Abstract
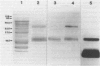
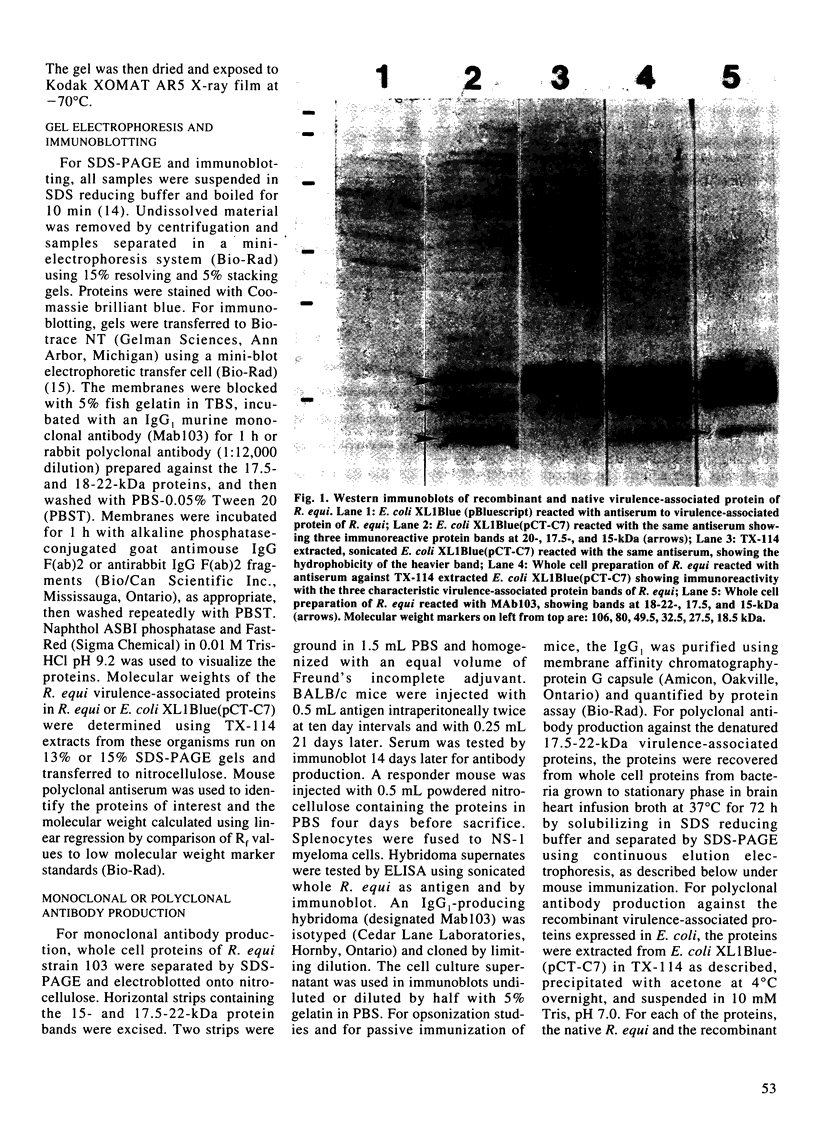
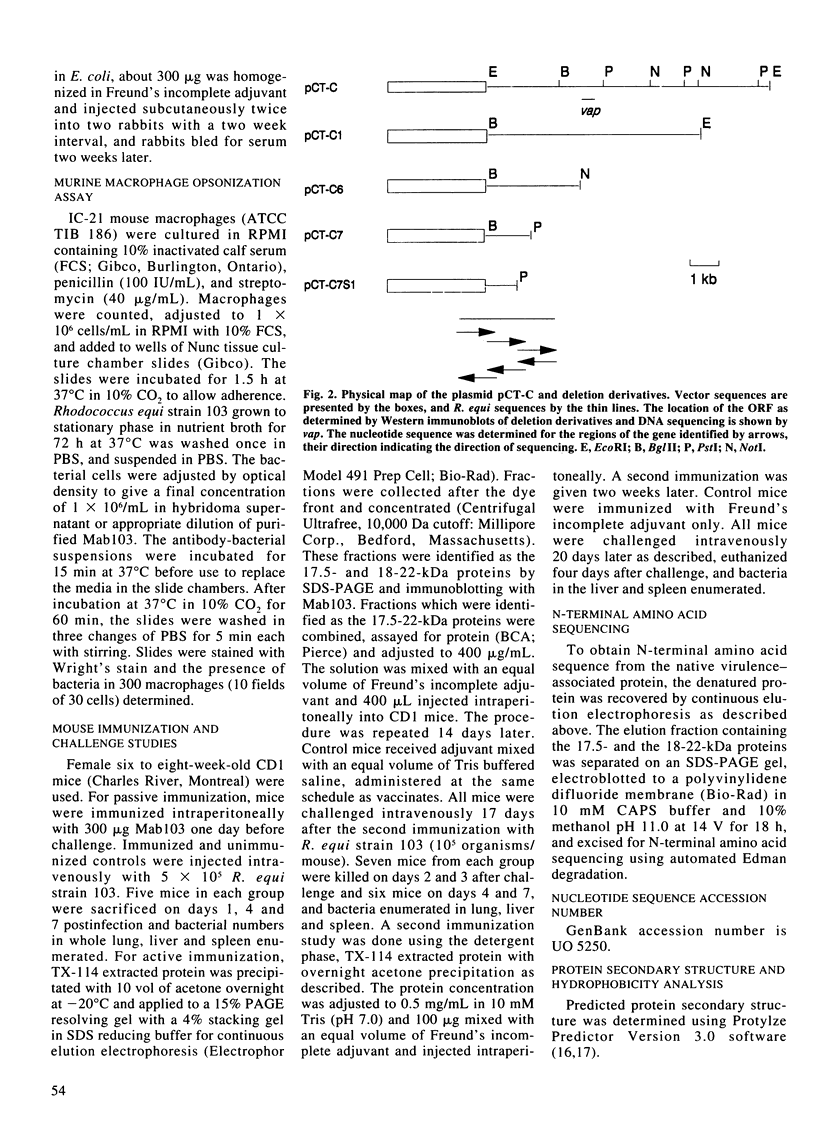
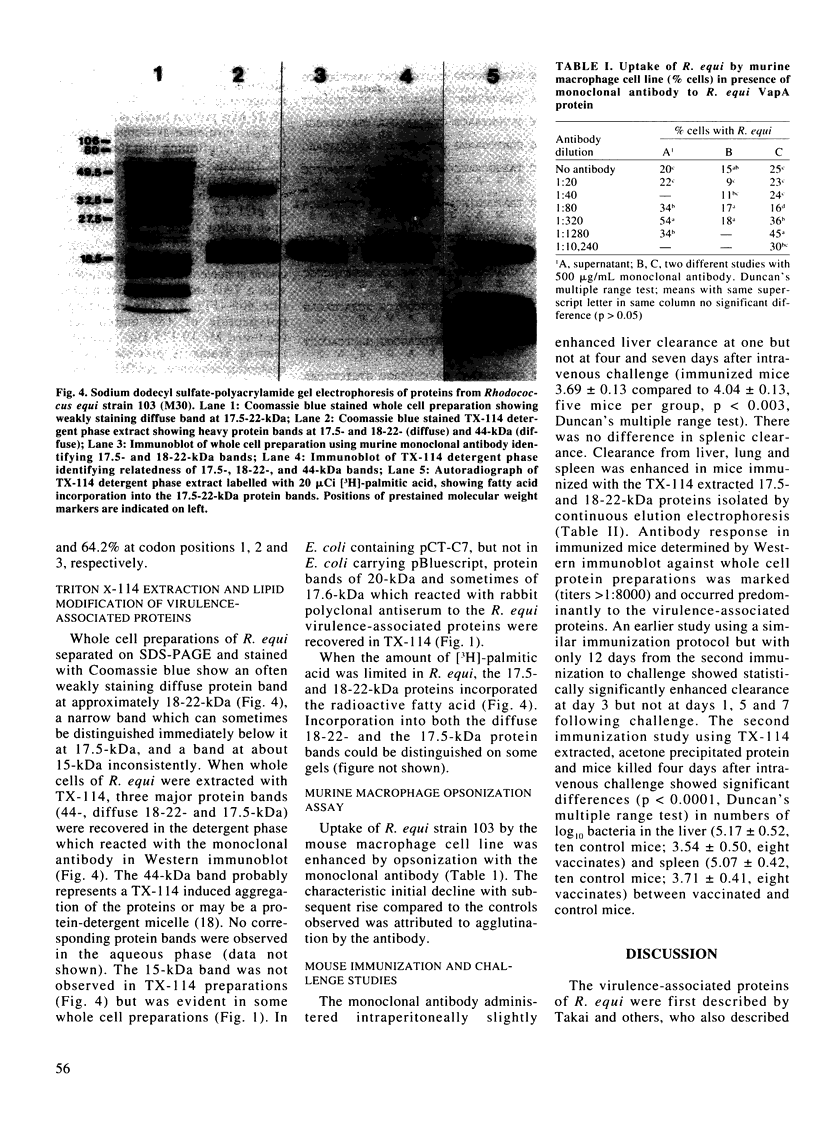
Virulent strains of Rhodococcus equi produce plasmid-mediated 15- and 17-kDa proteins, which are thermoregulated and apparently surface-expressed. We demonstrated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) that R. equi produce three antigenically-related virulence-associated proteins, a diffuse 18-22-kDa, a 17.5-kDa and a 15-kDa protein. Phase partitioning of whole cells of R. equi strain 103 with Triton X-114 (TX-114) and labelling with [3H]-labelled palmitic acid showed that the two higher molecular weight proteins are hydrophobic and lipid modified. The 15-kDa protein did not partition into TX-114 and was not lipid modified. Cloning and expression of a fragment of the R. equi virulence plasmid in Escherichia coli showed that the three proteins were expressed from a single gene. Sequence analysis of this gene (designated vapA) revealed a 570-bp open reading frame encoding a polypeptide of 189 amino acids with a calculated molecular mass of 19,175 Da. The mature, nonlipid modified protein had a calculated mass of 16,246 Da. The 17.5- and 18-22-kDa forms of the protein are therefore due to lipid modification. No significant sequence homology of the vapA gene with other reported nucleotide sequences were found. Opsonization of virulent R. equi with an IgG1 mouse monoclonal antibody (MAb103) to the VapA protein significantly enhanced uptake in the murine macrophage cell line IC-21. Intraperitoneal injection of mice with Mab103 enhanced initial clearance from the liver of mice challenged intravenously with R. equi. Immunization of mice with the lipid-modified VapA purified by SDS-PAGE fractionation or with acetone precipitated VapA protein following TX-114 extraction resulted in significantly enhanced clearance from the liver and spleen following intravenous challenge. The VapA protein of R. equi appears therefore to be a protective immunogen.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins D. R., Purcell B. K., Mitra M. M., Norgard M. V., Radolf J. D. Lipid modification of the 17-kilodalton membrane immunogen of Treponema pallidum determines macrophage activation as well as amphiphilicity. Infect Immun. 1993 Apr;61(4):1202–1210. doi: 10.1128/iai.61.4.1202-1210.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Deres K., Schild H., Wiesmüller K. H., Jung G., Rammensee H. G. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989 Nov 30;342(6249):561–564. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- Finnerty W. R. The biology and genetics of the genus Rhodococcus. Annu Rev Microbiol. 1992;46:193–218. doi: 10.1146/annurev.mi.46.100192.001205. [DOI] [PubMed] [Google Scholar]
- Hauschildt S., Hoffmann P., Beuscher H. U., Dufhues G., Heinrich P., Wiesmüller K. H., Jung G., Bessler W. G. Activation of bone marrow-derived mouse macrophages by bacterial lipopeptide: cytokine production, phagocytosis and Ia expression. Eur J Immunol. 1990 Jan;20(1):63–68. doi: 10.1002/eji.1830200110. [DOI] [PubMed] [Google Scholar]
- Hietala S. K., Ardans A. A. Interaction of Rhodococcus equi with phagocytic cells from R. equi-exposed and non-exposed foals. Vet Microbiol. 1987 Aug;14(3):307–320. doi: 10.1016/0378-1135(87)90118-0. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kanaly S. T., Hines S. A., Palmer G. H. Failure of pulmonary clearance of Rhodococcus equi infection in CD4+ T-lymphocyte-deficient transgenic mice. Infect Immun. 1993 Nov;61(11):4929–4932. doi: 10.1128/iai.61.11.4929-4932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno T., Asawa T., Ito H., Takai S., Tsubaki S., Sekizaki T. Restriction map of a virulence-associated plasmid of Rhodococcus equi. Plasmid. 1993 Nov;30(3):309–311. doi: 10.1006/plas.1993.1065. [DOI] [PubMed] [Google Scholar]
- Katona L. I., Beck G., Habicht G. S. Purification and immunological characterization of a major low-molecular-weight lipoprotein from Borrelia burgdorferi. Infect Immun. 1992 Dec;60(12):4995–5003. doi: 10.1128/iai.60.12.4995-5003.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundemose A. G., Rouch D. A., Penn C. W., Pearce J. H. The Chlamydia trachomatis Mip-like protein is a lipoprotein. J Bacteriol. 1993 Jun;175(11):3669–3671. doi: 10.1128/jb.175.11.3669-3671.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Weis J. J. Borrelia burgdorferi outer surface lipoproteins OspA and OspB possess B-cell mitogenic and cytokine-stimulatory properties. Infect Immun. 1993 Sep;61(9):3843–3853. doi: 10.1128/iai.61.9.3843-3853.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens R. J., Martens J. G., Fiske R. A., Hietala S. K. Rhodococcus equi foal pneumonia: protective effects of immune plasma in experimentally infected foals. Equine Vet J. 1989 Jul;21(4):249–255. doi: 10.1111/j.2042-3306.1989.tb02161.x. [DOI] [PubMed] [Google Scholar]
- Nielsen J. B., Lampen J. O. Glyceride-cysteine lipoproteins and secretion by Gram-positive bacteria. J Bacteriol. 1982 Oct;152(1):315–322. doi: 10.1128/jb.152.1.315-322.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J. F. Rhodococcus equi: an animal and human pathogen. Clin Microbiol Rev. 1991 Jan;4(1):20–34. doi: 10.1128/cmr.4.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouls L. M., Mout R., Dekker J., van Embden J. D. Characterization of lipid-modified immunogenic proteins of Treponema pallidum expressed in Escherichia coli. Microb Pathog. 1989 Sep;7(3):175–188. doi: 10.1016/0882-4010(89)90053-3. [DOI] [PubMed] [Google Scholar]
- Takai S., Iie M., Kobayashi C., Morishita T., Nishio T., Ishida T., Fujimura T., Sasaki Y., Tsubaki S. Monoclonal antibody specific to virulence-associated 15- to 17-kilodalton antigens of Rhodococcus equi. J Clin Microbiol. 1993 Oct;31(10):2780–2782. doi: 10.1128/jcm.31.10.2780-2782.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S., Iie M., Watanabe Y., Tsubaki S., Sekizaki T. Virulence-associated 15- to 17-kilodalton antigens in Rhodococcus equi: temperature-dependent expression and location of the antigens. Infect Immun. 1992 Jul;60(7):2995–2997. doi: 10.1128/iai.60.7.2995-2997.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S., Koike K., Ohbushi S., Izumi C., Tsubaki S. Identification of 15- to 17-kilodalton antigens associated with virulent Rhodococcus equi. J Clin Microbiol. 1991 Mar;29(3):439–443. doi: 10.1128/jcm.29.3.439-443.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S., Sekizaki T., Ozawa T., Sugawara T., Watanabe Y., Tsubaki S. Association between a large plasmid and 15- to 17-kilodalton antigens in virulent Rhodococcus equi. Infect Immun. 1991 Nov;59(11):4056–4060. doi: 10.1128/iai.59.11.4056-4060.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai S., Watanabe Y., Ikeda T., Ozawa T., Matsukura S., Tamada Y., Tsubaki S., Sekizaki T. Virulence-associated plasmids in Rhodococcus equi. J Clin Microbiol. 1993 Jul;31(7):1726–1729. doi: 10.1128/jcm.31.7.1726-1729.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachuk-Saad O., Prescott J. Rhodococcus equi plasmids: isolation and partial characterization. J Clin Microbiol. 1991 Dec;29(12):2696–2700. doi: 10.1128/jcm.29.12.2696-2700.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H. C., Tokunaga M. Biogenesis of lipoproteins in bacteria. Curr Top Microbiol Immunol. 1986;125:127–157. doi: 10.1007/978-3-642-71251-7_9. [DOI] [PubMed] [Google Scholar]
- Young D. B., Garbe T. R. Lipoprotein antigens of Mycobacterium tuberculosis. Res Microbiol. 1991 Jan;142(1):55–65. doi: 10.1016/0923-2508(91)90097-t. [DOI] [PubMed] [Google Scholar]