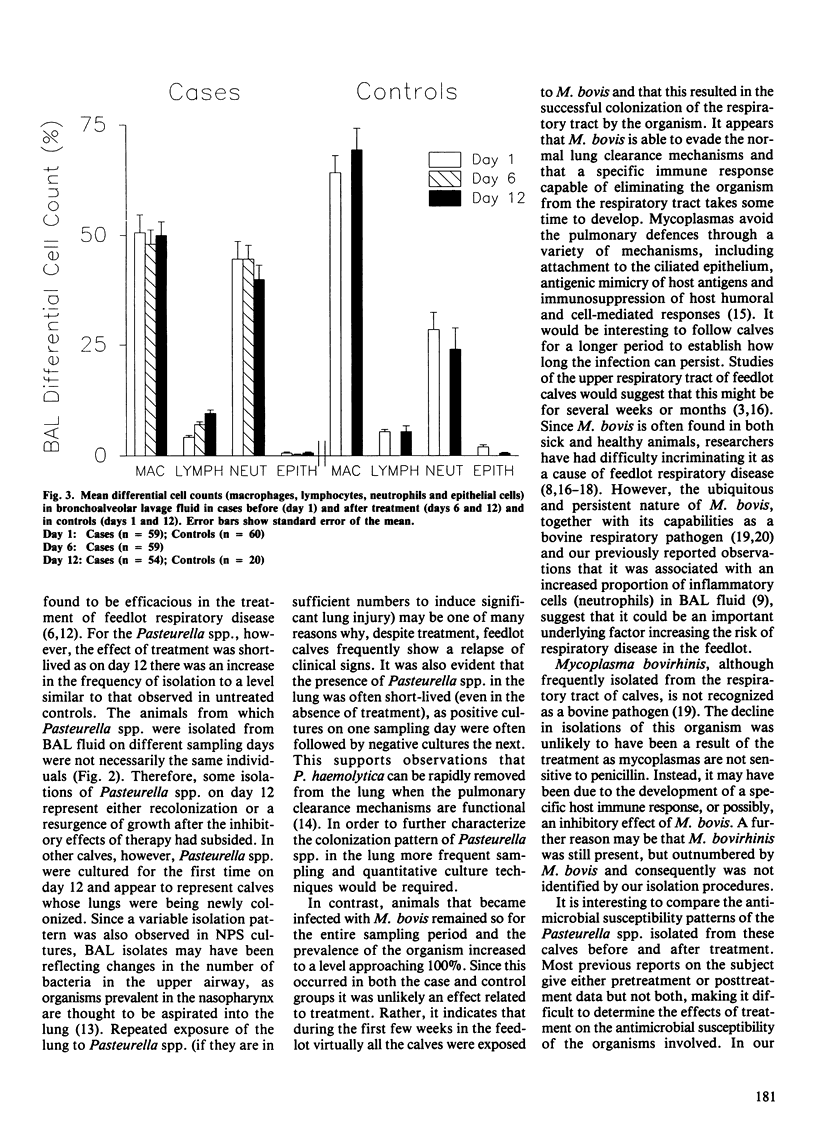
Serial nasopharyngeal swab and bronchoalveolar lavage cultures were used to estimate changes in the bacterial flora of the respiratory tracts of calves during the first month after arrival in the feedlot. Bronchoalveolar lavage (BAL) differential cell counts served to evaluate pulmonary inflammatory changes during this period. Two groups of calves were studied, one consisting of clinically normal controls (n = 60), the other, of cases (n = 59) which received treatment for respiratory disease (penicillin +/- trimethoprimsulfadoxine). A variety of organisms, including Pasteurella multocida, Pasteurella haemolytica, Haemophilus somnus, Mycoplasma bovis and Mycoplasma bovirhinis, were present in the upper and lower airways of both groups during the postarrival period. With the exception of M. bovis, an overall decline in the prevalence of these organisms was observed during the course of the study. In cases, there was a marked decrease in the number of Pasteurella spp. and H. somnus isolates immediately following treatment. For the Pasteurella spp., however, this effect was shortlived as they often appeared to recolonize the respiratory tract within eight days of terminating antimicrobial therapy. Treatment did not appear to affect the frequency of isolating M. bovis. Its prevalence, in both groups of calves, increased to levels approaching 100% during the course of the study. All Pasteurella spp. isolates were tested for susceptibility to several commonly used antimicrobials. Resistance was only evident among P. haemolytica isolated from cases and in every instance this was to a combination of penicillin, ampicillin and tetracycline. Significantly more isolates were resistant after treatment than before. There were BAL differential cell count abnormalities indicative of inflammation in both cases and controls.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. W., Viel L., Bateman K. G., Rosendal S., Shewen P. E. Cytological findings in bronchoalveolar lavage fluid from feedlot calves: associations with pulmonary microbial flora. Can J Vet Res. 1992 Apr;56(2):122–126. [PMC free article] [PubMed] [Google Scholar]
- Allen J. W., Viel L., Bateman K. G., Rosendal S., Shewen P. E., Physick-Sheard P. The microbial flora of the respiratory tract in feedlot calves: associations between nasopharyngeal and bronchoalveolar lavage cultures. Can J Vet Res. 1991 Oct;55(4):341–346. [PMC free article] [PubMed] [Google Scholar]
- Bateman K. G., Martin S. W., Shewen P. E., Menzies P. I. An evaluation of antimicrobial therapy for undifferentiated bovine respiratory disease. Can Vet J. 1990 Oct;31(10):689–696. [PMC free article] [PubMed] [Google Scholar]
- Boothby J. T., Jasper D. E., Zinkl J. G., Thomas C. B., Dellinger J. D. Prevalence of mycoplasmas and immune responses to Mycoplasma bovis in feedlot calves. Am J Vet Res. 1983 May;44(5):831–838. [PubMed] [Google Scholar]
- Boyce J. R., Morter R. L. Plasmid profile analysis of bovine isolates of Pasteurella haemolytica. Am J Vet Res. 1986 Jun;47(6):1204–1206. [PubMed] [Google Scholar]
- Corbeil L. B., Gogolewski R. P. Mechanisms of bacterial injury. Vet Clin North Am Food Anim Pract. 1985 Jul;1(2):367–376. doi: 10.1016/s0749-0720(15)31331-1. [DOI] [PubMed] [Google Scholar]
- Frank G. H., Smith P. C. Prevalence of Pasteurella haemolytica in transported calves. Am J Vet Res. 1983 Jun;44(6):981–985. [PubMed] [Google Scholar]
- Gourlay R. N., Howard C. J., Thomas L. H., Wyld S. G. Pathogenicity of some Mycoplasma and Acholeplasma species in the lungs of gnotobiotic calves. Res Vet Sci. 1979 Sep;27(2):233–237. [PubMed] [Google Scholar]
- Grey C. L., Thomson R. G. Pasteurella haemolytica in the tracheal air of calves. Can J Comp Med. 1971 Apr;35(2):121–128. [PMC free article] [PubMed] [Google Scholar]
- HOERLEIN A. B., SAXENA S. P., MANSFIELD M. E. Studies on shipping fever of cattle. II. Prevalence of Pasteurella species in nasal secretions from normal calves and calves with shipping fever. Am J Vet Res. 1961 May;22:470–472. [PubMed] [Google Scholar]
- Janzen E. D. Clinical management of bovine respiratory disease. Can Vet J. 1991 Apr;32(4):215–218. [PMC free article] [PubMed] [Google Scholar]
- Liggitt H. D. Defense mechanisms in the bovine lung. Vet Clin North Am Food Anim Pract. 1985 Jul;1(2):347–366. doi: 10.1016/s0749-0720(15)31330-x. [DOI] [PubMed] [Google Scholar]
- Lillie L. E., Thomson R. G. The pulmonary clearance of bacteria by calves and mice. Can J Comp Med. 1972 Apr;36(2):129–137. [PMC free article] [PubMed] [Google Scholar]
- Lopez A., Maxie M. G., Ruhnke L., Savan M., Thomson R. G. Cellular inflammatory response in the lungs of calves exposed to bovine viral diarrhea virus, Mycoplasma bovis, and Pasteurella haemolytica. Am J Vet Res. 1986 Jun;47(6):1283–1286. [PubMed] [Google Scholar]
- Martin S. W., Bateman K. G., Shewen P. E., Rosendal S., Bohac J. E. The frequency, distribution and effects of antibodies, to seven putative respiratory pathogens, on respiratory disease and weight gain in feedlot calves in Ontario. Can J Vet Res. 1989 Jul;53(3):355–362. [PMC free article] [PubMed] [Google Scholar]
- Martin S. W., Meek A. H., Curtis R. A. Antimicrobial use in feedlot calves: its association with culture rates and antimicrobial susceptibility. Can J Comp Med. 1983 Jan;47(1):6–10. [PMC free article] [PubMed] [Google Scholar]
- Mechor G. D., Jim G. K., Janzen E. D. Comparison of penicillin, oxytetracycline, and trimethoprim-sulfadoxine in the treatment of acute undifferentiated bovine respiratory disease. Can Vet J. 1988 May;29(5):438–443. [PMC free article] [PubMed] [Google Scholar]
- Pringle J. K., Viel L., Shewen P. E., Willoughby R. A., Martin S. W., Valli V. E. Bronchoalveolar lavage of cranial and caudal lung regions in selected normal calves: cellular, microbiological, immunoglobulin, serological and histological variables. Can J Vet Res. 1988 Apr;52(2):239–248. [PMC free article] [PubMed] [Google Scholar]
- Thomas L. H., Howard C. J., Parsons K. R., Anger H. S. Growth of Mycoplasma bovis in organ cultures of bovine foetal trachea and comparison with Mycoplasma dispar. Vet Microbiol. 1987 Feb;13(2):189–200. doi: 10.1016/0378-1135(87)90044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson R. G., Benson M. L., Savan M. Pneumonic pasteurellosis of cattle: microbiology and immunology. Can J Comp Med. 1969 Jul;33(3):194–206. [PMC free article] [PubMed] [Google Scholar]
- Walker R. D., Hopkins F. M., Schultz T. W., McCracken M. D., Moore R. N. Changes in leukocyte populations in pulmonary lavage fluids of calves after inhalation of Pasteurella haemolytica. Am J Vet Res. 1985 Dec;46(12):2429–2433. [PubMed] [Google Scholar]
- Yates W. D., Kingscote B. F., Bradley J. A., Mitchell D. The relationship of serology and nasal microbiology to pulmonary lesions in feedlot cattle. Can J Comp Med. 1983 Jul;47(3):375–378. [PMC free article] [PubMed] [Google Scholar]


