Abstract
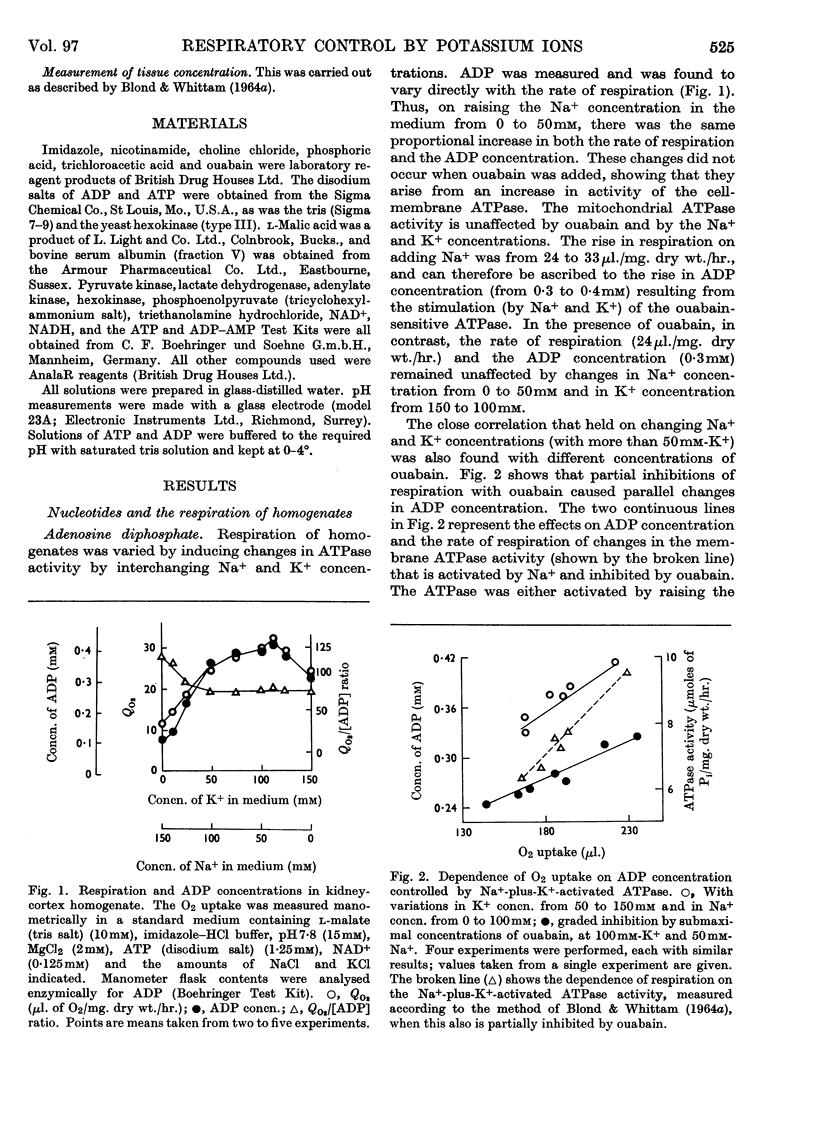
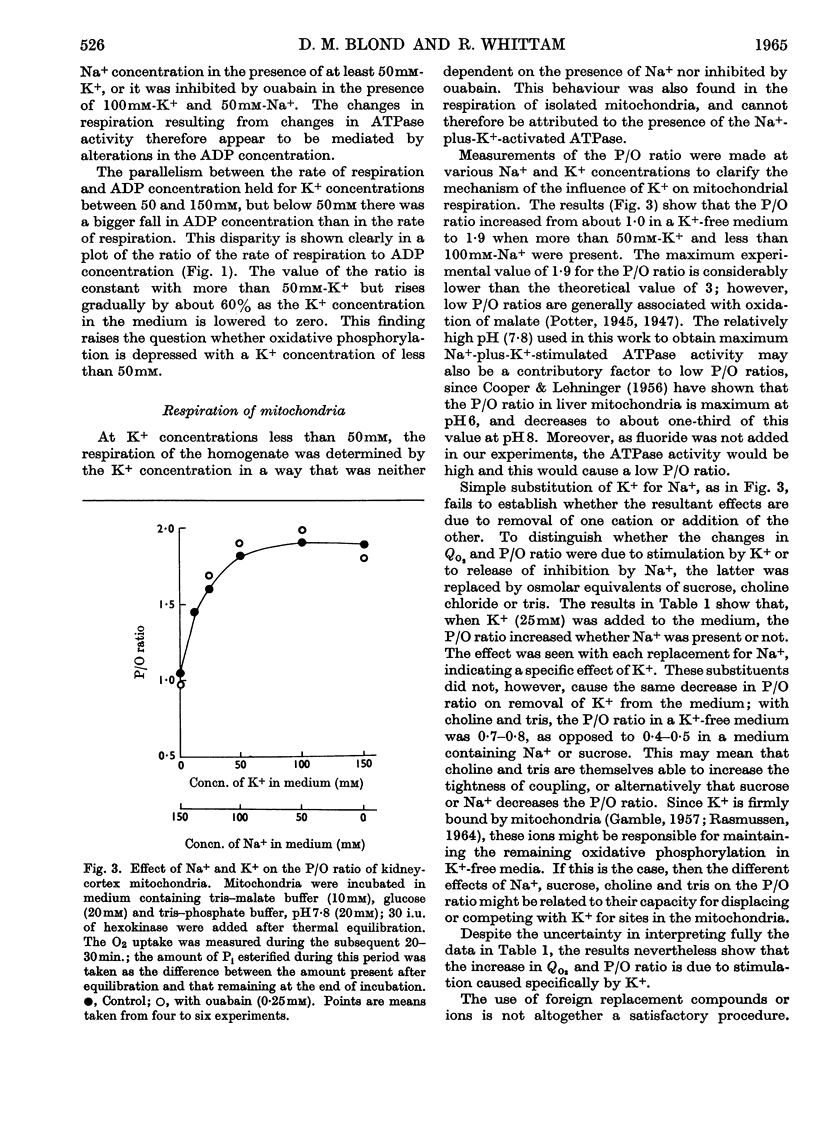
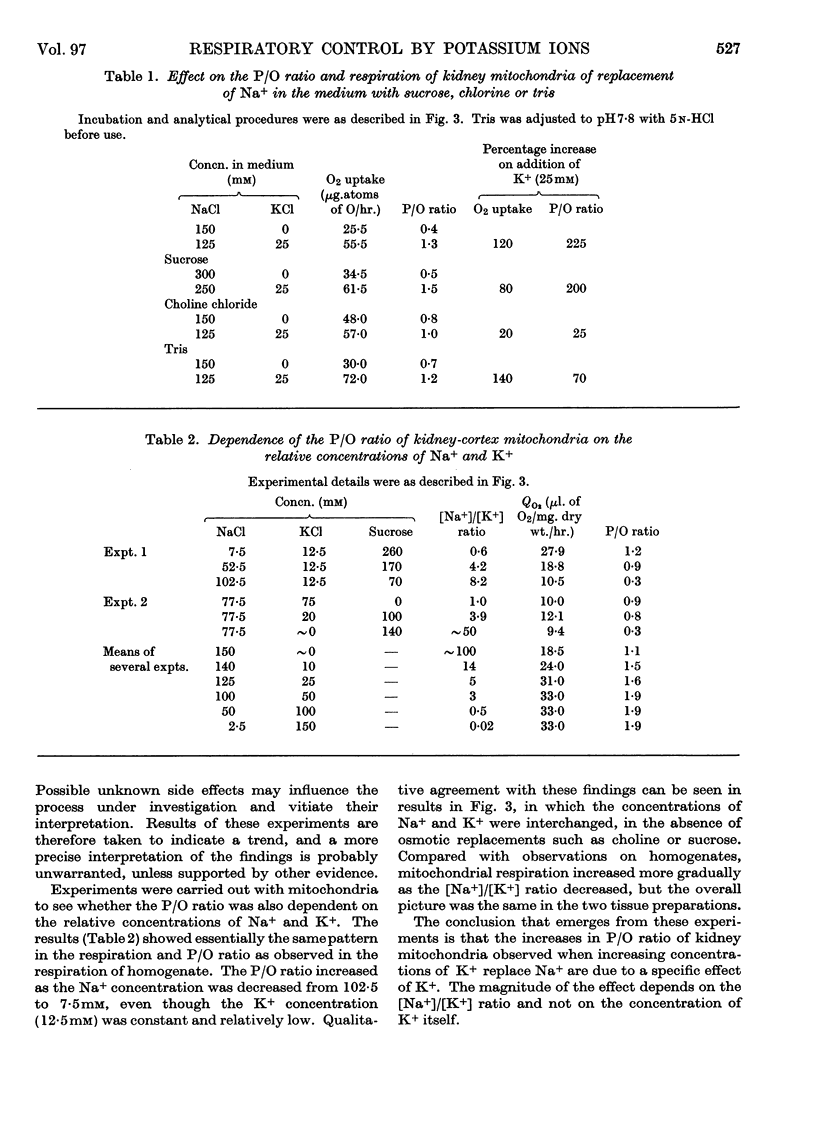
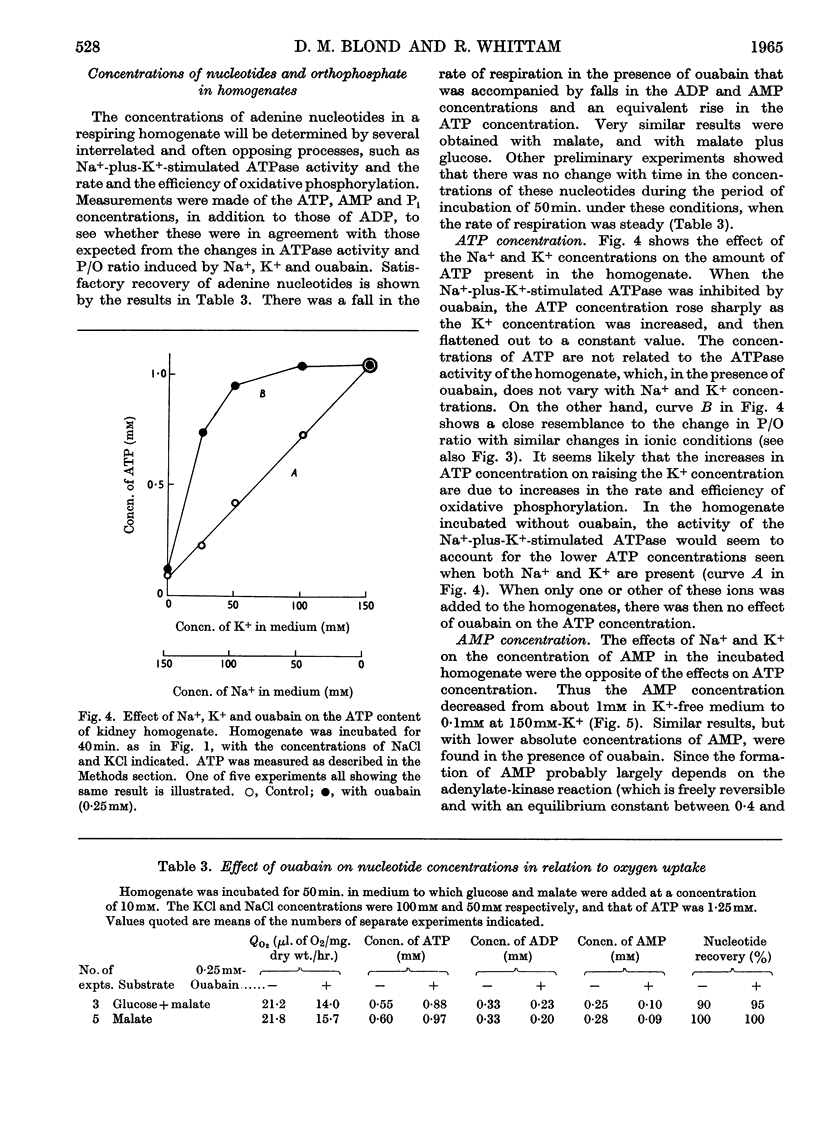
1. A study has been made of the oxygen consumption of kidney homogenates in relation to the ADP concentration as regulated by the cell-membrane adenosine triphosphatase. Stimulation of this enzymic activity by Na+ and K+ caused parallel increases in oxygen consumption and ADP concentration. Similarly, inhibition with ouabain caused a parallel fall. The membrane adenosine triphosphatase concerned in active transport therefore appears to regulate respiration through its control of ADP concentration. 2. The respiration of homogenates and mitochondria was also stimulated by K+ in a way independent of adenosine-triphosphatase activity. It was shown that K+ facilitates oxidative phosphorylation and the respiratory response to ADP. A K+ concentration of 25–50mm was needed for maximum oxidative phosphorylation in the presence of physiological concentration of Na+. Na+ counteracted K+ in the effects on mitochondria. It is concluded that K+ regulates cellular respiration at two structures, one directly in mitochondria, and the second indirectly through control of ADP production at the cell membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMOORE J. E. Exchange of potassium ions across a concentration difference by isolated rat-liver mitochondria. Biochem J. 1960 Sep;76:438–444. doi: 10.1042/bj0760438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGER M. Studies on the distribution of potassium in the rat liver cell and the mechanism of potassium accumulation. Biochim Biophys Acta. 1957 Mar;23(3):504–509. doi: 10.1016/0006-3002(57)90369-4. [DOI] [PubMed] [Google Scholar]
- BOWEN W. J., KERWIN T. D. The kinetics of myokinase. II. Studies of heat denaturation, the effects of salts and the state of equilibrium. Arch Biochem Biophys. 1956 Oct;64(2):278–284. doi: 10.1016/0003-9861(56)90270-3. [DOI] [PubMed] [Google Scholar]
- BURG M. B., ORLOFF J. Effect of strophanthidin on electrolyte content and PAH accumulation of rabbit kidney slices. Am J Physiol. 1962 Mar;202:565–571. doi: 10.1152/ajplegacy.1962.202.3.565. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem. 1955 Nov;217(1):383–393. [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- COOPER C., LEHNINGER A. L. Oxidative phosphorylation by an enzyme complex from extracts of mitochondria. I. The span beta-hydroxybutyrate to oxygen. J Biol Chem. 1956 Mar;219(1):489–506. [PubMed] [Google Scholar]
- Chappell J. B. The oxidation of citrate, isocitrate and cis-aconitate by isolated mitochondria. Biochem J. 1964 Feb;90(2):225–237. doi: 10.1042/bj0900225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUJIMOTO M., NASH F. D., KESSLER R. H. EFFECTS OF CYANIDE, QO, AND DINITROPHENOL ON RENAL SODIUM REABSORPTION AND OXYGEN CONSUMPTION. Am J Physiol. 1964 Jun;206:1327–1332. doi: 10.1152/ajplegacy.1964.206.6.1327. [DOI] [PubMed] [Google Scholar]
- KLINGENBERG M., SCHOLLMEYER P. [On the reversibility of oxidative phosphorylation. Adenosine triphosphate-dependent respiratory control and reduction of diphosphopyridine nucleotide in mitochondria]. Biochem Z. 1960;333:335–350. [PubMed] [Google Scholar]
- LARDY H. A., WELLMAN H. Oxidative phosphorylations; rôle of inorganic phosphate and acceptor systems in control of metabolic rates. J Biol Chem. 1952 Mar;195(1):215–224. [PubMed] [Google Scholar]
- LASSEN N. A., MUNCK O., THAYSEN J. H. Oxygen consumption and sodium reabsorption in the kidney. Acta Physiol Scand. 1961 Apr;51:371–384. doi: 10.1111/j.1748-1716.1961.tb02147.x. [DOI] [PubMed] [Google Scholar]
- MAITRA P. K., GHOSH A., SCHOENER B., CHANCE B. TRANSIENTS IN GLYCOLYTIC METABOLISM FOLLOWING ELECTRICAL ACTIVITY IN ELECTROPHORUS. Biochim Biophys Acta. 1964 Jul 29;88:112–119. doi: 10.1016/0926-6577(64)90159-7. [DOI] [PubMed] [Google Scholar]
- PRESSMAN B. C., LARDY H. A. Further studies on the potassium requirements of mitochondria. Biochim Biophys Acta. 1955 Dec;18(4):482–487. doi: 10.1016/0006-3002(55)90138-4. [DOI] [PubMed] [Google Scholar]
- SIEKEVITZ P., POTTER V. R. Biochemical structure of mitochondria. II. Radioactive labeling of intra-mitochondrial nucleotides during oxidative phosphorylation. J Biol Chem. 1955 Jul;215(1):237–255. [PubMed] [Google Scholar]
- STANBURY S. W., MUDGE G. H. Potassium metabolism of liver mitochondria. Proc Soc Exp Biol Med. 1953 Apr;82(4):675–681. doi: 10.3181/00379727-82-20216. [DOI] [PubMed] [Google Scholar]
- UTTER M. F. Mechanism of inhibition of anaerobic glycolysis of brain by sodium ions. J Biol Chem. 1950 Aug;185(2):499–517. [PubMed] [Google Scholar]
- WHITTAM R. Active cation transport as a pace-maker of respiration. Nature. 1961 Aug 5;191:603–604. doi: 10.1038/191603a0. [DOI] [PubMed] [Google Scholar]
- WHITTAM R., WILLIS J. S. ION MOVEMENTS AND OXYGEN CONSUMPTION IN KIDNEY CORTEX SLICES. J Physiol. 1963 Aug;168:158–177. doi: 10.1113/jphysiol.1963.sp007184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam R., Blond D. M. Respiratory control by an adenosine triphosphatase involved in active transport in brain cortex. Biochem J. 1964 Jul;92(1):147–158. doi: 10.1042/bj0920147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R. Possible role of citrate in the control of epinephrine-stimulated glycogenolysis in rat heart. Nature. 1965 May 1;206(983):473–475. doi: 10.1038/206473a0. [DOI] [PubMed] [Google Scholar]


