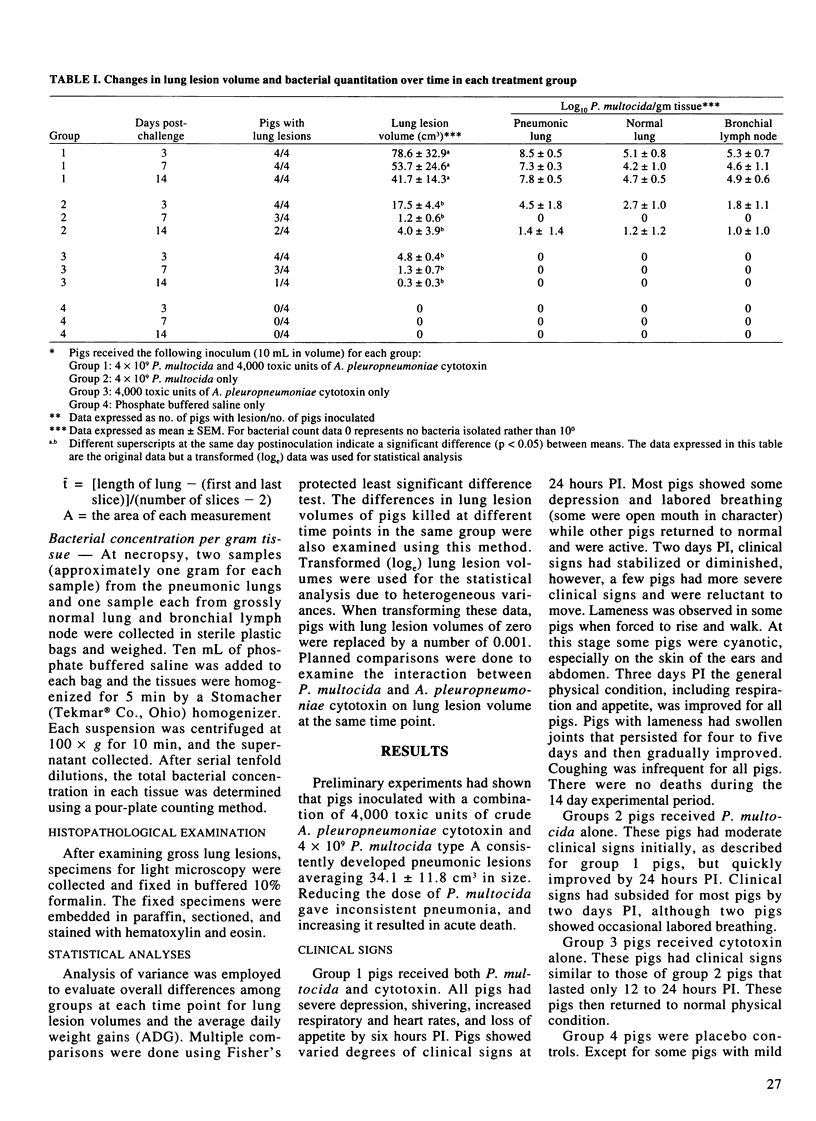
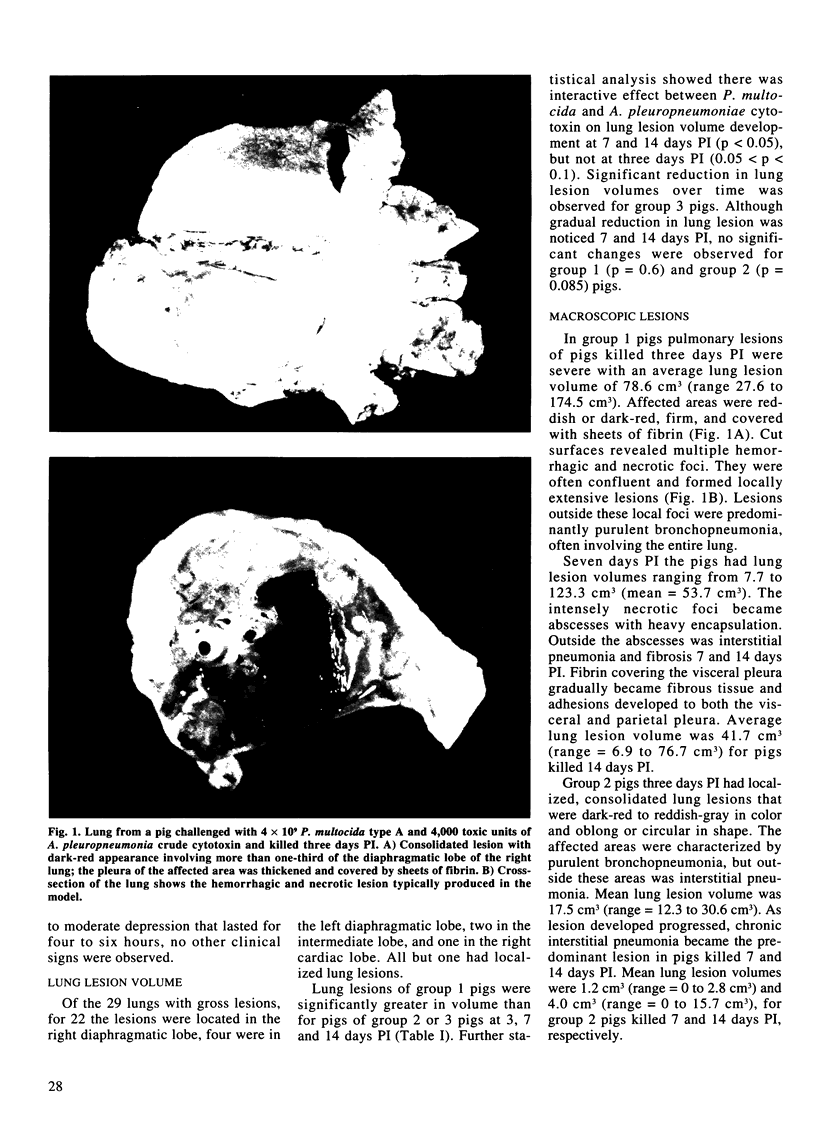
Abstract
This study was designed to develop and characterize a swine pneumonic pasteurellosis model by concurrent introduction of Pasteurella multocida type A and Actinobacillus pleuropneumoniae crude cytotoxin. After a series of preliminary experiments, a combination of 4 x 10(9) P. multocida and 4,000 toxic units of A. pleuropneumoniae crude cytotoxin was determined to produce optimal results. A total of 48 pigs were divided into four groups of 12 pigs each. The control group received buffered saline only. Four pigs from each group were randomly selected for necropsy 3, 7 and 14 days postinoculation (PI). Inoculation of pigs with P. multocida and A. pleuropneumoniae cytotoxin (group 1) resulted in moderate to severe pneumonia. Pasteurella multocida was isolated from pneumonic lesions, grossly normal lung, and bronchial lymph nodes of all group 1 pigs throughout the 14 day experimental period. Pathological changes typical of field cases of swine pneumonic pasteurellosis were produced. Pigs inoculated with P. multocida alone (group 2) had pneumonic lesions and P. multocida was reisolated from lungs at three days PI. Pasteurella multocida was not isolated from these pigs at 7 and 14 days PI, except for one pig in which an abscess developed in the thorax. Pulmonary lesions induced by A. pleuropneumoniae crude cytotoxin alone (group 3) were transient and resolved by seven days PI. Group 1 pigs had significantly greater lung lesion volumes than group 2 and 3 pigs at 3, 7 and 14 days PI. Statistical analysis indicated a significant interactive effect of P. multocida and A. pleuropneumoniae cytotoxin on the development of lung lesion volumes at 7 and 14 days PI (p < 0.05).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendixen P. H., Shewen P. E., Rosendal S., Wilkie B. N. Toxicity of Haemophilus pleuropneumoniae for porcine lung macrophages, peripheral blood monocytes, and testicular cells. Infect Immun. 1981 Sep;33(3):673–676. doi: 10.1128/iai.33.3.673-676.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley O. E., Farrington D. O. Evaluation of an induced Pasteurella multocida swine pneumonia model. Am J Vet Res. 1980 Nov;41(11):1870–1873. [PubMed] [Google Scholar]
- Chung W. B., Bäckström L. R., McDonald J., Collins M. T. The (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium) colorimetric assay for the quantitation of Actinobacillus pleuropneumoniae cytotoxin. Can J Vet Res. 1993 Jul;57(3):159–165. [PMC free article] [PubMed] [Google Scholar]
- Ciprián A., Pijoan C., Cruz T., Camacho J., Tórtora J., Colmenares G., López-Revilla R., de la Garza M. Mycoplasma hyopneumoniae increases the susceptibility of pigs to experimental Pasteurella multocida pneumonia. Can J Vet Res. 1988 Oct;52(4):434–438. [PMC free article] [PubMed] [Google Scholar]
- Fuentes M. C., Pijoan C. Pneumonia in pigs induced by intranasal challenge exposure with pseudorabies virus and Pasteurella multocida. Am J Vet Res. 1987 Oct;48(10):1446–1448. [PubMed] [Google Scholar]
- Gundersen H. J., Bendtsen T. F., Korbo L., Marcussen N., Møller A., Nielsen K., Nyengaard J. R., Pakkenberg B., Sørensen F. B., Vesterby A. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988 May;96(5):379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hall W. F., Bane D. P., Kilroy C. R., Essex-Sorlie D. L. A model for the induction of Pasteurella multocida type-A pneumonia in pigs. Can J Vet Res. 1990 Apr;54(2):238–243. [PMC free article] [PubMed] [Google Scholar]
- Heilmann P., Müller G. Bronchoalveoläre Clearance nach Inhalation von Pasteurella-multocida-Aerosolen bei Läuferschweinen. Zentralbl Veterinarmed B. 1987 Mar;34(2):126–137. [PubMed] [Google Scholar]
- Jericho K. W., Done S. H., Saunders R. W. Pneumonia and efficiency of pig production. Can Vet J. 1975 Feb;16(2):44–49. [PMC free article] [PubMed] [Google Scholar]
- Kielstein P., Martin J., Janetschke P. Experimentelle Pasteurella-multocida-Infektionen beim Schwein als ein Beitrag zur Atiologie der enzootischen Pneumonie des Schweines. Arch Exp Veterinarmed. 1977;31(4):609–619. [PubMed] [Google Scholar]
- Kielstein P. On the occurrence of toxin-producing Pasteurella-multocida-strains in atrophic rhinitis and in pneumonias of swine and cattle. Zentralbl Veterinarmed B. 1986 Aug;33(6):418–424. doi: 10.1111/j.1439-0450.1986.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Little T. W., Harding J. D. The interaction of Haemophilus parahaemolyticus and Pasteurella multocida in the respiratory tract of the pig. Br Vet J. 1980 Jul-Aug;136(4):371–383. doi: 10.1016/s0007-1935(17)32240-6. [DOI] [PubMed] [Google Scholar]
- MURTY D. K., KAUSHIK R. K. STUDIES ON AN OUTBREAK OF ACUTE SWINE PASTEURELLOSIS DUE TO PASTEURELLA MULTOCIDA TYPE B (CARTER, 1955). Vet Rec. 1965 Apr 10;77:411–416. [PubMed] [Google Scholar]
- Morrison R. B., Pijoan C., Hilley H. D., Rapp V. Microorganisms associated with pneumonia in slaughter weight swine. Can J Comp Med. 1985 Apr;49(2):129–137. [PMC free article] [PubMed] [Google Scholar]
- Osborne A. D., Saunders J. R., K-Sebunya T. An abattoir survey of the incidence of pneumonia in Saskatchewan swine and an investigation of the microbiology of affected lungs. Can Vet J. 1981 Apr;22(4):82–85. [PMC free article] [PubMed] [Google Scholar]
- Pakkenberg B. Post-mortem study of chronic schizophrenic brains. Br J Psychiatry. 1987 Dec;151:744–752. doi: 10.1192/bjp.151.6.744. [DOI] [PubMed] [Google Scholar]
- Pijoan C. Effect of Pasteurella multocida and Haemophilus pleuropneumoniae toxins on swine alveolar macrophages. Vet Immunol Immunopathol. 1986 Sep;13(1-2):141–149. doi: 10.1016/0165-2427(86)90055-3. [DOI] [PubMed] [Google Scholar]
- Pijoan C., Lastra A., Ramirez C., Leman A. D. Isolation of toxigenic strains of Pasteurella multocida from lungs of pneumonic swine. J Am Vet Med Assoc. 1984 Sep 1;185(5):522–523. [PubMed] [Google Scholar]
- Pijoan C., Ochoa G. Interaction between a hog cholera vaccine strain and Pasteurella multocida in the production of porcine pneumonia. J Comp Pathol. 1978 Apr;88(2):167–170. doi: 10.1016/0021-9975(78)90020-8. [DOI] [PubMed] [Google Scholar]
- Rosendal S., Devenish J., MacInnes J. I., Lumsden J. H., Watson S., Xun H. Evaluation of heat-sensitive, neutrophil-toxic, and hemolytic activity of Haemophilus (Actinobacillus) pleuropneumoniae. Am J Vet Res. 1988 Jul;49(7):1053–1058. [PubMed] [Google Scholar]
- Schimmel D. Ergebnisse experimenteller Infektionen von SPF-Ferkeln mit Pasteurella multocida--Vorschlag eines reproduzierbaren Infektionsmodells. Arch Exp Veterinarmed. 1987 May;41(3):455–462. [PubMed] [Google Scholar]
- Smith I. M., Hodges R. T., Betts A. O., Hayward A. H. Experimental infections of gnotobiotic piglets with Pasteurella septica (sero-group A) alone or with Mycoplasma hyopneumoniae. J Comp Pathol. 1973 Jul;83(3):307–321. doi: 10.1016/0021-9975(73)90055-8. [DOI] [PubMed] [Google Scholar]
- Straw B. E., Tuovinen V. K., Bigras-Poulin M. Estimation of the cost of pneumonia in swine herds. J Am Vet Med Assoc. 1989 Dec 15;195(12):1702–1706. [PubMed] [Google Scholar]
- Van Leengoed L. A., Kamp E. M., Pol J. M. Toxicity of Haemophilus pleuropneumoniae to porcine lung macrophages. Vet Microbiol. 1989 Apr;19(4):337–349. doi: 10.1016/0378-1135(89)90099-0. [DOI] [PubMed] [Google Scholar]
- Verma N. D. Pasteurella multocida B:2 in haemorrhagic septicaemia outbreak in pigs in India. Vet Rec. 1988 Jul 9;123(2):63–63. doi: 10.1136/vr.123.2.63-a. [DOI] [PubMed] [Google Scholar]