Abstract
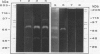
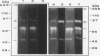
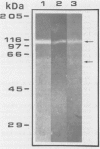
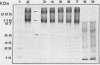
It was found that 48 hour cultures of Actinobacillus pleuropneumoniae secreted proteases into the medium. Electrophoresis in polyacrylamide gels (10%) copolymerized with porcine gelatin (0.1%), of the 70% (NH4)2SO4 precipitate from the culture supernatants, displayed protease activities of different molecular weights: > 200, 200, 90, 80, 70 and 50 kDa. They had activity over a broad range of pHs (4-8), with an optimal pH of 6-7. All were inhibited by 10 mM EDTA, and reactivated by 10 mM calcium. They were stable at -20 degrees C for more than a month. The proteases also degraded porcine IgA and porcine, human, and bovine hemoglobin, although they appeared to be less active against the hemoglobins. The IgA was totally cleaved in 48 h, using supernatants concentrated with polyvinyl pyrrolidone or the 70% (NH4)2SO4. Extracellular proteases could play a role in virulence.
Full text
PDF
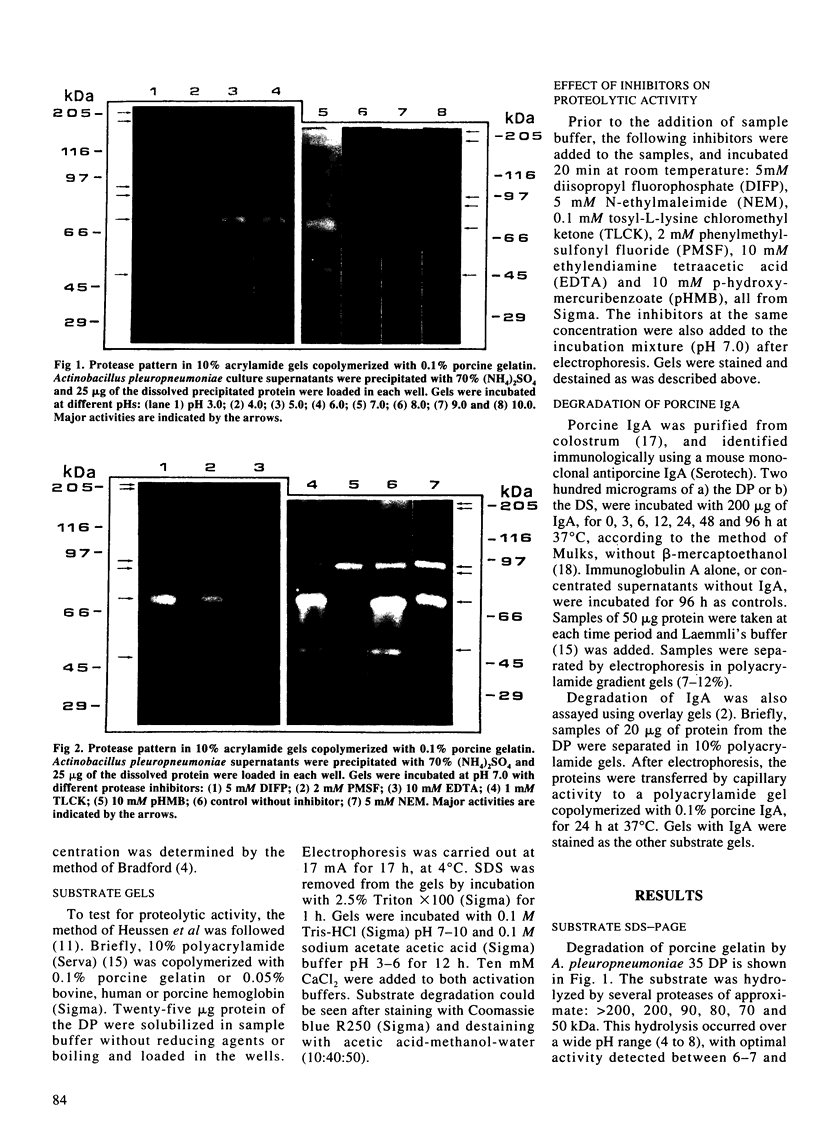
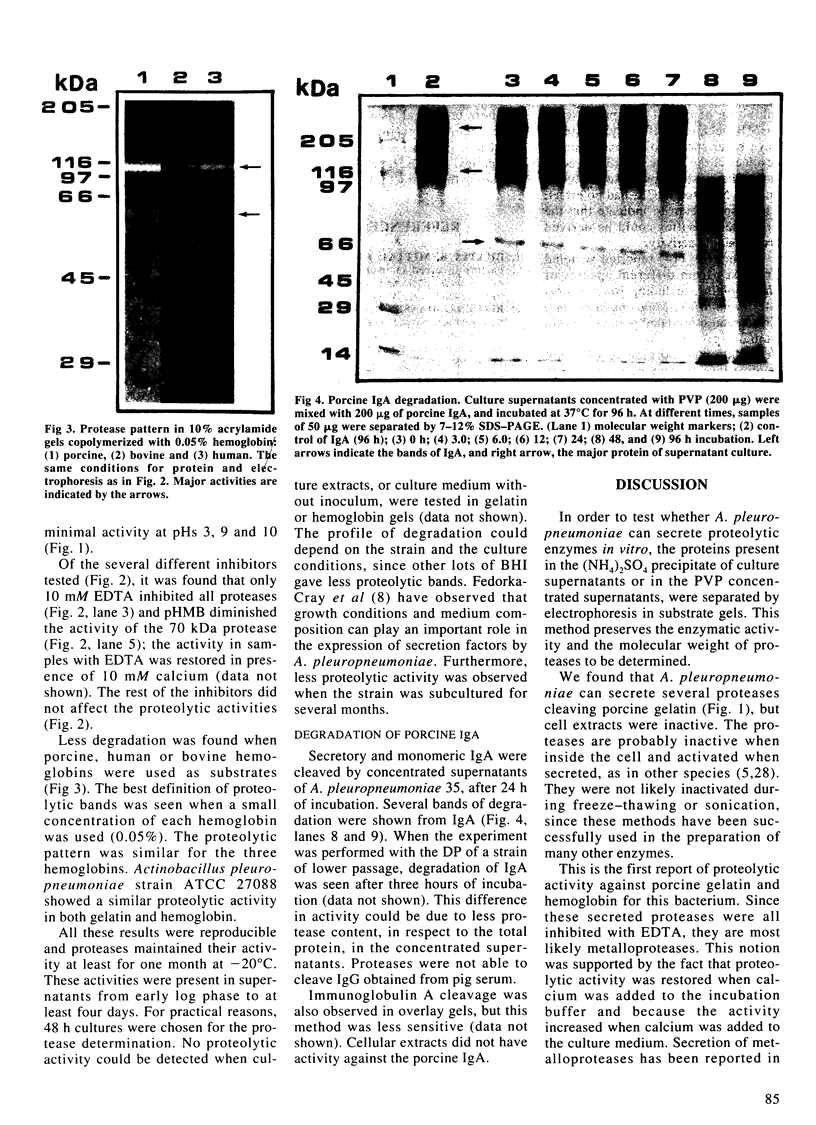

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhatia B., Mittal K. R., Frey J. Factors involved in immunity against Actinobacillus pleuropneumoniae in mice. Vet Microbiol. 1991 Oct;29(2):147–158. doi: 10.1016/0378-1135(91)90122-v. [DOI] [PubMed] [Google Scholar]
- Birkedal-Hansen H., Taylor R. E. Detergent-activation of latent collagenase and resolution of its component molecules. Biochem Biophys Res Commun. 1982 Aug 31;107(4):1173–1178. doi: 10.1016/s0006-291x(82)80120-4. [DOI] [PubMed] [Google Scholar]
- Bond J. S., Butler P. E. Intracellular proteases. Annu Rev Biochem. 1987;56:333–364. doi: 10.1146/annurev.bi.56.070187.002001. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Delepelaire P., Wandersman C. Protease secretion by Erwinia chrysanthemi. Proteases B and C are synthesized and secreted as zymogens without a signal peptide. J Biol Chem. 1989 May 25;264(15):9083–9089. [PubMed] [Google Scholar]
- Deneer H. G., Potter A. A. Effect of iron restriction on the outer membrane proteins of Actinobacillus (Haemophilus) pleuropneumoniae. Infect Immun. 1989 Mar;57(3):798–804. doi: 10.1128/iai.57.3.798-804.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devenish J., Brown J. E., Rosendal S. Association of the RTX proteins of Actinobacillus pleuropneumoniae with hemolytic, CAMP, and neutrophil-cytotoxic activities. Infect Immun. 1992 May;60(5):2139–2142. doi: 10.1128/iai.60.5.2139-2142.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey J., Nicolet J. Hemolysin patterns of Actinobacillus pleuropneumoniae. J Clin Microbiol. 1990 Feb;28(2):232–236. doi: 10.1128/jcm.28.2.232-236.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. R. Pseudomonas aeruginosa elastase and elastolysis revisited: recent developments. Mol Microbiol. 1991 Oct;5(10):2315–2321. doi: 10.1111/j.1365-2958.1991.tb02076.x. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Inzana T. J. Virulence properties of Actinobacillus pleuropneumoniae. Microb Pathog. 1991 Nov;11(5):305–316. doi: 10.1016/0882-4010(91)90016-4. [DOI] [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Schrohenloher R. E. Pathogenic species of the genus Haemophilus and Streptococcus pneumoniae produce immunoglobulin A1 protease. Infect Immun. 1979 Oct;26(1):143–149. doi: 10.1128/iai.26.1.143-149.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lonon M. K., Woods D. E., Straus D. C. The effects of purified 25-kDa lipase from a clinical isolate of Pseudomonas cepacia in the lungs of rats. Curr Microbiol. 1992 Aug;25(2):89–93. doi: 10.1007/BF01570965. [DOI] [PubMed] [Google Scholar]
- Mestecky J., Kulhavy R., Kraus F. W. Studies on human secretory immunoglobulin A. II. Subunit structure. J Immunol. 1972 Mar;108(3):738–747. [PubMed] [Google Scholar]
- Mulks M. H., Kornfeld S. J., Frangione B., Plaut A. G. Relationship between the specificity of IgA proteases and serotypes in Haemophilus influenzae. J Infect Dis. 1982 Aug;146(2):266–274. doi: 10.1093/infdis/146.2.266. [DOI] [PubMed] [Google Scholar]
- Mulks M. H., Moxon E. R., Bricker J., Wright A., Plaut A. G. Examination of Haemophilus pleuropneumoniae for immunoglobulin A protease activity. Infect Immun. 1984 Jul;45(1):276–277. doi: 10.1128/iai.45.1.276-277.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishina Y., Miyoshi S., Nagase A., Shinoda S. Significant role of an exocellular protease in utilization of heme by Vibrio vulnificus. Infect Immun. 1992 May;60(5):2128–2132. doi: 10.1128/iai.60.5.2128-2132.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes E. P., Feeney D. A., Pijoan C. Comparison of the effect of pneumonia detected during lifetime with pneumonia detected at slaughter on growth in swine. J Am Vet Med Assoc. 1990 Oct 15;197(8):1025–1029. [PubMed] [Google Scholar]
- Otulakowski G. L., Shewen P. E., Udoh A. E., Mellors A., Wilkie B. N. Proteolysis of sialoglycoprotein by Pasteurella haemolytica cytotoxic culture supernatant. Infect Immun. 1983 Oct;42(1):64–70. doi: 10.1128/iai.42.1.64-70.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaut A. G. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- Rechnitzer C., Williams A., Wright J. B., Dowsett A. B., Milman N., Fitzgeorge R. B. Demonstration of the intracellular production of tissue-destructive protease by Legionella pneumophila multiplying within guinea-pig and human alveolar macrophages. J Gen Microbiol. 1992 Aug;138(Pt 8):1671–1677. doi: 10.1099/00221287-138-8-1671. [DOI] [PubMed] [Google Scholar]
- Udeze F. A., Kadis S. Effects of Actinobacillus pleuropneumoniae hemolysin on porcine neutrophil function. Infect Immun. 1992 Apr;60(4):1558–1567. doi: 10.1128/iai.60.4.1558-1567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utrera V., Pijoan C. Fimbriae in A pleuropneumoniae strains isolated from pig respiratory tracts. Vet Rec. 1991 Apr 13;128(15):357–358. doi: 10.1136/vr.128.15.357. [DOI] [PubMed] [Google Scholar]
- Wandersman C. Secretion, processing and activation of bacterial extracellular proteases. Mol Microbiol. 1989 Dec;3(12):1825–1831. doi: 10.1111/j.1365-2958.1989.tb00169.x. [DOI] [PubMed] [Google Scholar]
- Wolf U., Bauer D., Traub W. H. Metalloproteases of Serratia liquefaciens: degradation of purified human serum proteins. Zentralbl Bakteriol. 1991 Dec;276(1):16–26. doi: 10.1016/s0934-8840(11)80214-8. [DOI] [PubMed] [Google Scholar]