Abstract
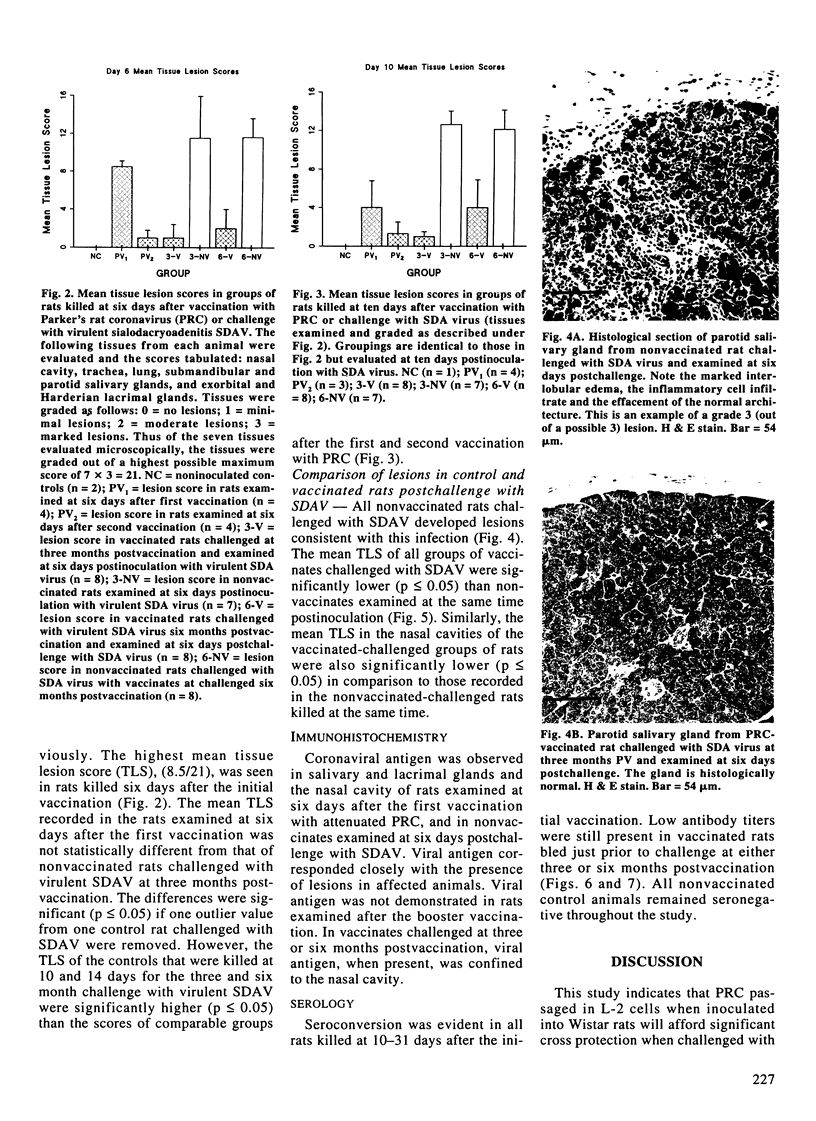
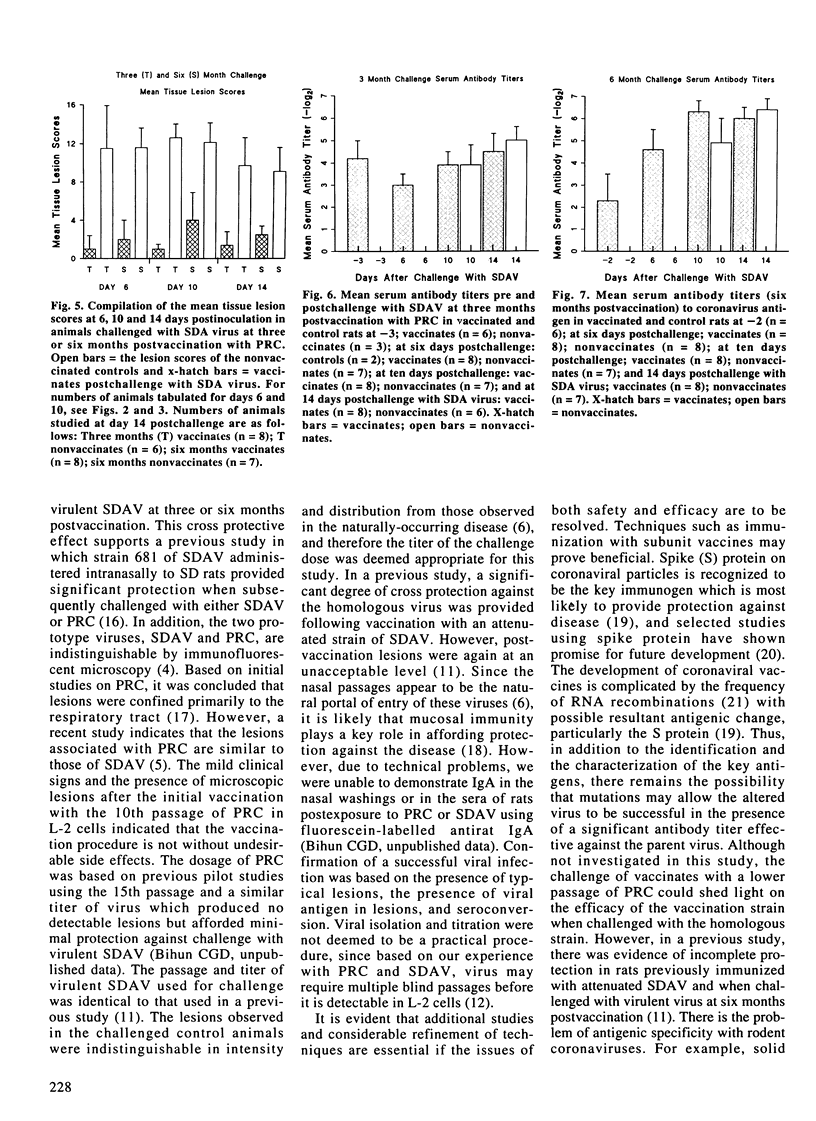
One hundred and twenty-one specific pathogen-free male Wistar rats eight to ten weeks of age were used to evaluate the efficacy of Parker's rat coronavirus (PRC) in affording cross protection on subsequent challenge with virulent sialodacryoadenitis (SDA) virus. Sixty-two animals were inoculated intranasally on day 0 and 21 days later with approximately 10(2) median tissue culture infective doses (TCID50) of the tenth passage of PRC replicated in L-2 cells. Animals were selected at random postvaccination to evaluate the safety and efficacy of PRC by histopathology, immunohistochemistry and serology. At three and six months postvaccination (PV), vaccinated and seronegative control rats were inoculated intranasally with approximately 10(3) TCID50 doses of virulent SDA virus. Challenged rats were then killed at 6, 10 and 14 days postchallenge and necropsied. Evaluations were based on lesion indices in lacrimal and salivary glands and respiratory tract, the presence of viral antigen by immunohistochemistry, and antibody response. Lesions were observed in rats killed PV, but in general, they were significantly reduced compared with those present in seronegative animals post-exposure to virulent SDA virus (p < or = 0.05). However, they were still considered to be an unacceptable level for a routine vaccination procedure. Potvaccination antibody titers to rat coronavirus were evident in all animals tested at three or six months prior to challenge with SDA virus.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barthold S. W., Smith A. L. Virus strain specificity of challenge immunity to coronavirus. Arch Virol. 1989;104(3-4):187–196. doi: 10.1007/BF01315542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt P. N., Jacoby R. O. Experimental infection of adult axenic rats with Parker's rat coronavirus. Arch Virol. 1977;54(4):345–352. doi: 10.1007/BF01314779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienenstock J., Befus A. D. Mucosal immunology. Immunology. 1980 Oct;41(2):249–270. [PMC free article] [PubMed] [Google Scholar]
- Casebolt D. B., Lindsey J. R., Cassell G. H. Prevalence rates of infectious agents among commercial breeding populations of rats and mice. Lab Anim Sci. 1988 Jun;38(3):327–329. [PubMed] [Google Scholar]
- Compton S. R., Barthold S. W., Smith A. L. The cellular and molecular pathogenesis of coronaviruses. Lab Anim Sci. 1993 Feb;43(1):15–28. [PubMed] [Google Scholar]
- Daniel C., Talbot P. J. Protection from lethal coronavirus infection by affinity-purified spike glycoprotein of murine hepatitis virus, strain A59. Virology. 1990 Jan;174(1):87–94. doi: 10.1016/0042-6822(90)90057-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby R. O., Bhatt P. N., Jonas A. M. Pathogenesis of sialodacryoadenitis in gnotobiotic rats. Vet Pathol. 1975;12(3):196–209. doi: 10.1177/030098587501200305. [DOI] [PubMed] [Google Scholar]
- Kraft V., Meyer B. Seromonitoring in small laboratory animal colonies. A five year survey: 1984-1988. Z Versuchstierkd. 1990;33(1):29–35. [PubMed] [Google Scholar]
- Lussier G., Descôteaux J. P. Prevalence of natural virus infections in laboratory mice and rats used in Canada. Lab Anim Sci. 1986 Apr;36(2):145–148. [PubMed] [Google Scholar]
- Makino S., Keck J. G., Stohlman S. A., Lai M. M. High-frequency RNA recombination of murine coronaviruses. J Virol. 1986 Mar;57(3):729–737. doi: 10.1128/jvi.57.3.729-737.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy D. H., Hayes M. A., Kocal T. E., Wojcinski Z. W. Depletion of salivary gland epidermal growth factor by sialodacryoadenitis virus infection in the Wistar rat. Vet Pathol. 1988 May;25(3):183–192. doi: 10.1177/030098588802500301. [DOI] [PubMed] [Google Scholar]
- Percy D. H., Scott R. A. Coronavirus infection in the laboratory rat: immunization trials using attenuated virus replicated in L-2 cells. Can J Vet Res. 1991 Jan;55(1):60–66. [PMC free article] [PubMed] [Google Scholar]
- Percy D. H., Williams K. L., Bond S. J., MacInnes J. I. Characteristics of Parker's rat coronavirus (PRC) replicated in L-2 cells. Arch Virol. 1990;112(3-4):195–202. doi: 10.1007/BF01323164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy D. H., Williams K. L., Paturzo F. X. A comparison of the sensitivity and specificity of sialodacryoadenitis virus, Parker's rat coronavirus, and mouse hepatitis virus-infected cells as a source of antigen for the detection of antibody to rat coronaviruses. Arch Virol. 1991;119(3-4):175–180. doi: 10.1007/BF01310668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeb T. R., Lindsey J. R. Exacerbation of murine respiratory mycoplasmosis by sialodacryoadenitis virus infection in gnotobiotic F344 rats. Vet Pathol. 1987 Sep;24(5):392–399. doi: 10.1177/030098588702400505. [DOI] [PubMed] [Google Scholar]
- Smith A. L. An immunofluorescence test for detection of serum antibody to rodent coronaviruses. Lab Anim Sci. 1983 Apr;33(2):157–160. [PubMed] [Google Scholar]
- Stoscheck C. M., King L. E., Jr Role of epidermal growth factor in carcinogenesis. Cancer Res. 1986 Mar;46(3):1030–1037. [PubMed] [Google Scholar]
- Weir E. C., Jacoby R. O., Paturzo F. X., Johnson E. A. Infection of SDAV-immune rats with SDAV and rat coronavirus. Lab Anim Sci. 1990 Jul;40(4):363–366. [PubMed] [Google Scholar]
- Wojcinski Z. W., Percy D. H. Sialodacryoadenitis virus-associated lesions in the lower respiratory tract of rats. Vet Pathol. 1986 May;23(3):278–286. doi: 10.1177/030098588602300308. [DOI] [PubMed] [Google Scholar]
- Young J. T. Histopathologic examination of the rat nasal cavity. Fundam Appl Toxicol. 1981 Jul-Aug;1(4):309–312. doi: 10.1016/s0272-0590(81)80037-1. [DOI] [PubMed] [Google Scholar]