Abstract
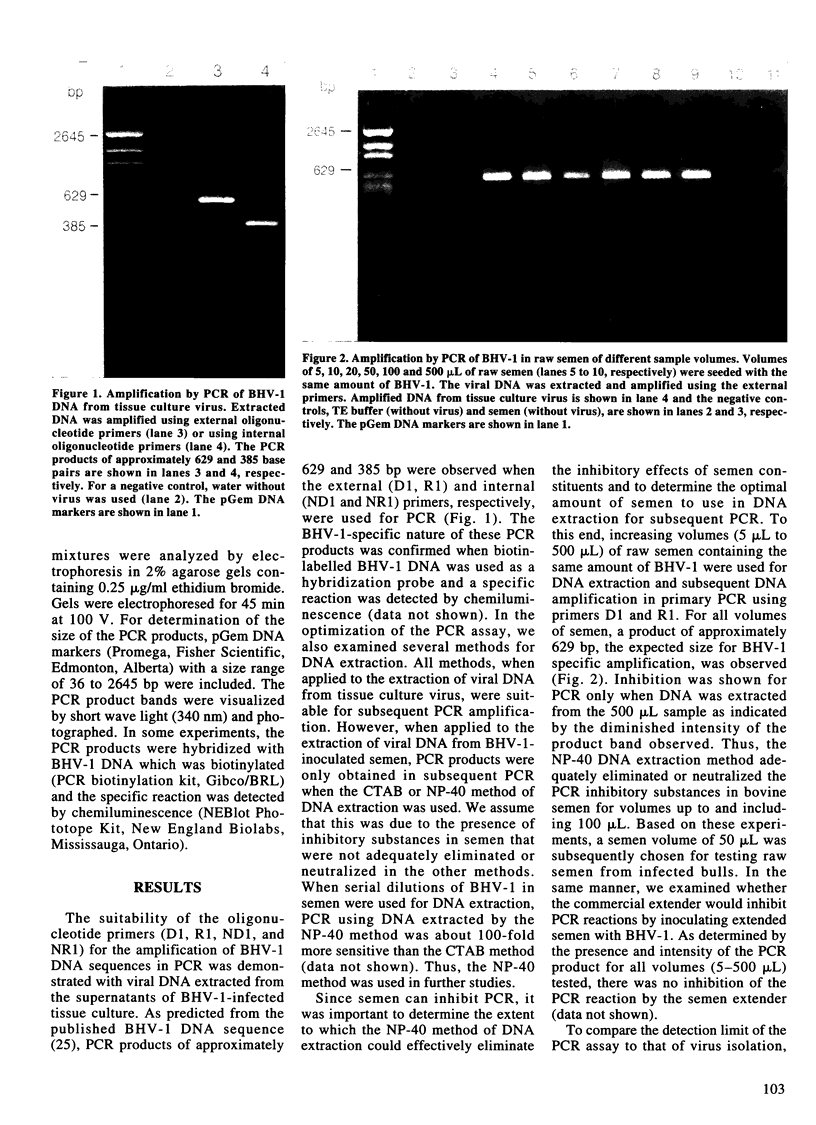
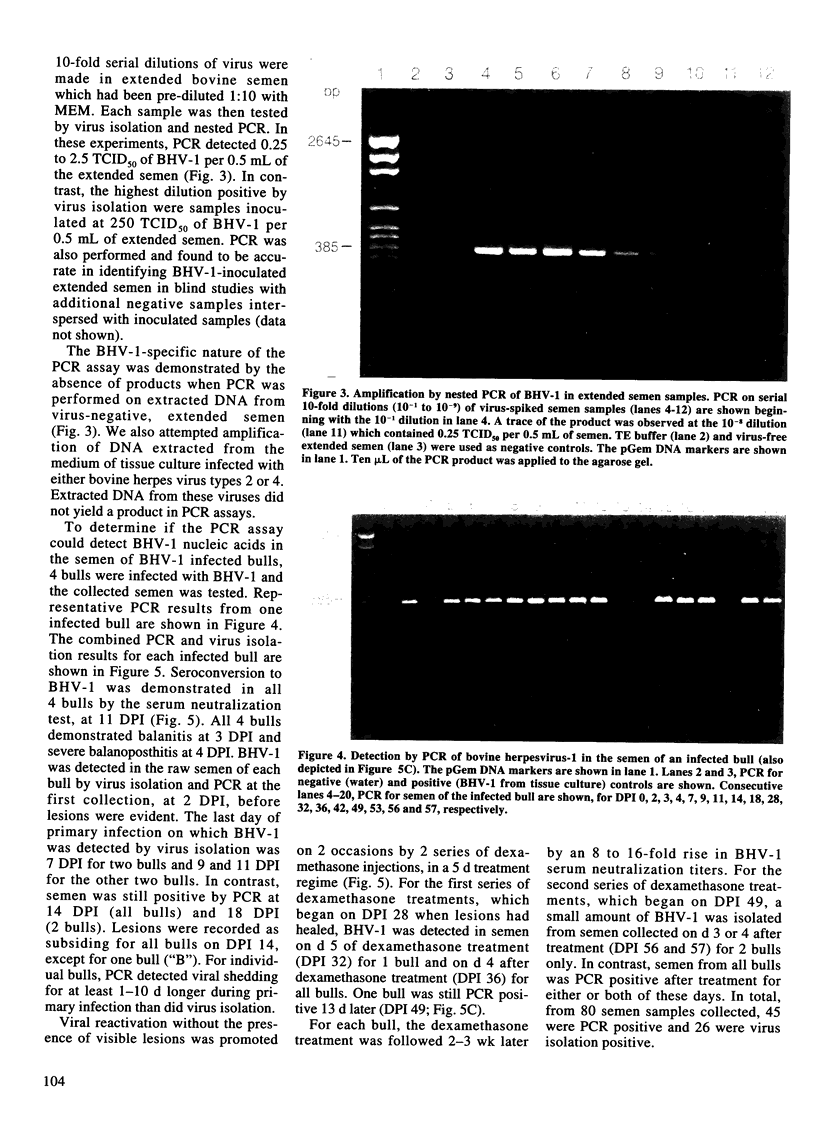
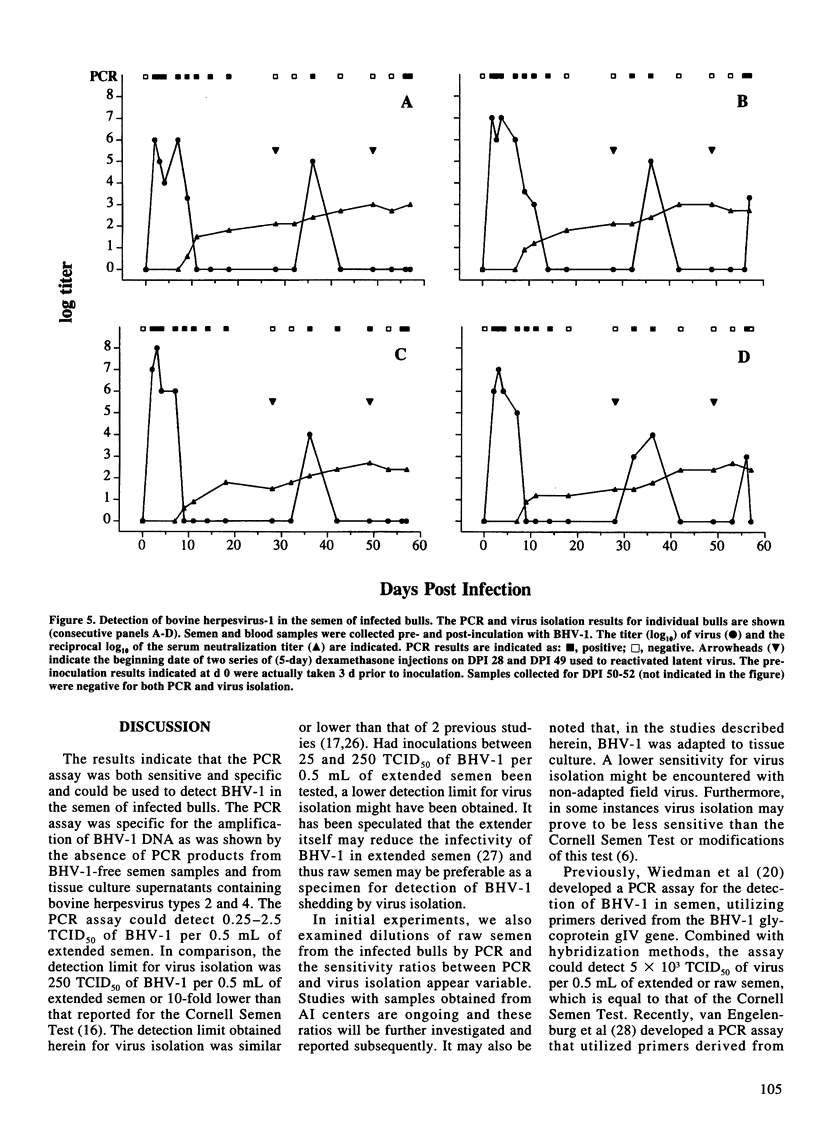
A nested polymerase chain reaction (PCR) assay was developed for the detection of bovine herpesvirus 1 (BHV-1) in bovine semen and compared with the virus isolation method. When extended semen, commonly used in the bovine artificial insemination industry, was inoculated with BHV-1, the PCR assay detected BHV-1 DNA in semen inoculated at 0.25-2.5 TCID50 per 0.5 mL. In contrast, the lower limit of detection for virus isolation was 250 TCID50 of BHV-1 inoculated in 0.5 mL of extended semen. These methods were also used to detect BHV-1 in the semen of four bulls which were experimentally infected with BHV-1. All infected bulls demonstrated balanitis at 3 d post-inoculation (DPI) and severe balanoposthitis at 4 DPI. BHV-1 was detected in raw semen by virus isolation and PCR at 2 DPI, before balanitis was evident. For virus isolation, the last day that BHV-1 was detected during primary infection was 7 DPI for two bulls and 9 and 11 DPI for the other two bulls. In contrast, PCR detected BHV-1 in the bulls' semen until 14 or 18 DPI. For individual animals, PCR detected BHV-1 during primary infection for at least 1-10 d longer than virus isolation. Reactivation of BHV-1 from latency without the presence of visible lesions was promoted twice by two series of 5 d dexamethasone injections. For the first series of dexamethasone treatments, a positive virus isolation result was obtained on the 5th d of treatment for only one bull. In contrast, two bulls demonstrated evidence of viral reactivation on this day by PCR. All bulls shed BHV-1 in semen on d 4 after dexamethasone treatment, as evidenced by positive virus isolation and PCR results. One bull was still PCR positive 13 d later. For the second series of dexamethasone treatments, a small amount of virus was isolated from semen collected on d 3 or 4 after treatment for two bulls but not from the other two bulls. In contrast, semen samples from all bulls were PCR positive for either or both of these 2 d. In total, from 80 semen samples, 45 were PCR positive and 26 were virus isolation positive. Thus, the PCR assay detected BHV-1 shedding in bulls earlier, more often, and for a longer duration, than did the virus isolation method.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M., Wyler R. The DNA of an IPV strain of bovid herpesvirus 1 in sacral ganglia during latency after intravaginal infection. Vet Microbiol. 1984 Feb;9(1):53–63. doi: 10.1016/0378-1135(84)90078-6. [DOI] [PubMed] [Google Scholar]
- Bielanski A., Loewen K. G., Hare W. C. Inactivation of bovine herpesvirus-1 (BHV-I) from in vitro infected bovine semen. Theriogenology. 1988 Oct;30(4):649–657. doi: 10.1016/0093-691x(88)90300-7. [DOI] [PubMed] [Google Scholar]
- Bradley J. A. Eradication of infectious bovine rhinotracheitis virus (bovine herpesvirus 1) from a herd of beef cattle. Can Vet J. 1985 Jun;26(6):195–198. [PMC free article] [PubMed] [Google Scholar]
- Cho H. J., Bohac J. G. Sensitivity and specificity of an enzyme-linked immunosorbent assay for the detection of infectious bovine rhinotracheitis viral antibody in cattle. Can J Comp Med. 1985 Apr;49(2):189–194. [PMC free article] [PubMed] [Google Scholar]
- Deas D. W., Johnston W. S. The isolation and transmission of the virus of infectious bovine rhinotracheitis-infectious pustular vulvo-vaginitis. Vet Rec. 1973 Jun 16;92(24):636–639. doi: 10.1136/vr.92.24.636. [DOI] [PubMed] [Google Scholar]
- Deregt D., Cho H. J., Kozub G. C. A comparative evaluation of two sensitive serum neutralization tests for bovine herpesvirus-1 antibodies. Can J Vet Res. 1993 Jan;57(1):56–59. [PMC free article] [PubMed] [Google Scholar]
- Deregt D., Masri S. A., Cho H. J., Bielefeldt Ohmann H. Monoclonal antibodies to the p80/125 gp53 proteins of bovine viral diarrhea virus: their potential use as diagnostic reagents. Can J Vet Res. 1990 Jun;54(3):343–348. [PMC free article] [PubMed] [Google Scholar]
- Drew T. W., Hewitt-Taylor C., Watson L., Edwards S. Effect of storage conditions and culture technique on the isolation of infectious bovine rhinotracheitis virus from bovine semen. Vet Rec. 1987 Dec 5;121(23):547–548. [PubMed] [Google Scholar]
- Israel B. A., Herber R., Gao Y., Letchworth G. J., 3rd Induction of a mucosal barrier to bovine herpesvirus 1 replication in cattle. Virology. 1992 May;188(1):256–264. doi: 10.1016/0042-6822(92)90755-e. [DOI] [PubMed] [Google Scholar]
- Kahrs R. F., Gibbs E. P., Larsen R. E. The search for viruses in bovine semen, a review. Theriogenology. 1980 Aug;14(2):151–165. doi: 10.1016/0093-691x(80)90101-6. [DOI] [PubMed] [Google Scholar]
- Kibenge F. S., Harris L. M., McKenna P. K., Wadowska D., Yason C. V. Amplification of strains of bovine herpesvirus 1 by use of polymerase chain reaction with primers in the thymidine kinase region. Am J Vet Res. 1994 Sep;55(9):1206–1212. [PubMed] [Google Scholar]
- Kupferschmied H. U., Kihm U., Bachmann P., Müller K. H., Ackermann M. Transmission of IBR/IPV virus in bovine semen: A case report. Theriogenology. 1986 Mar;25(3):439–443. doi: 10.1016/0093-691x(86)90052-x. [DOI] [PubMed] [Google Scholar]
- Pastoret P. P., Babiuk L. A., Misra V., Griebel P. Reactivation of temperature-sensitive and non-temperature-sensitive infectious bovine rhinotracheitis vaccine virus with dexamethasone. Infect Immun. 1980 Aug;29(2):483–488. doi: 10.1128/iai.29.2.483-488.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin B., Bitsch V., Cordioli P., Edwards S., Eloit M., Guérin B., Lenihan P., Perrin M., Rønsholt L., Van Oirschot J. T. A European comparative study of serological methods for the diagnosis of infectious bovine rhinotracheitis. Rev Sci Tech. 1993 Sep;12(3):969–984. doi: 10.20506/rst.12.3.724. [DOI] [PubMed] [Google Scholar]
- Roizmann B., Desrosiers R. C., Fleckenstein B., Lopez C., Minson A. C., Studdert M. J. The family Herpesviridae: an update. The Herpesvirus Study Group of the International Committee on Taxonomy of Viruses. Arch Virol. 1992;123(3-4):425–449. doi: 10.1007/BF01317276. [DOI] [PubMed] [Google Scholar]
- Schultz R. D., Adams L. S., Letchworth G., Sheffy B. E., Manning T., Bean B. A method to test large numbers of bovine semen samples for viral contamination and results of a study using this method. Theriogenology. 1982 Feb;17(2):115–123. doi: 10.1016/0093-691x(82)90071-1. [DOI] [PubMed] [Google Scholar]
- St-Laurent G., Morin G., Archambault D. Detection of equine arteritis virus following amplification of structural and nonstructural viral genes by reverse transcription-PCR. J Clin Microbiol. 1994 Mar;32(3):658–665. doi: 10.1128/jcm.32.3.658-665.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub O. C. BHV1 infections: relevance and spread in Europe. Comp Immunol Microbiol Infect Dis. 1991;14(2):175–186. doi: 10.1016/0147-9571(91)90130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Engelenburg F. A., Van Schie F. W., Rijsewijk F. A., Van Oirschot J. T. Excretion of bovine herpesvirus 1 in semen is detected much longer by PCR than by virus isolation. J Clin Microbiol. 1995 Feb;33(2):308–312. doi: 10.1128/jcm.33.2.308-312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek S. Detection of the bovine herpesvirus-1 (BHV-1) genome by PCR. J Virol Methods. 1993 Feb;41(2):245–247. doi: 10.1016/0166-0934(93)90132-b. [DOI] [PubMed] [Google Scholar]
- Whitbeck J. C., Bello L. J., Lawrence W. C. Comparison of the bovine herpesvirus 1 gI gene and the herpes simplex virus type 1 gB gene. J Virol. 1988 Sep;62(9):3319–3327. doi: 10.1128/jvi.62.9.3319-3327.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedmann M., Brandon R., Wagner P., Dubovi E. J., Batt C. A. Detection of bovine herpesvirus-1 in bovine semen by a nested PCR assay. J Virol Methods. 1993 Sep;44(1):129–139. doi: 10.1016/0166-0934(93)90015-j. [DOI] [PubMed] [Google Scholar]
- Yates W. D. A review of infectious bovine rhinotracheitis, shipping fever pneumonia and viral-bacterial synergism in respiratory disease of cattle. Can J Comp Med. 1982 Jul;46(3):225–263. [PMC free article] [PubMed] [Google Scholar]
- van Engelenburg F. A., Maes R. K., van Oirschot J. T., Rijsewijk F. A. Development of a rapid and sensitive polymerase chain reaction assay for detection of bovine herpesvirus type 1 in bovine semen. J Clin Microbiol. 1993 Dec;31(12):3129–3135. doi: 10.1128/jcm.31.12.3129-3135.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oirschot J. T., Straver P. J., van Lieshout J. A., Quak J., Westenbrink F., van Exsel A. C. A subclinical infection of bulls with bovine herpesvirus type 1 at an artificial insemination centre. Vet Rec. 1993 Jan 9;132(2):32–35. doi: 10.1136/vr.132.2.32. [DOI] [PubMed] [Google Scholar]