Abstract
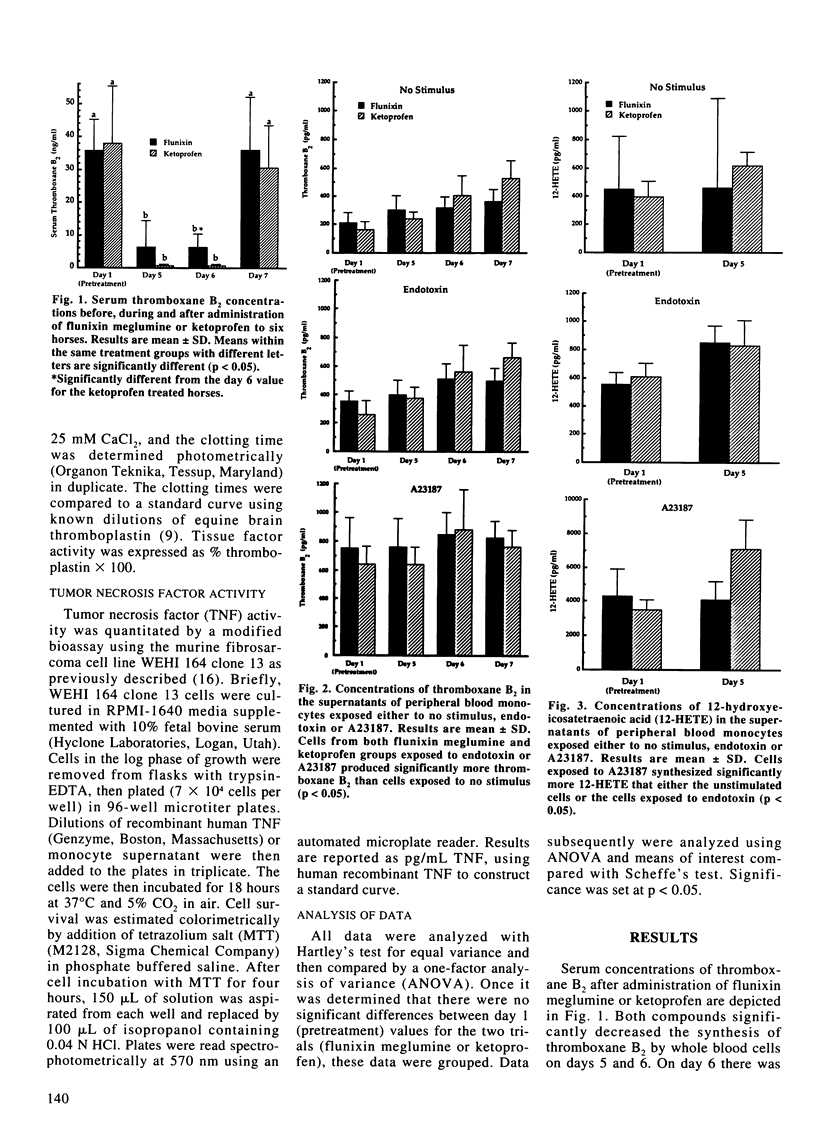
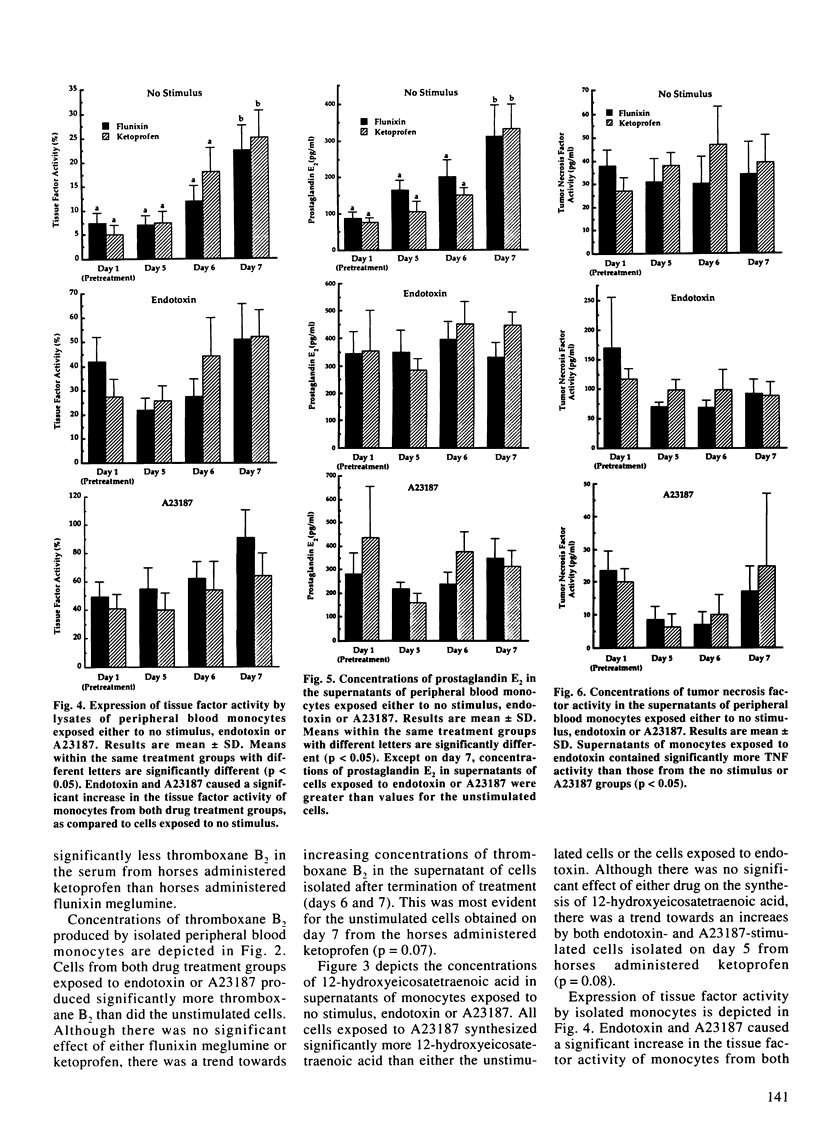
The purpose of this study was to investigate the in vitro effects of flunixin meglumine, a cyclo-oxygenase inhibitor, and ketoprofen, a reported cyclo-oxygenase and lipoxygenase inhibitor, on the synthesis of cyclo-oxygenase end-products thromboxane B2 and prostaglandin E2, lipoxygenase derived 12-hydroxyeicosatetraenoic acid, tumor necrosis factor and tissue factor. Six adult horses were each randomly administered flunixin meglumine (1.1 mg/kg) or ketoprofen (2.2 mg/kg) intravenously every 12 hours with the drug treatments separated by two weeks. Blood samples were obtained prior to initiating treatment, the last day of treatment and for two consecutive days after the termination of treatment for measurement of serum concentrations of thromboxane B2 as well as isolation of peripheral blood monocytes. Quantitation of unstimulated, endotoxin- and calcium ionophore-induced synthesis of thromboxane B2, prostaglandin E2, 12-hydroxyeicosatetraenoic acid, tumor necrosis factor and tissue factor by peripheral blood monocytes was performed in vitro. Both flunixin meglumine and ketoprofen significantly decreased serum concentrations of thromboxane B2 demonstrating in vivo cyclo-oxygenase inhibition. There were no significant differences between drug treatment groups in the in vitro production of thromboxane B2, prostaglandin E2, 12-hydroxy-eicosatetraenoic acid, tumor necrosis factor or tissue factor. This study does not identify significant differences between the effects of flunixin meglumine and ketoprofen.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottoms G. D., Fessler J. F., Roesel O. F., Moore A. B., Frauenfelder H. C. Endotoxin-induced hemodynamic changes in ponies: effects of flunixin meglumine. Am J Vet Res. 1981 Sep;42(9):1514–1518. [PubMed] [Google Scholar]
- Dawson W., Boot J. R., Harvey J., Walker J. R. The pharmacology of benoxaprofen with particular to effects on lipoxygenase product formation. Eur J Rheumatol Inflamm. 1982;5(2):61–68. [PubMed] [Google Scholar]
- Edwards R. L., Rickles F. R. Macrophage procoagulants. Prog Hemost Thromb. 1984;7:183–209. [PubMed] [Google Scholar]
- Henry M. M., Moore J. N. Clinical relevance of monocyte procoagulant activity in horses with colic. J Am Vet Med Assoc. 1991 Mar 1;198(5):843–848. [PubMed] [Google Scholar]
- Henry M. M., Moore J. N. Endotoxin-induced procoagulant activity in equine peripheral blood monocytes. Circ Shock. 1988 Nov;26(3):297–309. [PubMed] [Google Scholar]
- Hetland O., Brovold A. B., Holme R., Gaudernack G., Prydz H. Thromboplastin (tissue factor) in plasma membranes of human monocytes. Biochem J. 1985 Jun 15;228(3):735–743. doi: 10.1042/bj2280735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel S. L., Spengler M., May M. A., Spengler R., Larrick J., Remick D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J Biol Chem. 1988 Apr 15;263(11):5380–5384. [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F., Soberman R. J. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990 Sep 6;323(10):645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F. The biologically active leukotrienes. Biosynthesis, metabolism, receptors, functions, and pharmacology. J Clin Invest. 1984 Apr;73(4):889–897. doi: 10.1172/JCI111312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li E. J., Cook J. A., Wise W. C., Jackson W. T., Halushka P. V. Effect of LTB4 receptor antagonists in endotoxic shock in the rat. Circ Shock. 1991 Aug;34(4):385–392. [PubMed] [Google Scholar]
- Lorenzet R., Niemetz J., Marcus A. J., Broekman M. J. Enhancement of mononuclear procoagulant activity by platelet 12-hydroxyeicosatetraenoic acid. J Clin Invest. 1986 Aug;78(2):418–423. doi: 10.1172/JCI112592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAllister C. G., Morgan S. J., Borne A. T., Pollet R. A. Comparison of adverse effects of phenylbutazone, flunixin meglumine, and ketoprofen in horses. J Am Vet Med Assoc. 1993 Jan 1;202(1):71–77. [PubMed] [Google Scholar]
- Marcinkiewicz J. In vitro cytokine release by activated murine peritoneal macrophages: role of prostaglandins in the differential regulation of tumor necrosis factor alpha, interleukin 1, and interleukin 6. Cytokine. 1991 Jul;3(4):327–332. doi: 10.1016/1043-4666(91)90501-4. [DOI] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Moore J. N., Garner H. E., Shapland J. E., Hatfield D. G. Prevention of endotoxin-induced arterial hypoxaemia and lactic acidosis with flunixin meglumine in the conscious pony. Equine Vet J. 1981 Apr;13(2):95–98. doi: 10.1111/j.2042-3306.1981.tb04122.x. [DOI] [PubMed] [Google Scholar]
- Moore J. N., Hardee M. M., Hardee G. E. Modulation of arachidonic acid metabolism in endotoxic horses: comparison of flunixin meglumine, phenylbutazone, and a selective thromboxane synthetase inhibitor. Am J Vet Res. 1986 Jan;47(1):110–113. [PubMed] [Google Scholar]
- Morris D. D., Moore J. N., Fischer K., Tarleton R. L. Endotoxin-induced tumor necrosis factor activity production by equine peritoneal macrophages. Circ Shock. 1990 Mar;30(3):229–236. [PubMed] [Google Scholar]
- Semrad S. D., Hardee G. E., Hardee M. M., Moore J. N. Flunixin meglumine given in small doses: pharmacokinetics and prostaglandin inhibition in healthy horses. Am J Vet Res. 1985 Dec;46(12):2474–2479. [PubMed] [Google Scholar]
- Semrad S. D., Hardee G. E., Hardee M. M., Moore J. N. Low dose flunixin meglumine: effects on eicosanoid production and clinical signs induced by experimental endotoxaemia in horses. Equine Vet J. 1987 May;19(3):201–206. doi: 10.1111/j.2042-3306.1987.tb01380.x. [DOI] [PubMed] [Google Scholar]
- Sitrin R. G., Kaltreider H. B., Goldyne M. E. Prostaglandin E is required for the augmentation of procoagulant activity of LPS-stimulated rabbit alveolar macrophages. J Immunol. 1984 Feb;132(2):867–871. [PubMed] [Google Scholar]
- Walker J. L. Interrelationships of SRS-A production and arachidonic acid metabolism in human lung tissue. Adv Prostaglandin Thromboxane Res. 1980;6:115–119. [PubMed] [Google Scholar]


