Abstract
1. Homogenates of the mucosa of the small intestine of the guinea pig were separated by fractional sedimentation into seven different fractions. The enzymic properties of some of these subcellular fractions were compared with those obtained from the mucosa of the small intestine of the rabbit and cat. 2. The enzymic properties of the low-speed sediment (15000g-min.) were investigated and it was shown that invertase and alkaline ribonuclease were predominantly located in this subcellular fraction, whereas alkaline phosphatase, aryl-amidase, acid phosphatase, acid ribonuclease and phosphoprotein phosphatase, though true constituents of this fraction, occurred to varying degrees in other subcellular structures also. 3. It was shown that the most probable source of the enzymic activities observed in the low-speed sediment was the brush border. Electron micrographs of the purified brush-border fraction indicated vesicles derived from the brush-border membrane. 4. A method is described for the fractionation of mucosal homogenates into a brush border-plus-nuclei fraction, a mitochondrial fraction, a microsomal fraction and a particle-free supernatant. The fractions were shown to be relatively pure, as indicated by the distribution of invertase, DNA, succinate dehydrogenase, glucose 6-phosphatase and 6-phosphogluconate dehydrogenase. 5. Most of the activity of four lysosomal enzymes present in the nuclei-free homogenate was sedimented at 375000g-min., suggesting the occurrence of lysosomal particles in mucosal homogenates. 6. Further fractionation of the microsomal membranes into three fractions is described. The enzymic composition of the membrane fractions is given and discussed in relation to their structure as seen in electron micrographs.
Full text
PDF


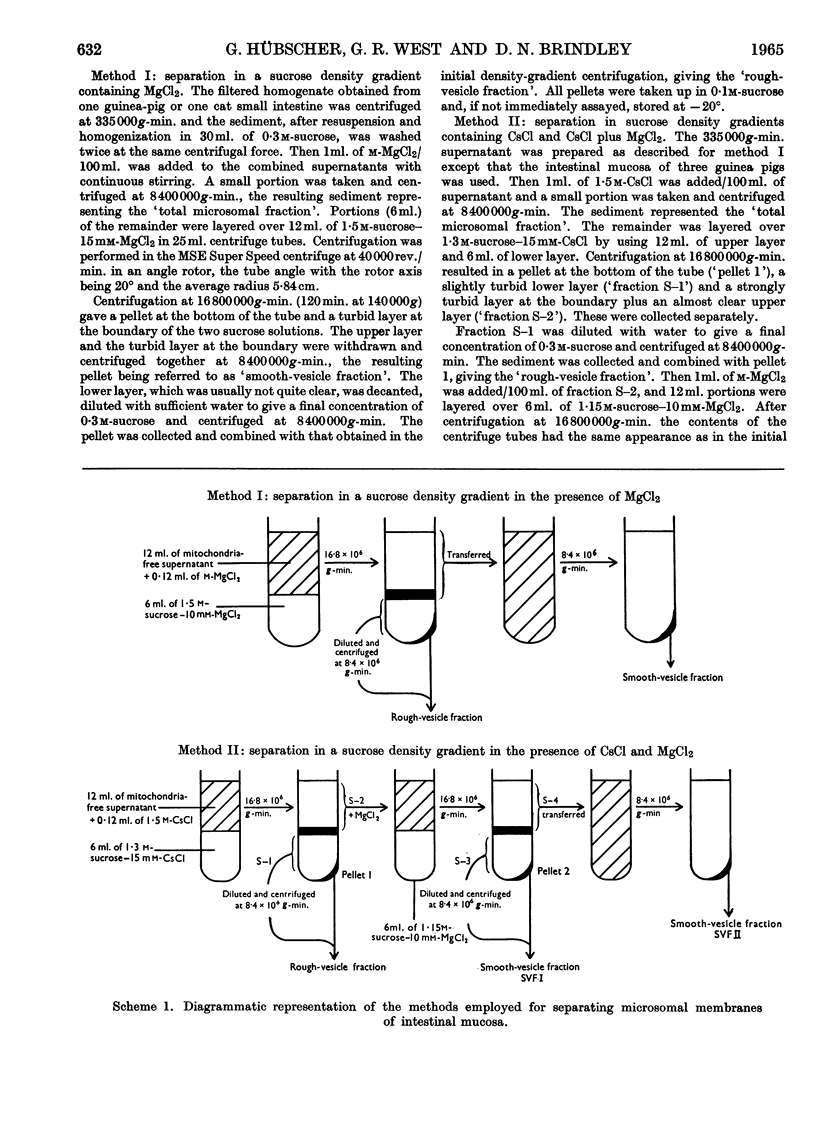







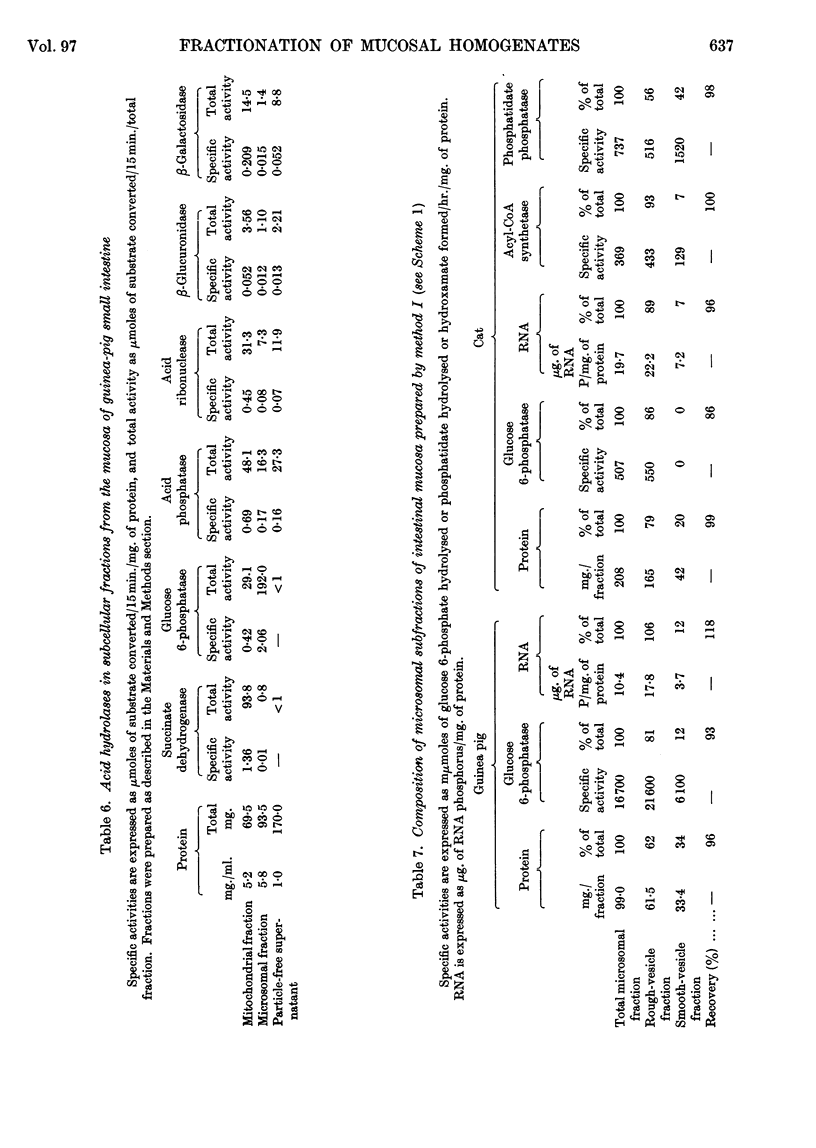





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AILHAUD G., SAMUEL D., DESNUELLE P. [Subcellular localization of acyl-CoA synthetase from intestinal mucosa]. Biochim Biophys Acta. 1963 Jan 8;67:150–152. doi: 10.1016/0006-3002(63)91806-7. [DOI] [PubMed] [Google Scholar]
- ALLARD C., DE LAMIRANDE G., CANTERO A. Enzymes and cytological studies in rat hepatoma transplants, primary liver tumors, and in liver following azo dye feeding or partial hepatectomy. Cancer Res. 1957 Oct;17(9):862–879. [PubMed] [Google Scholar]
- BAKER R. V. Observations on the localization of 5-hydroxytryptamine. J Physiol. 1958 Aug 6;142(3):563–570. doi: 10.1113/jphysiol.1958.sp006036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAUFAY H., DE DUVE C., HOLT S. J., UNDERHAY E. Intracellular localization of esterase in rat liver. J Biophys Biochem Cytol. 1956 Sep 25;2(5):635–637. doi: 10.1083/jcb.2.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRANDES D., ZETTERQVIST H., SHELDON H. Histochemical techniques for electron microscopy: alkaline phosphatase. Nature. 1956 Feb 25;177(4504):382–383. doi: 10.1038/177382a0. [DOI] [PubMed] [Google Scholar]
- BROWN J. L., JOHNSTON J. M. THE MECHANISM OF INTESTINAL UTILIZATION OF MONOGLYCERIDES. Biochim Biophys Acta. 1964 Jun 15;84:264–274. doi: 10.1016/0926-6542(64)90055-1. [DOI] [PubMed] [Google Scholar]
- BUELL G. C., REISER R. Glyceride-glycerol precursors in the intestinal mucosa. J Biol Chem. 1959 Feb;234(2):217–219. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSCH H., DAVIS J. R., ANDERSON D. C. Labeling of histones and other nuclear proteins with L-lysine-U-C14 in tissues of tumor-bearing rats. Cancer Res. 1958 Sep;18(8 Pt 1):916–926. [PubMed] [Google Scholar]
- CARNIE J. A., PORTEOUS J. W. The invertase activity of rabbit small intestine. Biochem J. 1962 Dec;85:450–456. doi: 10.1042/bj0850450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARNIE J. A., PORTEOUS J. W. The solubilization, thermolability, chromatographic purification and intracellular distribution of some glycosidases of rabbit small intestine. Biochem J. 1962 Dec;85:620–629. doi: 10.1042/bj0850620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. Rat-intestinal dextranase. Localization and relation to the other carbohydrases of the digestive tract. Biochem J. 1963 Jan;86:72–76. doi: 10.1042/bj0860072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALLNER G., ORRENIUS S., BERGSTRAND A. Isolation and properties of rough and smooth vesicles from rat liver. J Cell Biol. 1963 Feb;16:426–430. doi: 10.1083/jcb.16.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOELL R. G., KRETCHMER N. Studies of small intestine during development. I. Distribution and activity of beta-galactosidase. Biochim Biophys Acta. 1962 Aug 13;62:353–362. doi: 10.1016/0006-3002(62)90097-5. [DOI] [PubMed] [Google Scholar]
- EPSTEIN B., SHAPIRO B. Lecithinase and lysolecithinase of intestinal mucosa. Biochem J. 1959 Apr;71(4):615–619. doi: 10.1042/bj0710615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEISHER G. A., PANKOW M., WARMKA C. LEUCINE AMINOPEPTIDASE IN HUMAN SERUM: COMPARISON OF HYDROLYSIS OF L-LEUCYLGLYCINE AND L-LEUCYL-BETA-NAPHTHYLAMIDE. Clin Chim Acta. 1964 Mar;9:259–268. doi: 10.1016/0009-8981(64)90105-6. [DOI] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOVER J., GREEN C. Sterol metabolism. 3. The distribution and transport of sterols across the intestinal mucosa of the guinea pig. Biochem J. 1957 Oct;67(2):308–316. doi: 10.1042/bj0670308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBARG J. A., RUTENBURG A. M. The colorimetric determination of leucine aminopeptidase in urine and serum of normal subjects and patients with cancer and other diseases. Cancer. 1958 Mar-Apr;11(2):283–291. doi: 10.1002/1097-0142(195803/04)11:2<283::aid-cncr2820110209>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- HOLT J. H., MILLER D. The localization of phosphomonoesterase and aminopeptidase in brush borders isolated from intestinal epithelial cells. Biochim Biophys Acta. 1962 Apr 9;58:239–243. doi: 10.1016/0006-3002(62)91004-1. [DOI] [PubMed] [Google Scholar]
- HSU L., TAPPEL A. L. LYSOSOMAL ENZYMES OF RAT INTESTINAL MUCOSA. J Cell Biol. 1964 Nov;23:233–240. doi: 10.1083/jcb.23.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUEBSCHER G., WEST G. R. SPECIFIC ASSAYS OF SOME PHOSPHATASES IN SUBCELLULAR FRACTIONS OF SMALL INTESTINAL MUCOSA. Nature. 1965 Feb 20;205:799–800. doi: 10.1038/205799a0. [DOI] [PubMed] [Google Scholar]
- JOHNSON F. R., KUGLER J. H. The distribution of alkaline phosphatase in the mucosal cells of the small intestine of the rat, cat and dog. J Anat. 1953 Jul;87(3):247–256. [PMC free article] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER D., CRANE R. K. The digestive function of the epithelium of the small intestine. I. An intracellular locus of disaccharide and sugar phosphate ester hydrolysis. Biochim Biophys Acta. 1961 Sep 16;52:281–293. doi: 10.1016/0006-3002(61)90677-1. [DOI] [PubMed] [Google Scholar]
- MORTON R. K. The purification of aklaline phosphatases of animal tissues. Biochem J. 1954 Aug;57(4):595–603. doi: 10.1042/bj0570595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Ruge W. Vergleichende Untersuchungen über die histotopochemischen Qualitäten der Tetrazolsalze MTT, Nitro-BT und TNBT in der Enzymhistochemie. Histochemie. 1965 Jan 12;4(5):438–445. doi: 10.1007/BF00306253. [DOI] [PubMed] [Google Scholar]
- NACHLAS M. M., MONIS B., ROSENBATT D., SELIGMAN A. M. Improvement in the histochemical localization of leucine aminopeptidase with a new substrate, L-leucyl-4-methoxy-2-naphthylamide. J Biophys Biochem Cytol. 1960 Apr;7:261–264. doi: 10.1083/jcb.7.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAIGEN K. The properties of particulate phosphoprotein phosphatase. J Biol Chem. 1958 Aug;233(2):388–394. [PubMed] [Google Scholar]
- PATTERSON E. K., HSIAO S. H., KEPPEL A. STUDIES ON DIPEPTIDASES AND AMINOPEPTIDASES. I. DISTINCTION BETWEEN LEUCINE AMINOPEPTIDASE AND ENZYMES THAT HYDROLYZE L-LEUCYL-BETA-NAPHTHYLAMIDE. J Biol Chem. 1963 Nov;238:3611–3620. [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINSON G. B. The distribution of peptidases in subcellular fractions from the mucosa of the small intestine of the rat. Biochem J. 1963 Jul;88:162–168. doi: 10.1042/bj0880162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT G., BESSMAN M. J., THANNHAUSER S. J. Enzymic hydrolysis of cephalin in rat intestinal mucosa. Biochim Biophys Acta. 1957 Jan;23(1):127–138. doi: 10.1016/0006-3002(57)90294-9. [DOI] [PubMed] [Google Scholar]
- SENIOR J. R., ISSELBACHER K. J. Direct esterification of monoglycerides with palmityl coenzyme A by intestinal epithelial subcellular fractions. J Biol Chem. 1962 May;237:1454–1459. [PubMed] [Google Scholar]
- SHERRATT H. S., HUBSCHER G. Properties of mitochondrial preparations from the small-intestinal mucosa of the guinea-pig. Biochim Biophys Acta. 1963 Feb 5;69:403–405. doi: 10.1016/0006-3002(63)91274-5. [DOI] [PubMed] [Google Scholar]
- SYLVEN B., SNELLMAN O. STUDIES ON THE HISTOCHEMICAL "LEUCINE AMINOPEPTIDASE" REACTION. 3. ON THE DIFFERENT LNA-SPLITTING ENZYMES FROM SPLEEN. Z Zellforch Microsk Anat Histochem. 1964 Apr 10;78:484–486. doi: 10.1007/BF00736629. [DOI] [PubMed] [Google Scholar]
- TRIANTAPHYLLOPOULOS E., TUBA J. Studies on the distribution and kinetics of the alkaline phosphatase of rat small intestine. Can J Biochem Physiol. 1959 May;37(5):699–709. [PubMed] [Google Scholar]





