Abstract
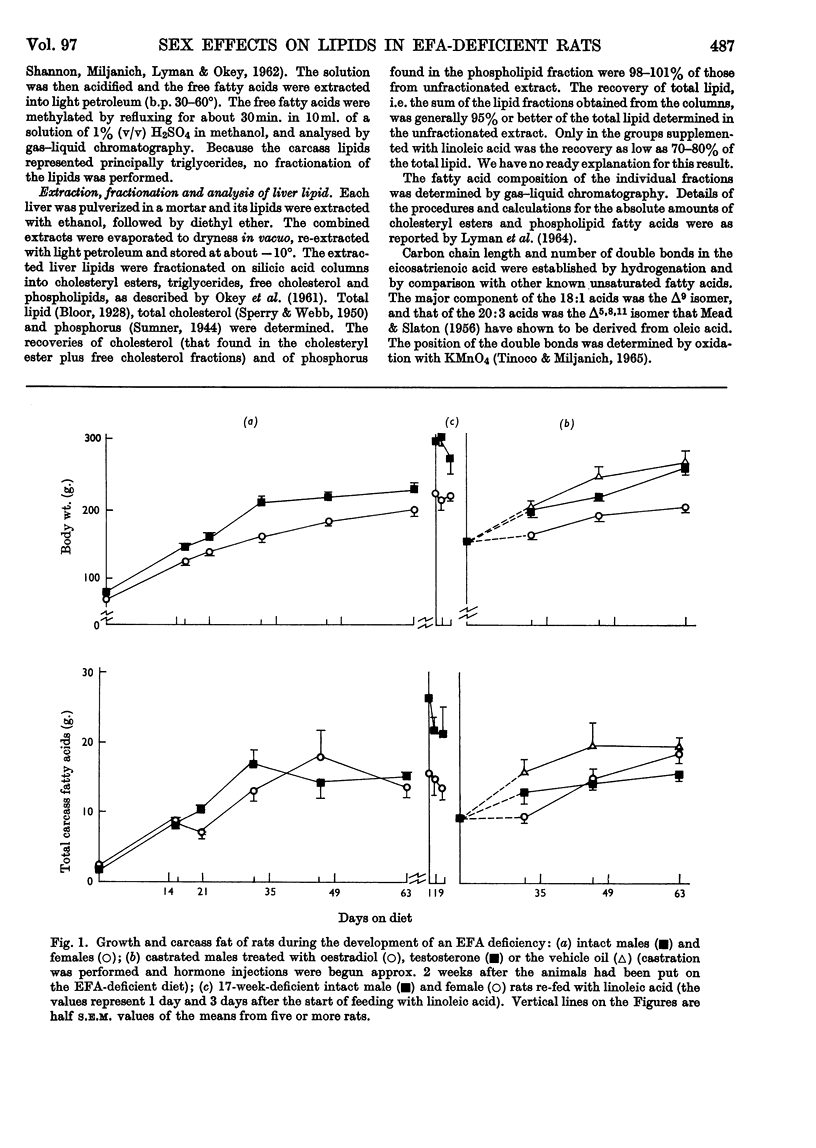
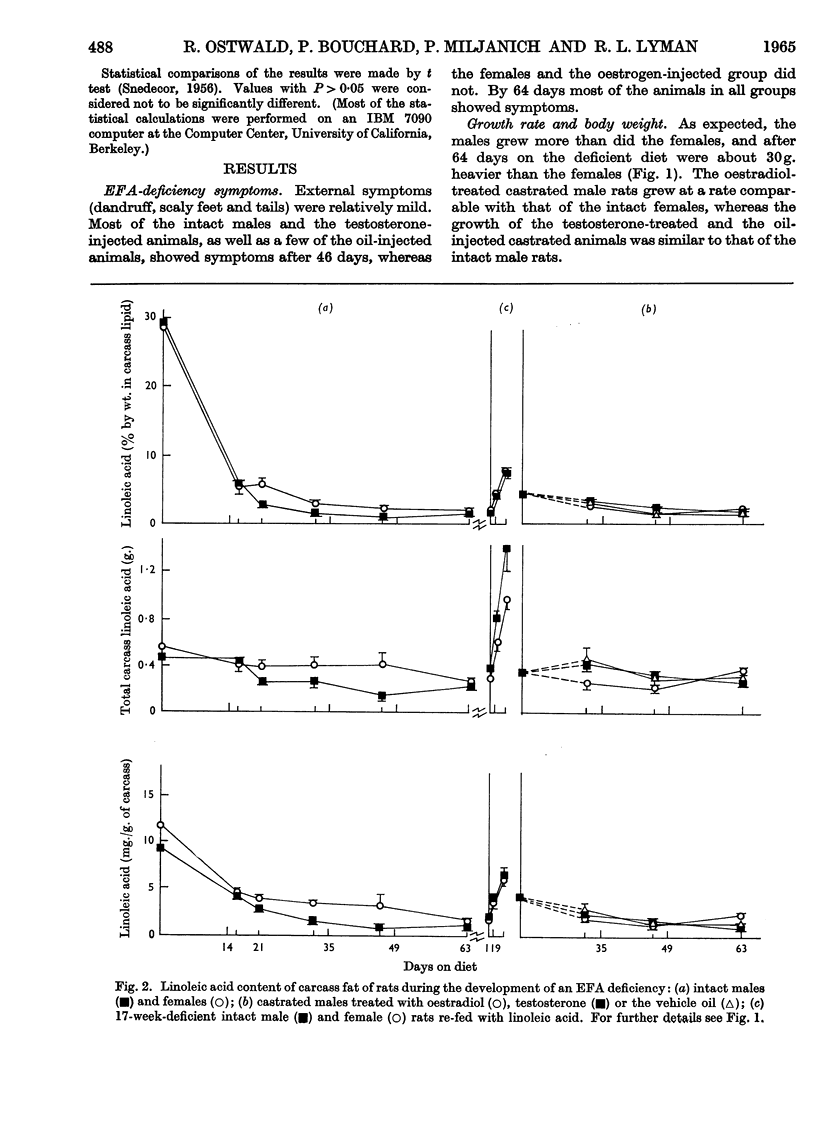
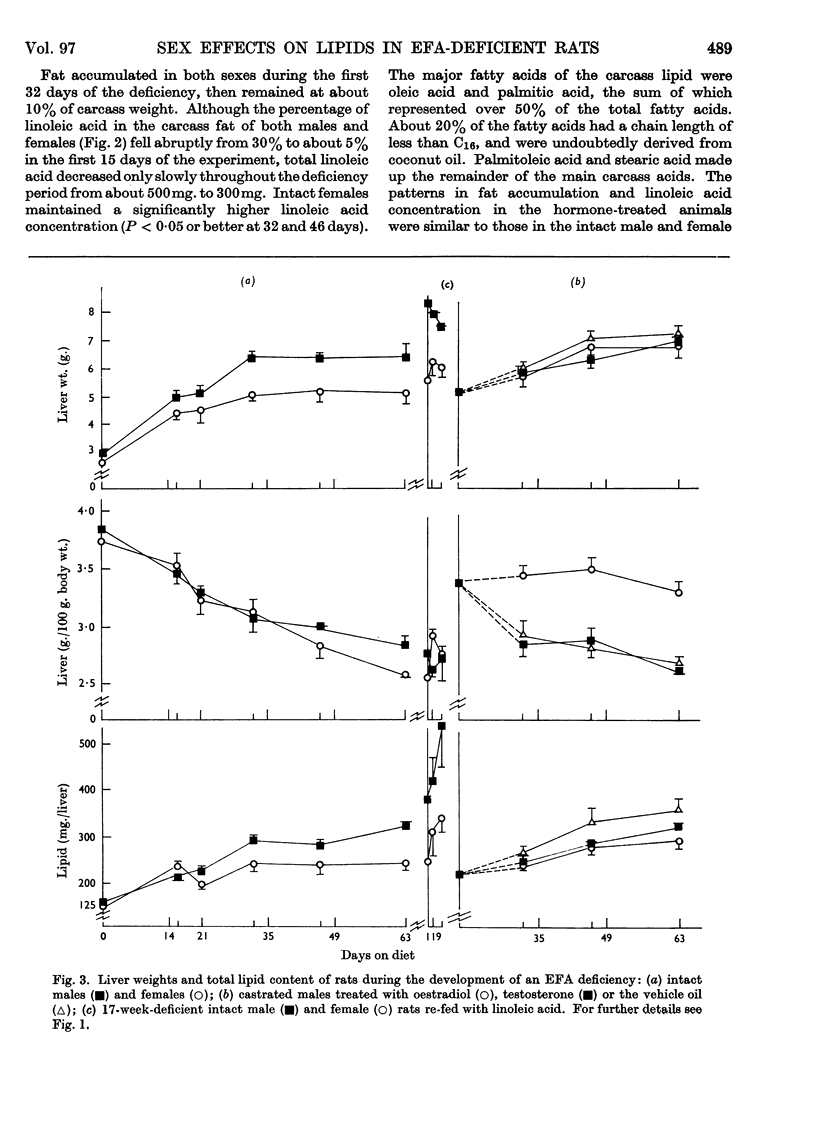
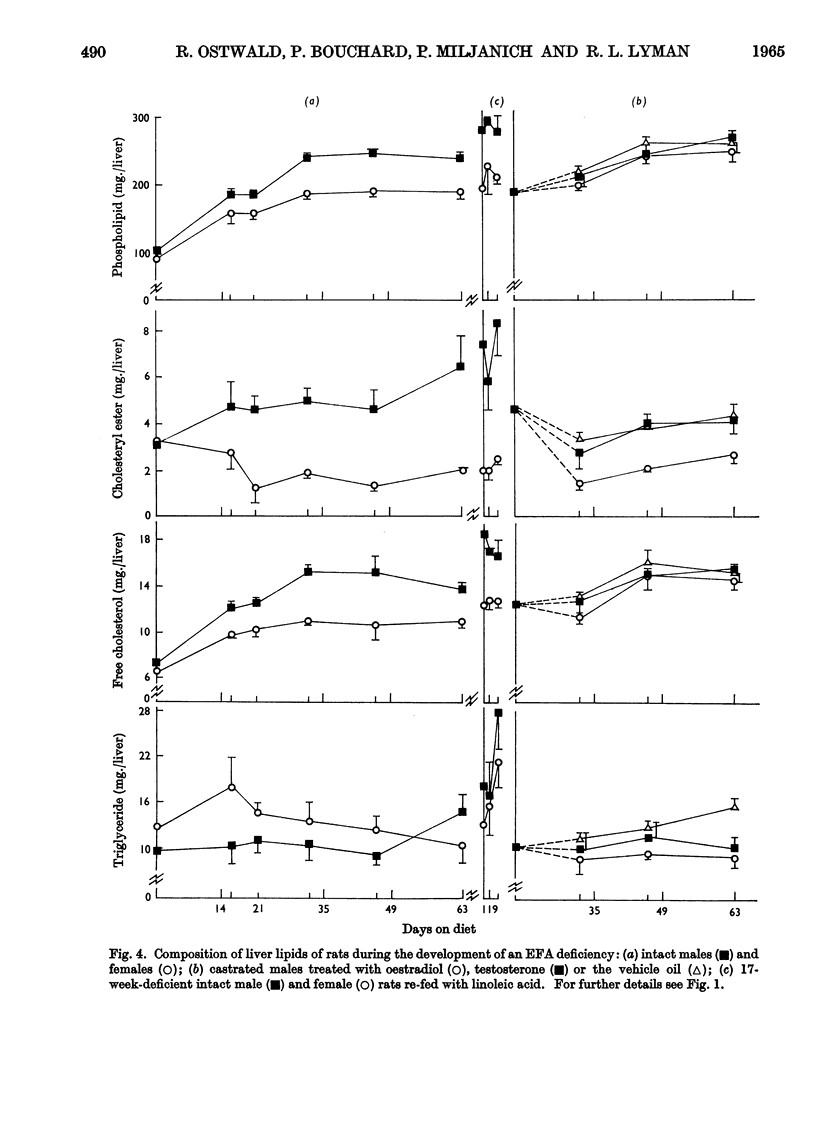
1. Groups of intact male and female rats and castrated rats injected with oestradiol or testosterone were given a diet containing hydrogenated coconut oil for 9 weeks, and at intervals the amounts and fatty acid compositions of the carcass and liver lipids were determined. 2. Male rats grew faster and larger, and exhibited typical external essential fatty acid deficiency symptoms sooner than did females. Testosterone-treated castrated male rats were similar to males, and oestradiol-injected castrated male rats resembled females. 3. Intact females maintained a higher linoleic acid concentration in their carcass than did males. Total amounts of carcass linoleic acid remained similar for all groups, only 200mg. being removed in 9 weeks regardless of body size. 4. The amounts of total cholesteryl esters were independent of liver size. They were higher in males and testosterone-treated castrated male rats than in females and oestrogen-treated castrated male rats. 5. Phospholipids represented about 80% of the liver lipids. The total amounts of the phospholipid linoleic acid and arachidonic acid were similar for all groups regardless of liver size, and were not affected appreciably by the deficiency. Females and oestrogen-treated castrated male rats maintained a higher proportion of phospholipid arachidonic acid for longer periods than did their male counterparts. Both the total amounts and the proportions of eicosatrienoic acid and palmitic acid were higher in males than in females. 6. Supplementation of the essential fatty acid-deficient diet with linoleic acid caused a rapid loss of eicosatrienoic acid and palmitic acid with a concomitant increase in stearic acid and arachidonic acid. 7. There were no obvious differences in the way that the essential fatty acids were metabolized or mobilized from adipose tissue of male or female rats during essential fatty acid deficiency. 8. The results indicated that the greater growth rate of the male rats caused them to require and synthesize more phospholipids than did the females. In the absence of adequate amounts of arachidonic acid, eicosatrienoic acid was substituted into the additional phospholipid. The earlier symptoms of essential fatty acid deficiency in the male rat could therefore be ascribed to the higher tissue concentrations of this unnatural phospholipid and its inability to perform the normal metabolic functions of phospholipids.
Full text
PDF


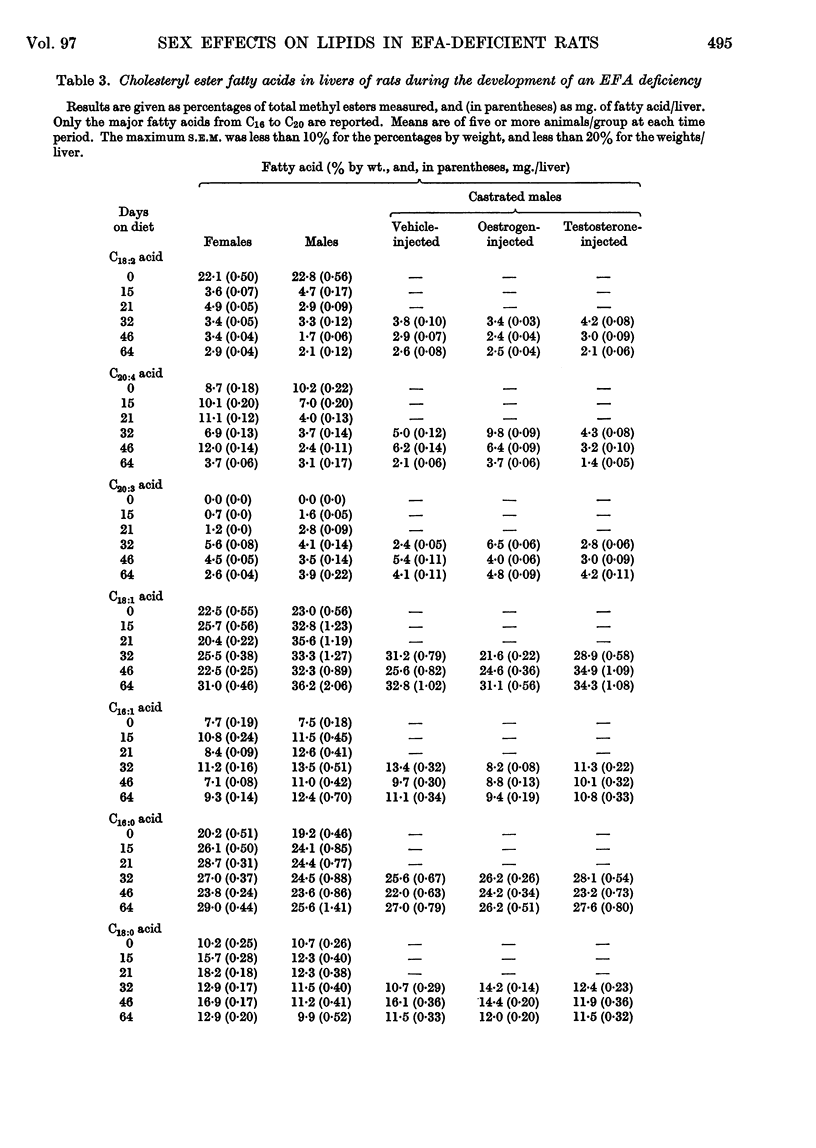



Selected References
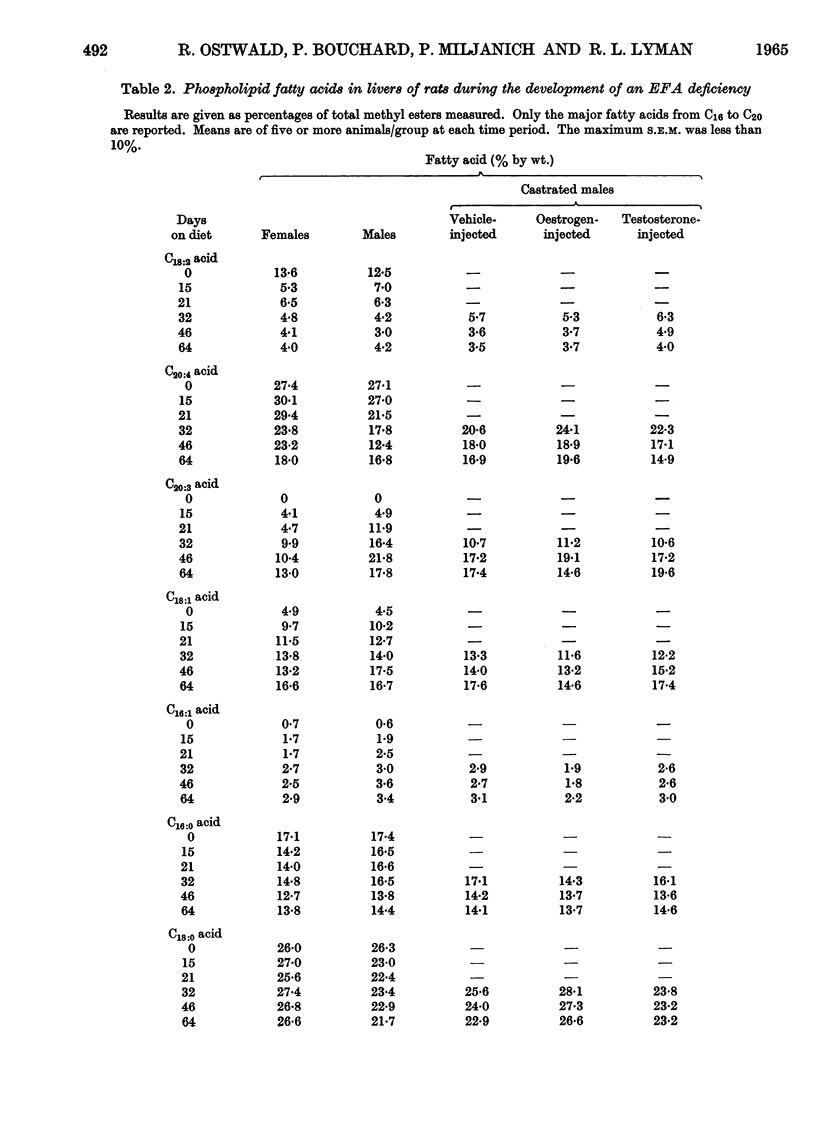
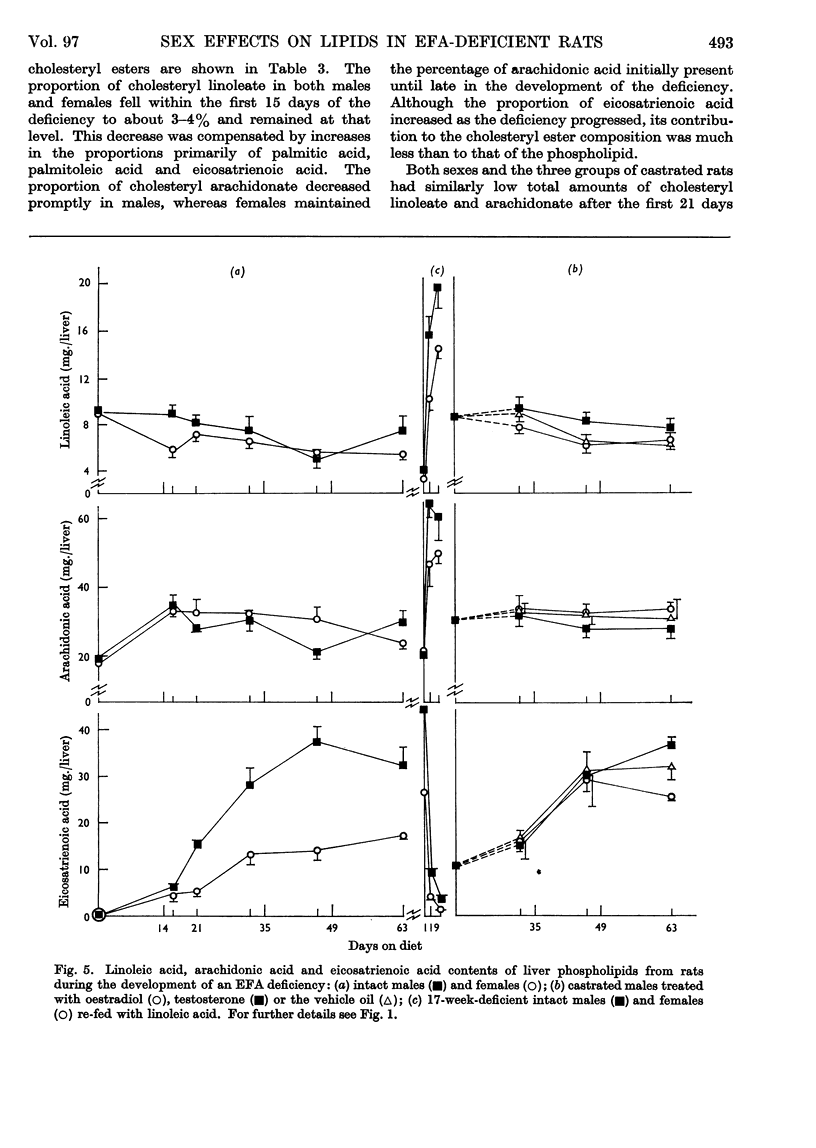
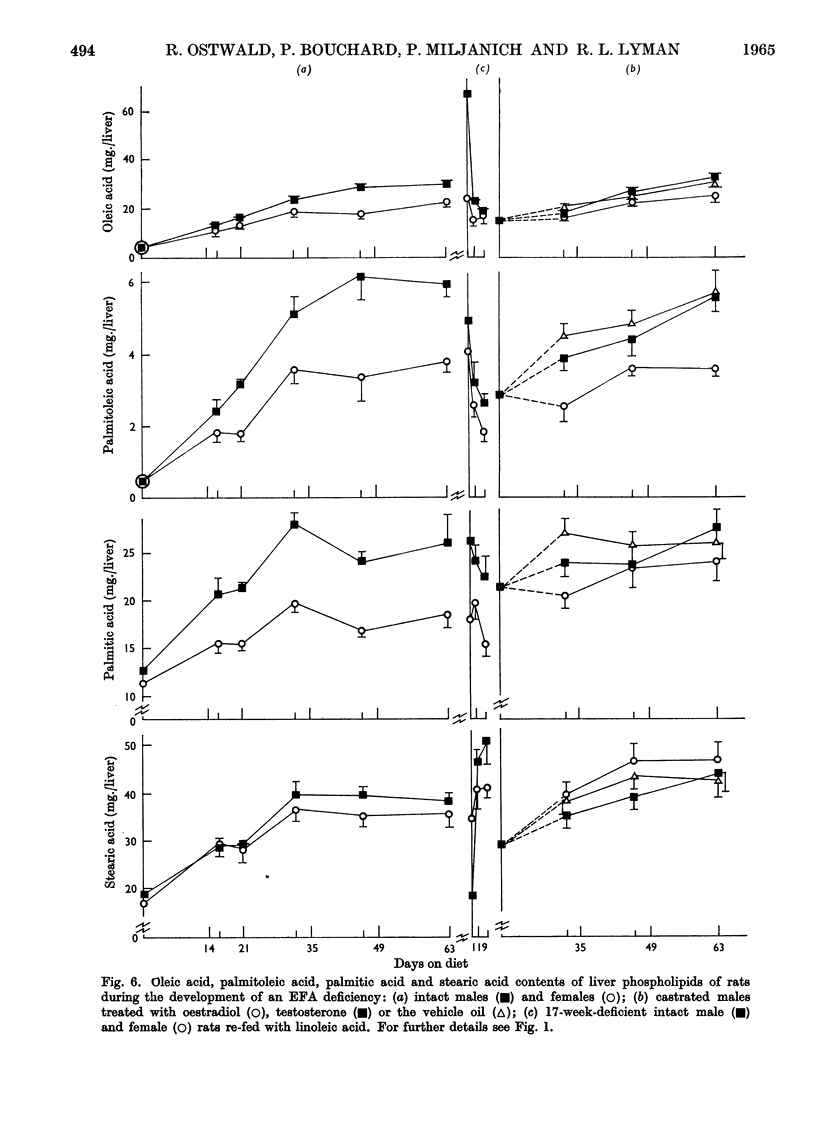
These references are in PubMed. This may not be the complete list of references from this article.
- AFTERGOOD L., ALFIN-SLATER R. B. DIETARY AND GONADAL HORMONE EFFECTS ON LIPID METABOLISM IN THE RAT. J Lipid Res. 1965 Apr;6:287–294. [PubMed] [Google Scholar]
- COLLINS F. D. Heterogeneity of lecithins labelled with phosphorus-32. Nature. 1960 Apr 30;186:366–367. doi: 10.1038/186366b0. [DOI] [PubMed] [Google Scholar]
- DEUEL H. J., Jr, ALFIN-SLATER R. B., WELLS A. F., KRYDER G. D., AFTERGOOD L. The effect of fat level of the diet on general nutrition. XIV. Further studies of the effect of hydrogenated coconut oil on essential fatty acid deficiency in the rat. J Nutr. 1955 Feb 10;55(2):337–346. doi: 10.1093/jn/55.2.337. [DOI] [PubMed] [Google Scholar]
- GREENBERG S. M., CALBERT C. E., SAVAGE E. E., DEUEL H. J., Jr The effect of fat level of the diet on general nutrition. VI. The interrelation of linoleate and linolenate in supplying the essential fatty acid requirement in the rat. J Nutr. 1950 Jul;41(3):473–486. doi: 10.1093/jn/41.3.473. [DOI] [PubMed] [Google Scholar]
- HARRIS P. M., ROBINSON D. S. Heterogeneity of liver lecithin isolated by chromatography on silicic acid columns. Nature. 1960 Nov 26;188:742–743. doi: 10.1038/188742a0. [DOI] [PubMed] [Google Scholar]
- HORNER A. A., MORTON R. A. The liver-lipid constituents of male and female rats. 2. Effects of the fat-deficiency syndrome. aggravated by dietary cholesterol. Biochem J. 1961 Jun;79:636–642. doi: 10.1042/bj0790636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRITCHEVSKY D., TEPPER S. A., STAPLE E., WHITEHOUSE M. W. INFLUENCE OF SEX AND SEX HORMONES ON THE OXIDATION OF CHOLESTEROL-26-C14 BY RAT LIVER MITOCHONDRIA. J Lipid Res. 1963 Apr;4:188–192. [PubMed] [Google Scholar]
- LYMAN R. L., SHANNON A., OSTWALD R., MILJANICH P. EFFECT OF ESTRADIOL AND TESTOSTERONE ON THE FATTY ACIDS OF PLASMA CHOLESTERYL ESTERS AND PHOSPHOLIPIDS IN THE CASTRATED RAT. Can J Biochem. 1964 Mar;42:365–376. doi: 10.1139/o64-044. [DOI] [PubMed] [Google Scholar]
- MEAD J. F., SLATON W. H., Jr Metabolism of essential fatty acids. III. Isolation of 5,8,11-eicosatrienoic acid from fat-deficient rats. J Biol Chem. 1956 Apr;219(2):705–709. [PubMed] [Google Scholar]
- MONSEN E. R., OKEY R., LYMAN R. L. Effect of diet and sex on the relative lipid composition of plasma and red blood cells in the rat. Metabolism. 1962 Oct;11:1113–1124. [PubMed] [Google Scholar]
- MUKHERJEE S., ACHAYA K. T., DEUEL H. J., Jr, ALFIN-SLATER R. B. The effect of dietary fat on the fatty acid composition of cholesterol esters in rat liver. J Nutr. 1958 Jul 10;65(3):469–479. doi: 10.1093/jn/65.3.469. [DOI] [PubMed] [Google Scholar]
- OKEY R., OSTWALD R., SHANNON A., TINOCO J. Changes in tissue lipids in response to diet. II. Fatty acid composition of liver and plasma lipids in relation to fed and stored fat. J Nutr. 1962 Apr;76:353–364. doi: 10.1093/jn/76.4.353. [DOI] [PubMed] [Google Scholar]
- OKEY R., SHANNON A., TINOCO J., OSTWALD R., MILJANICH P. Fatty acid components of rat liver lipids: effect of composition of the diet and of restricted access to food. J Nutr. 1961 Sep;75:51–60. doi: 10.1093/jn/75.1.51. [DOI] [PubMed] [Google Scholar]
- OSTWALD R., OKEY R., SHANNON A., TINOCO J. Changes in tissue lipids in response to diet. I. Fatty acids of subcutaneous, mesenteric and interscapular fat. J Nutr. 1962 Apr;76:341–352. doi: 10.1093/jn/76.4.341. [DOI] [PubMed] [Google Scholar]
- PINTER K. G., MILLER O. N., HAMILTON J. G. EFFECT OF MONOGLYCERIDES ON ABSORPTION OF CHOLESTEROL FROM THE INTESTINE AND TURNOVER RATE OF CHOLESTEROL ESTERS IN PLASMA AND LIVER OF THE RAT. Proc Soc Exp Biol Med. 1964 Feb;115:318–323. doi: 10.3181/00379727-115-28901. [DOI] [PubMed] [Google Scholar]
- SPERRY W. M., WEBB M. A revision of the Schoenheimer-Sperry method for cholesterol determination. J Biol Chem. 1950 Nov;187(1):97–106. [PubMed] [Google Scholar]
- Sumner J. B. A METHOD FOR THE COLORIMETRIC DETERMINATION OF PHOSPHORUS. Science. 1944 Nov 3;100(2601):413–414. doi: 10.1126/science.100.2601.413. [DOI] [PubMed] [Google Scholar]
- TINOCO J., MILJANICH P., LYMAN R. L. STABILITY OF LIPIDS IN LYOPHILIZED RAT LIVERS. J Lipid Res. 1963 Jul;4:359–361. [PubMed] [Google Scholar]
- TINOCO J., SHANNON A., MILJANICHP, LYMAN R. L., OKEY R. Analysis of fatty acid mixtures: comparison of two "absolute" methods of determination. Anal Biochem. 1962 Jun;3:514–518. doi: 10.1016/0003-2697(62)90084-2. [DOI] [PubMed] [Google Scholar]
- Tinoco J., Miljanich P. G. A rapid procedure for locating double bonds in unsaturated fatty acids. Anal Biochem. 1965 Jun;11(3):548–554. doi: 10.1016/0003-2697(65)90072-2. [DOI] [PubMed] [Google Scholar]


