Abstract
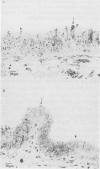
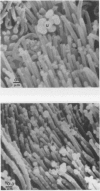
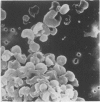
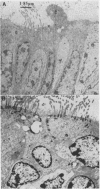
Calmodulin (CAM) acts as an intracellular regulator of calcium, an important mediator of many cell processes. We used the CAM assay and electron microscopy to investigate the effects of Ureaplasma diversum on bovine oviductal explants obtained aseptically from slaughtered cows. A stock suspension of U. diversum (treated specimens) and sterile broth (controls) was added to replicates of cultured explants and incubated at 38 degrees C in an atmosphere of 5.5% CO2 for 48 hours. Explants were examined for ciliary activity, extracellular CAM loss, and for histological and ultrastructural changes. Explants and their culture media were examined for changes in CAM concentration. All experiments were replicated three times. In addition, U. diversum, medium and broth were assayed for CAM content. The concentrations of CAM in explants and media changed significantly (p < 0.05) in samples which were inoculated with U. diversum when compared to controls. The controls and infected specimens did not differ histologically or ultrastructurally, but U. diversum was seen to be closely associated with infected explant tissue. In view of this close affinity it is assumed the loss of CAM from the oviductal cells was causally related, but this was not proven. The failure to show cell membrane injury on light and electron microscopic examination was probably related to the short duration of the experiment and may only point out the sensitivity of the CAM assay in detecting early cell membrane injury. Compromise in characteristics of the medium to support both, the viability of oviductal cells and U. diversum limited the experimental time to 48 hours.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




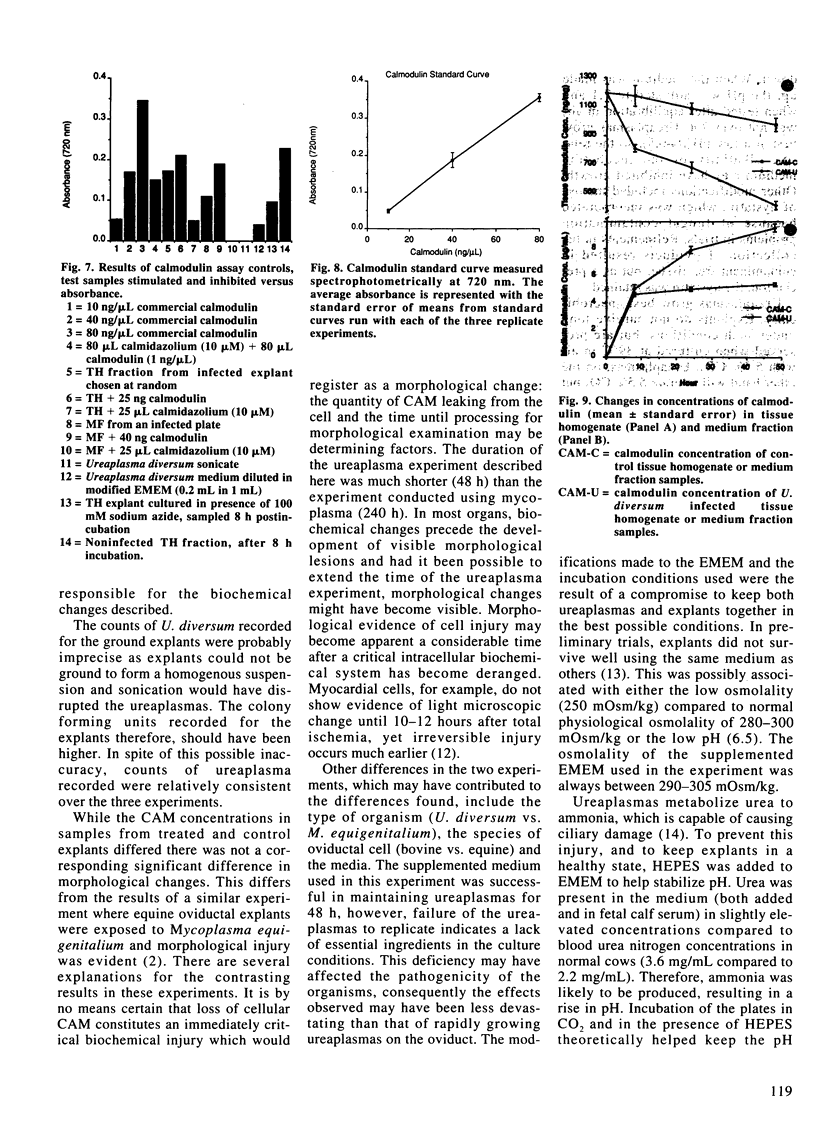


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bermúdez V. M., Miller R. B., Rosendal S., Fernando M. A., Johnson W. H., O'Brien P. J. Measurement of the cytotoxic effects of different strains of Mycoplasma equigenitalium on the equine uterine tube using a calmodulin assay. Can J Vet Res. 1992 Oct;56(4):331–338. [PMC free article] [PubMed] [Google Scholar]
- Doig P. A., Ruhnke H. L., Palmer N. C. Experimental bovine genital ureaplasmosis. II. Granular vulvitis, endometritis and salpingitis following uterine inoculation. Can J Comp Med. 1980 Jul;44(3):259–266. [PMC free article] [PubMed] [Google Scholar]
- Herdson P. B., Kaltenbach J. P., Jennings R. B. Fine structural and biochemical changes in dog myocardium during autolysis. Am J Pathol. 1969 Dec;57(3):539–557. [PMC free article] [PubMed] [Google Scholar]
- Leese H. J. The formation and function of oviduct fluid. J Reprod Fertil. 1988 Mar;82(2):843–856. doi: 10.1530/jrf.0.0820843. [DOI] [PubMed] [Google Scholar]
- O'Brien P. J., Forsyth G. W., Olexson D. W., Thatte H. S., Addis P. B. Canine malignant hyperthermia susceptibility: erythrocytic defects--osmotic fragility, glucose-6-phosphate dehydrogenase deficiency and abnormal Ca2+ homeostasis. Can J Comp Med. 1984 Oct;48(4):381–389. [PMC free article] [PubMed] [Google Scholar]
- Sharma R. K., Wang J. H. Preparation and assay of the Ca2+--dependent modulator protein. Adv Cyclic Nucleotide Res. 1979;10:187–198. [PubMed] [Google Scholar]
- Shepard M. C., Lunceford C. D. Differential agar medium (A7) for identification of Ureaplasma urealyticum (human T mycoplasmas) in primary cultures of clinical material. J Clin Microbiol. 1976 Jun;3(6):613–625. doi: 10.1128/jcm.3.6.613-625.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalheim O. H., Gallagher J. E., Deyoe B. L. Scanning electron microscopy of the bovine, equine, porcine, and caprine uterine tube (oviduct). Am J Vet Res. 1975 Aug;36(08):1069–1075. [PubMed] [Google Scholar]
- Stalheim O. H., Gallagher J. E. Ureaplasmal epithelial lesions related to ammonia. Infect Immun. 1977 Mar;15(3):995–996. doi: 10.1128/iai.15.3.995-996.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalheim O. H., Proctor S. J., Gallagher J. E. Growth and effects of ureaplasmas (T mycoplasmas) in bovine oviductal organ cultures. Infect Immun. 1976 Mar;13(3):915–925. doi: 10.1128/iai.13.3.915-925.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdugo P., Raess B. V., Villalon M. The role of calmodulin in the regulation of ciliary movement in mammalian epithelial cilia. J Submicrosc Cytol. 1983 Jan;15(1):95–96. [PubMed] [Google Scholar]
- Waelchli-Suter R. O., Doig P. A., Ruhnke H. L., Palmer N. C., Barker C. A. Experimental genital ureaplasmosis in the bull. Schweiz Arch Tierheilkd. 1982 Jun;124(6):273–295. [PubMed] [Google Scholar]