Abstract
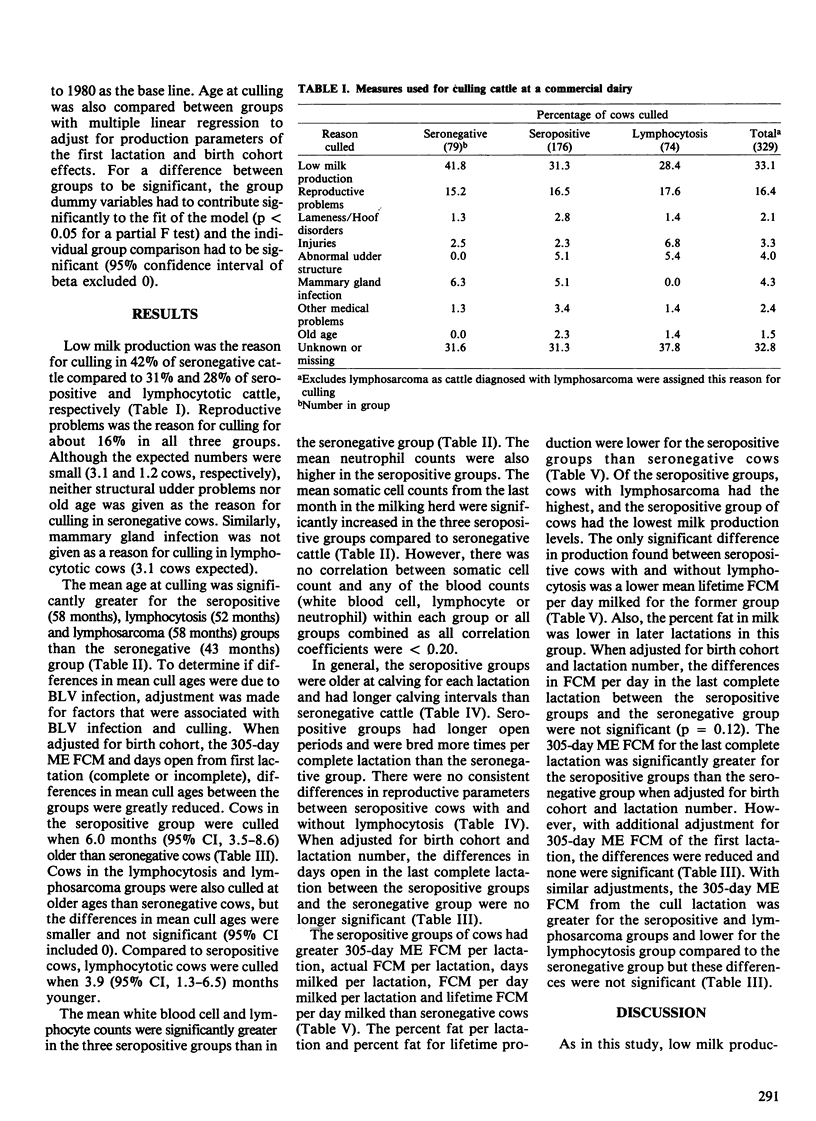
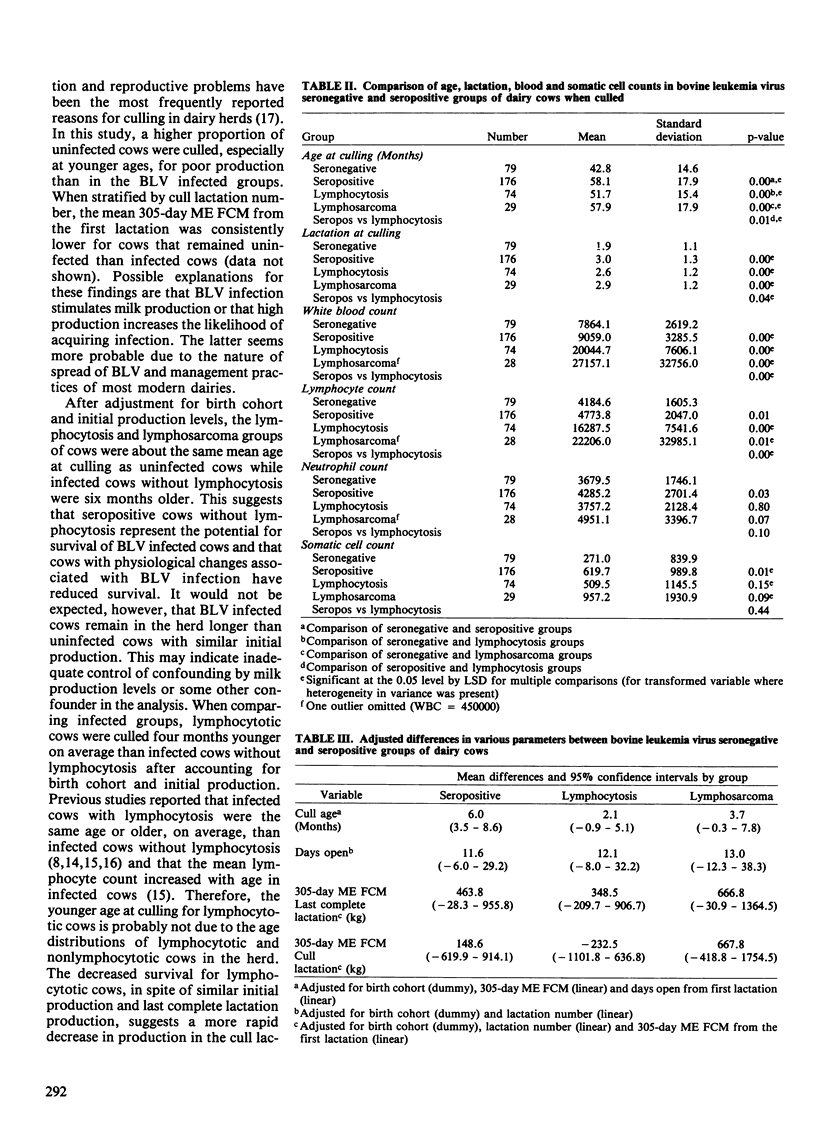
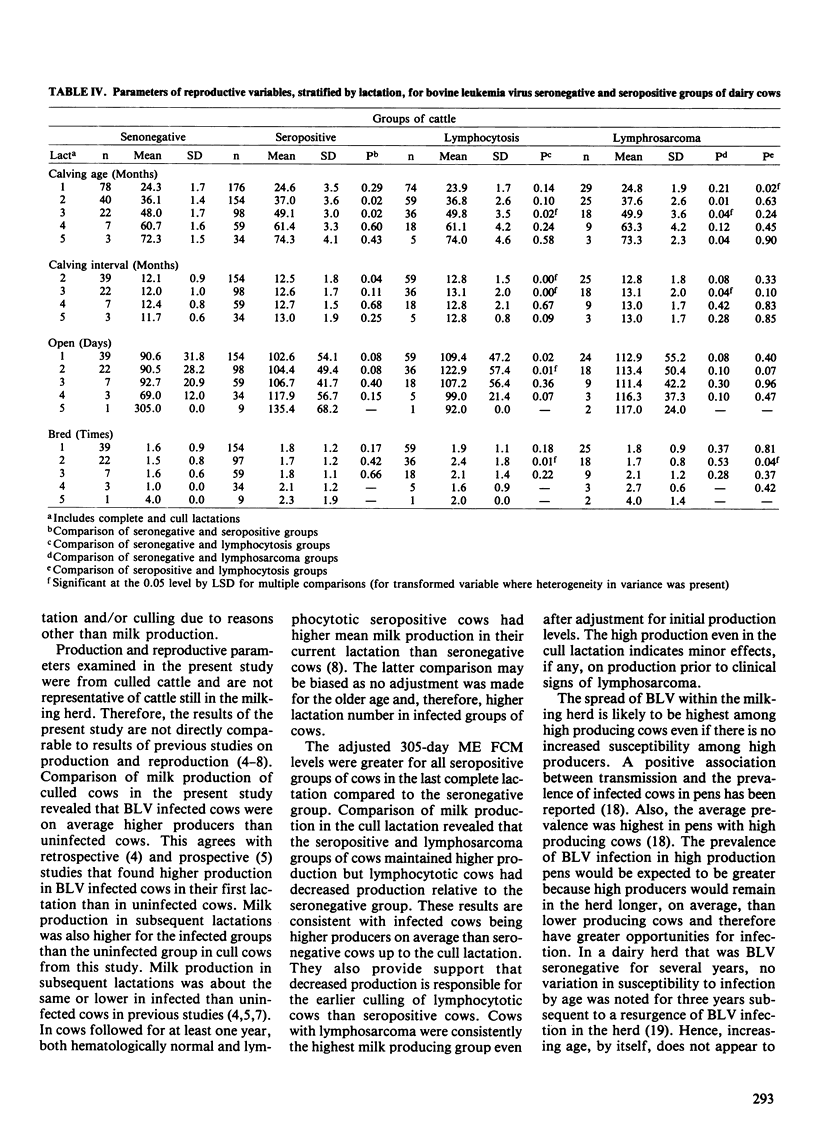
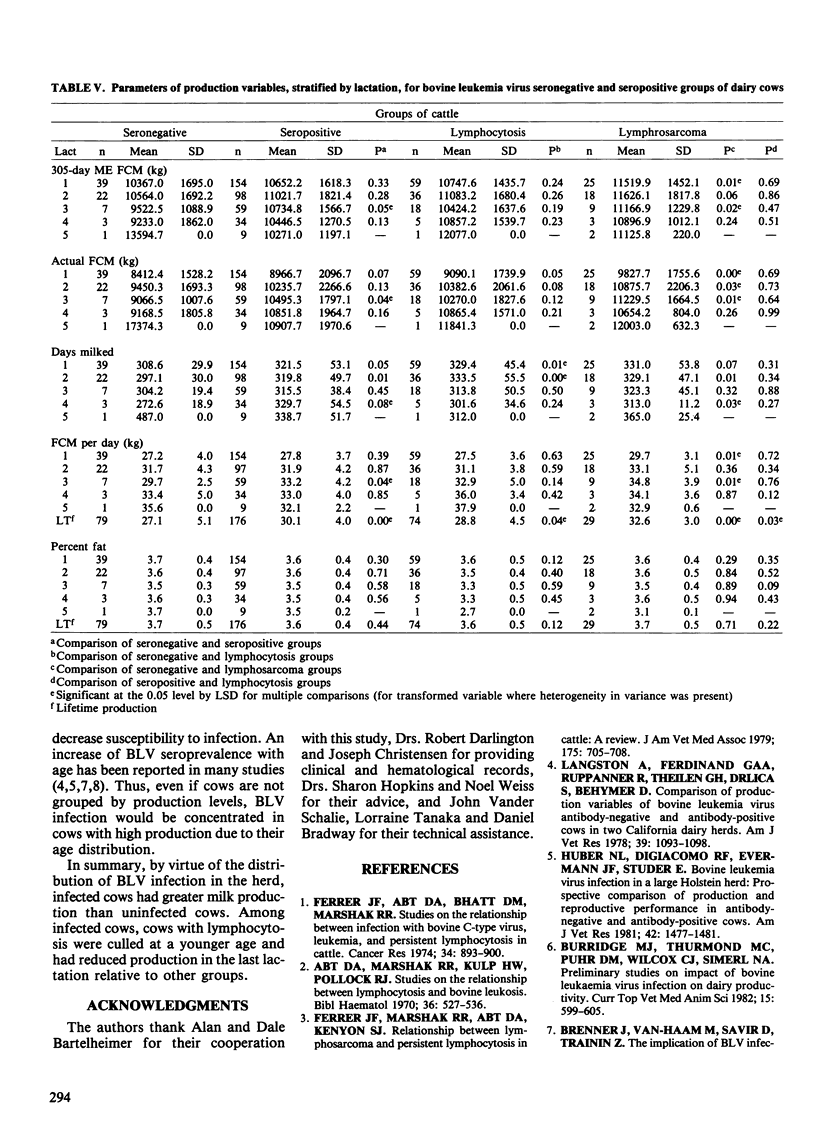
The purpose of this study was to determine the effects of bovine leukemia virus (BLV) infection on production, reproduction and longevity in dairy cattle. The study population was a commercial Holstein dairy herd of approximately 400 milking cows. Cattle were tested for antibodies to BLV at least annually for three years and when culled. Four groups of culled cows were compared: seronegative cows (n = 79), seropositive cows without lymphocytosis (n = 176), seropositive cows with lymphocytosis (> or = 9,000 lymphocytes/microliter) (n = 74), and seropositive cows with lymphosarcoma (n = 29). Seropositive groups of cows were bred more times and had longer calving intervals than seronegative cows. The seropositive groups had greater 305-day ME (mature equivalent) FCM (3.5% fat-corrected milk) per lactation and were older when culled than seronegative cows. However, the percent fat per lactation was greater in seronegative cows. In the last complete lactation, differences in 305-day ME FCM, days open and cull age between groups were reduced and none were significant (p > 0.05). In the cull lactation, only cows with lymphocytosis had reduced milk production relative to seronegative cows, although this difference was not significant. After adjustment for initial production and reproductive values, only seropositive nonlymphocytotic cows were culled at a significantly older age than seronegative cattle. Lymphocytotic cows were culled four months younger on average than nonlymphocytotic seropositive cows. Hence, BLV infected cows had greater milk production on average than uninfected cows. Adverse effects of BLV infection were primarily limited to lymphocytotic cows which were culled earlier and had reduced milk production in the cull lactation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abt D. A., Marshak R. R., Kulp H. W., Pollock R. J., Jr Studies on the relationship between lymphocytosis and bovine leukosis. Bibl Haematol. 1970;(36):527–536. doi: 10.1159/000391747. [DOI] [PubMed] [Google Scholar]
- Brenner J., Van-Haam M., Savir D., Trainin Z. The implication of BLV infection in the productivity, reproductive capacity and survival rate of a dairy cow. Vet Immunol Immunopathol. 1989 Oct;22(3):299–305. doi: 10.1016/0165-2427(89)90017-2. [DOI] [PubMed] [Google Scholar]
- Ferrer J. F., Abt D. A., Bhatt D. M., Marshak R. R. Studies on the relationship between infection with bovine C-type virus, leukemia, and persistent lymphocytosis in cattle. Cancer Res. 1974 Apr;34(4):893–900. [PubMed] [Google Scholar]
- Ferrer J. F., Marshak R. R., Abt D. A., Kenyon S. J. Relationship between lymphosarcoma and persistent lymphocytosis in cattle: a review. J Am Vet Med Assoc. 1979 Oct 1;175(7):705–708. [PubMed] [Google Scholar]
- Huber N. L., DiGiacomo R. F., Evermann J. F., Studer E. Bovine leukemia virus infection in a large Holstein herd: prospective comparison of production and reproductive performance in antibody-negative and antibody-positive cows. Am J Vet Res. 1981 Sep;42(9):1477–1481. [PubMed] [Google Scholar]
- Jacobsen K. L., Kaneene J. B., Miller J. M., Bull R. W. Comparison of the commercial agar-gel immunodiffusion test and radioimmunoprecipitation assay for detection of antibodies to bovine leukemia virus. Am J Vet Res. 1985 Jul;46(7):1430–1433. [PubMed] [Google Scholar]
- Langston A., Ferdinand G. A., Ruppanner R., Theilen G. H., Drlica S., Behymer D. Comparison of production variables of bovine leukemia virus antibody-negative and antibody-positive cows in two California dairy herds. Am J Vet Res. 1978 Jul;39(7):1093–1098. [PubMed] [Google Scholar]
- Lassauzet M. L., Thurmond M. C., Johnson W. O., Stevens F., Picanso J. P. Factors associated with transmission of bovine leukemia virus by contact in cows on a California dairy. Am J Epidemiol. 1991 Jan 15;133(2):164–176. doi: 10.1093/oxfordjournals.aje.a115855. [DOI] [PubMed] [Google Scholar]
- Lewin H. A., Bernoco D. Evidence for BoLA-linked resistance and susceptibility to subclinical progression of bovine leukaemia virus infection. Anim Genet. 1986;17(3):197–207. doi: 10.1111/j.1365-2052.1986.tb03191.x. [DOI] [PubMed] [Google Scholar]
- Lewin H. A., Wu M. C., Nolan T. J., Stewart J. A. Peripheral B lymphocyte percentage as an indicator of subclinical progression of bovine leukemia virus infection. J Dairy Sci. 1988 Sep;71(9):2526–2534. doi: 10.3168/jds.S0022-0302(88)79841-0. [DOI] [PubMed] [Google Scholar]
- Lewin H. A., Wu M. C., Stewart J. A., Nolan T. J. Association between BoLA and subclinical bovine leukemia virus infection in a herd of Holstein-Friesian cows. Immunogenetics. 1988;27(5):338–344. doi: 10.1007/BF00395129. [DOI] [PubMed] [Google Scholar]
- Thurmond M. C., Carter R. L., Picanso J. P., Stralka K. Upper-normal prediction limits of lymphocyte counts for cattle not infected with bovine leukemia virus. Am J Vet Res. 1990 Mar;51(3):466–470. [PubMed] [Google Scholar]
- Wilesmith J. W., Straub O. C., Lorenz R. J. Some observations on the epidemiology of bovine leucosis virus infection in a large dairy herd. Res Vet Sci. 1980 Jan;28(1):10–16. [PubMed] [Google Scholar]
- Wu M. C., Shanks R. D., Lewin H. A. Milk and fat production in dairy cattle influenced by advanced subclinical bovine leukemia virus infection. Proc Natl Acad Sci U S A. 1989 Feb;86(3):993–996. doi: 10.1073/pnas.86.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]


