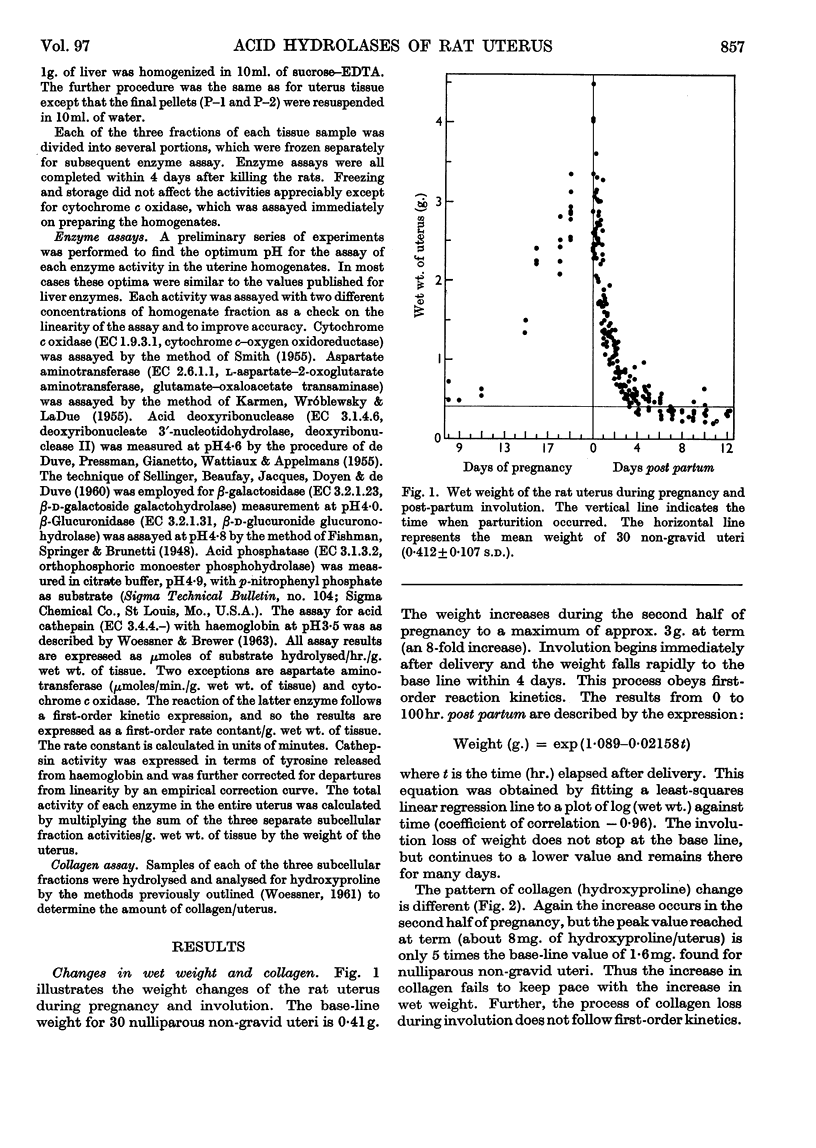
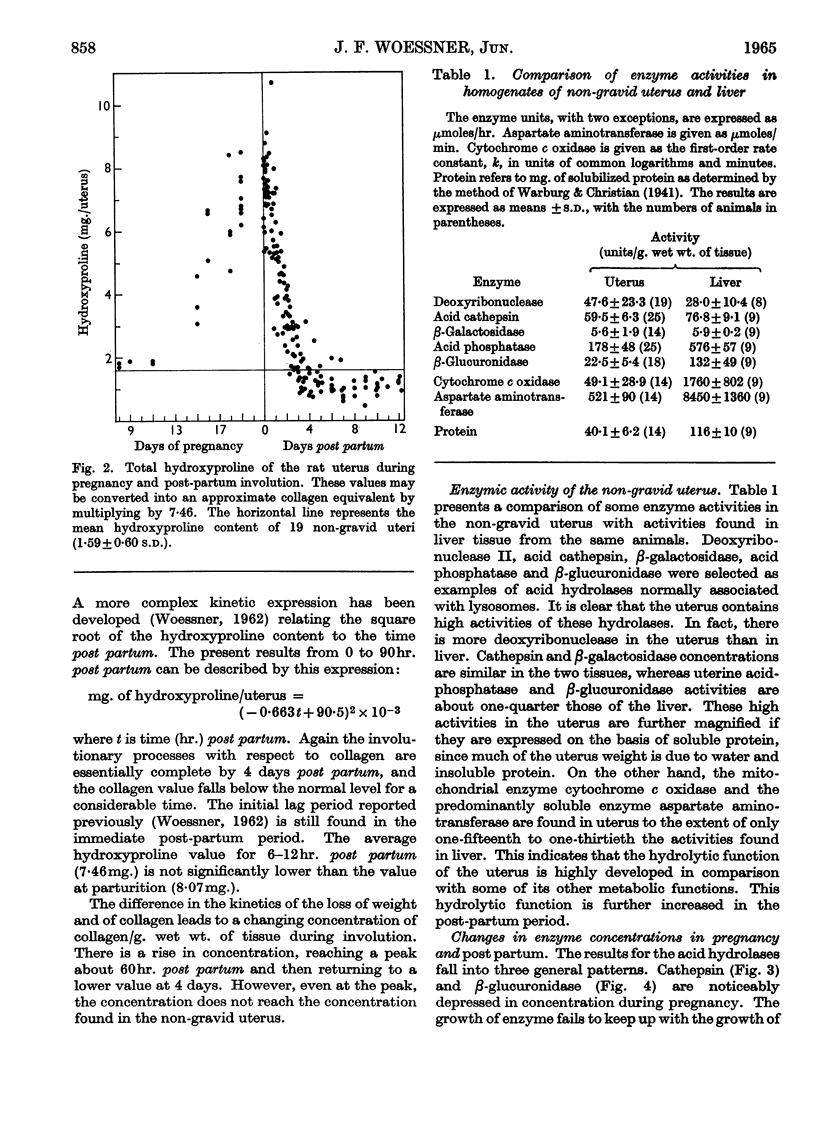
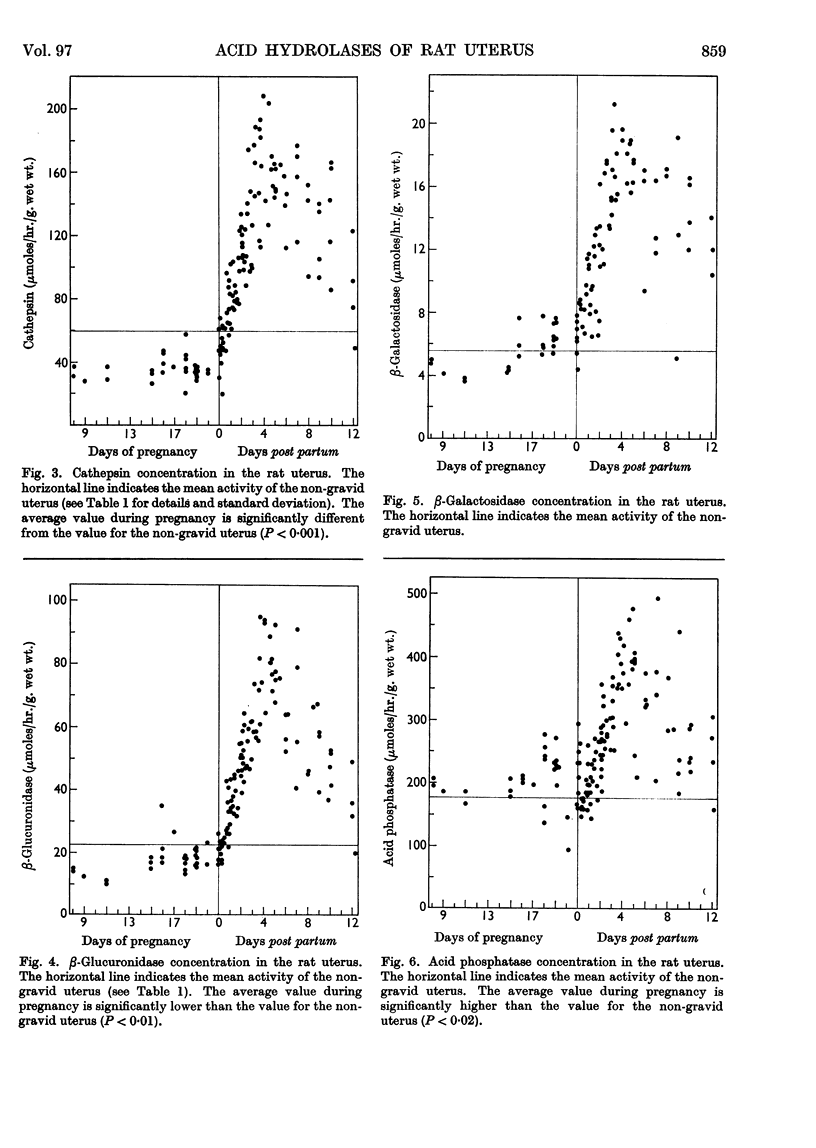
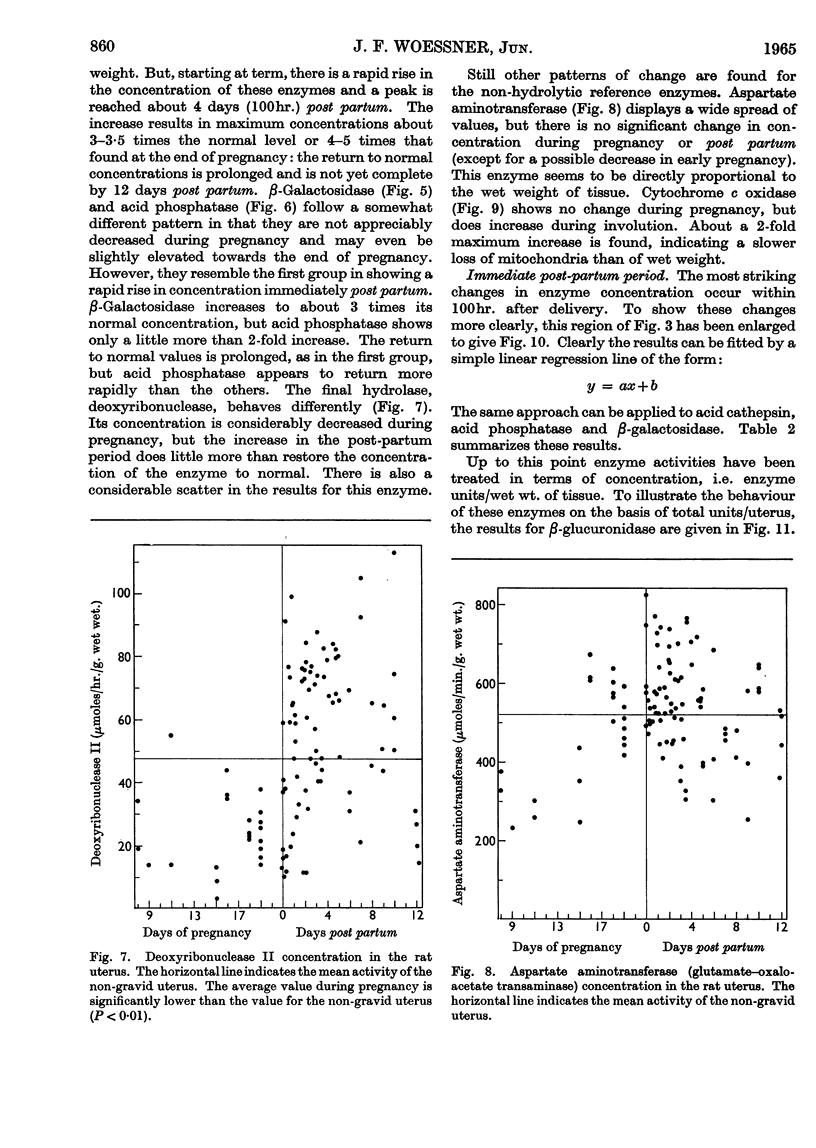
Abstract
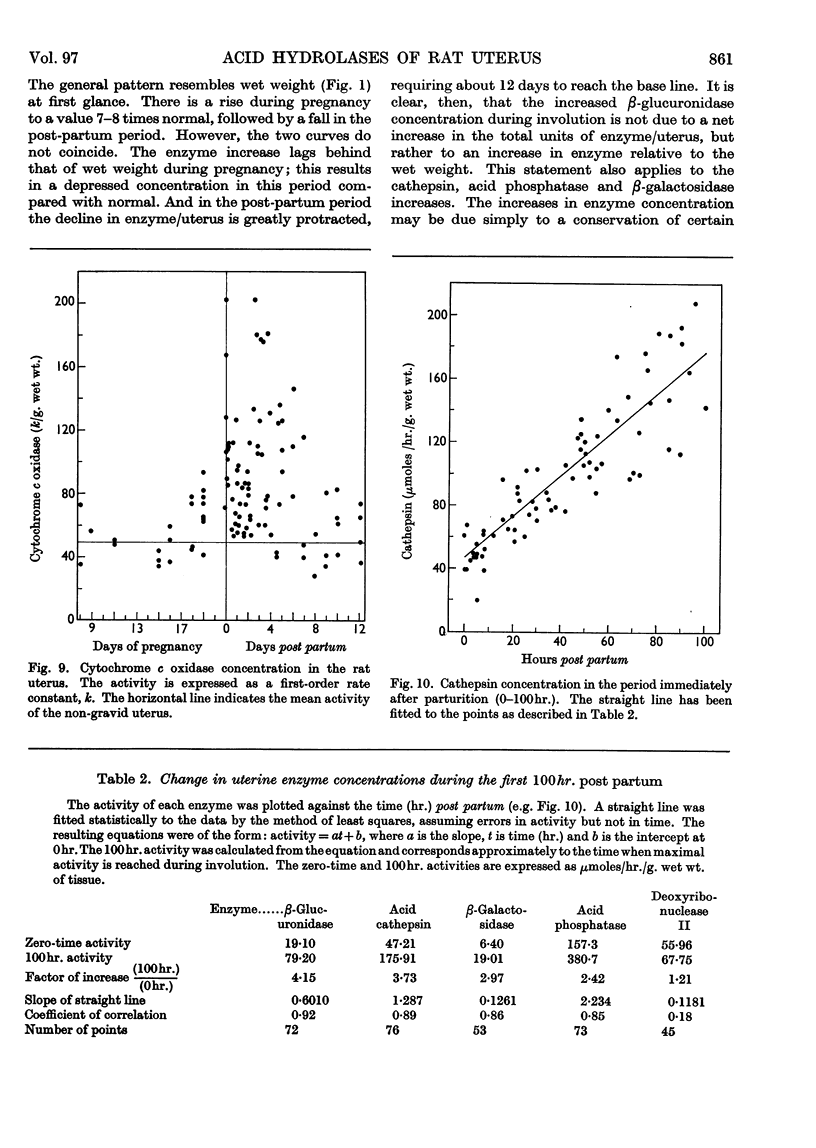
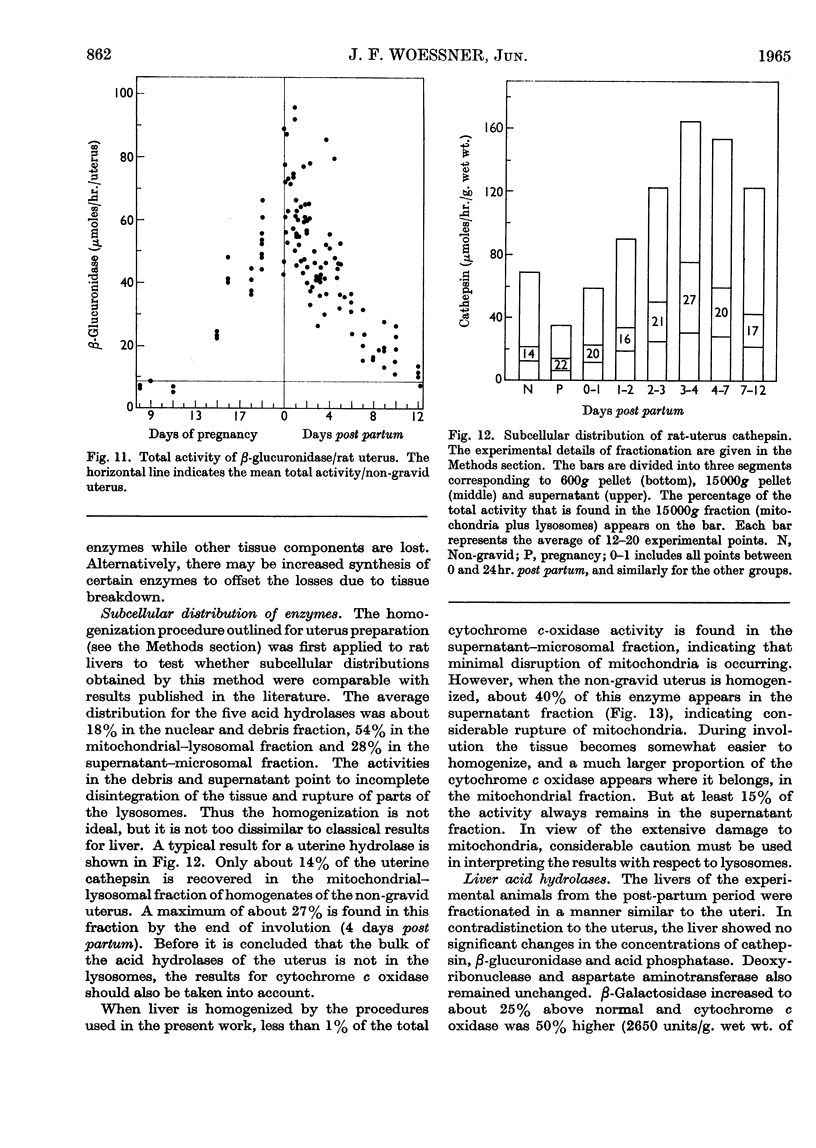
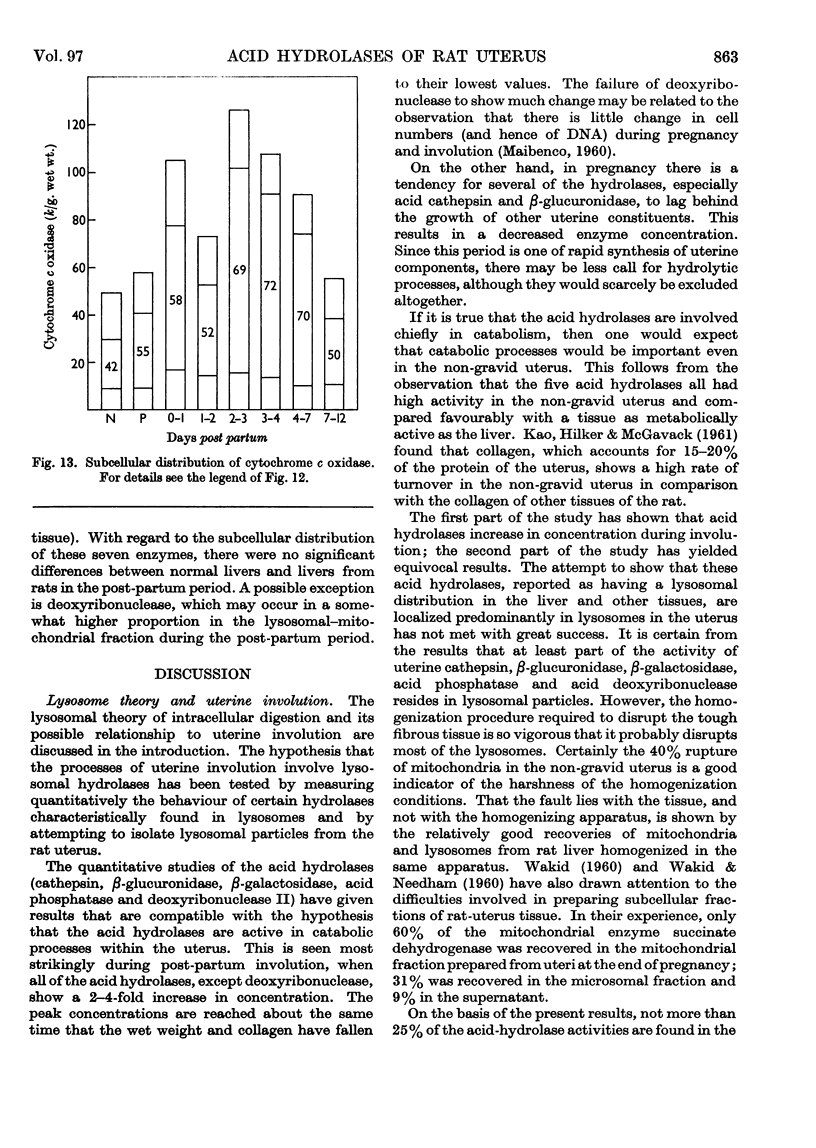
1. The rat uterus contains acid cathepsin, β-glucuronidase, β-galactosidase, acid phosphatase and deoxyribonuclease II at concentrations comparable with those found in liver. Two non-hydrolytic uterine enzymes, cytochrome c oxidase and aspartate aminotransferase, display only 2–6% of the activity found in liver. 2. The concentrations of acid cathepsin and β-glucuronidase are significantly decreased in pregnancy and increase 3–4-fold during post-partum involution. 3. The concentrations of β-galactosidase and acid phosphatase are not decreased in pregnancy and increase only 2–3-fold during involution. 4. The concentrations of these four acid hydrolases increase linearly during the first 4 days post partum and reach their peak values at the same time that wet weight and collagen content fall to their lowest point. 5. The concentration of deoxyribonuclease is depressed in pregnancy but does not rise above normal in the post-partum period. 6. Only a small proportion of each hydrolytic activity can be isolated in the mitochondrial–lysosomal fraction of sucrose homogenates of the rat uterus. This proportion increases during involution. However, the extensive mitochondrial rupture occurring during homogenization indicates that the technique is probably too harsh to obtain a true measure of the proportion of lysosomes present in the intact tissue. 7. There are no significant changes in either the concentration or subcellular distribution of the five acid hydrolases in the livers of the experimental rats during pregnancy or involution. In each case the largest proportion of the activity is found in the mitochondrial–lysosomal fraction of liver homogenates. 8. The results are interpreted in terms of the lysosomal theory of intracellular digestion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRACHET J., DECROLY-BRIERS M., HOYEZ J. Contribution à l'étude des lysosomes au cours du développement embryonnaire. Bull Soc Chim Biol (Paris) 1958;40(12):2039–2048. [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. I. COMPARATIVE ENZYMOLOGY, ISOLATION, AND PROPERTIES. J Exp Med. 1963 Dec 1;118:991–1008. doi: 10.1084/jem.118.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A., WIENER E. THE PARTICULATE HYDROLASES OF MACROPHAGES. II. BIOCHEMICAL AND MORPHOLOGICAL RESPONSE TO PARTICLE INGESTION. J Exp Med. 1963 Dec 1;118:1009–1020. doi: 10.1084/jem.118.6.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE DUVE C., WATTIAUX R., BAUDHUIN P. Distribution of enzymes between subcellular fractions in animal tissues. Adv Enzymol Relat Subj Biochem. 1962;24:291–358. doi: 10.1002/9780470124888.ch6. [DOI] [PubMed] [Google Scholar]
- DEDUVE C. FROM CYTASES TO LYSOSOMES. Fed Proc. 1964 Sep-Oct;23:1045–1049. [PubMed] [Google Scholar]
- Eeckhout Y. Le comportement des hydrolases acides de la queue du têtard de Xenopus laevis pendant la métamorphose. Arch Int Physiol Biochim. 1964 Mar;72(2):316–318. [PubMed] [Google Scholar]
- FRANKLAND D. M., WYNN C. H. The degradation of acidsoluble collagen by rat-liver preparations. Biochem J. 1962 Nov;85:276–282. doi: 10.1042/bj0850276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J., LAPIERE C. M. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARKNESS R. D., MORALEE B. E. The time-course and route of loss of collagen from the rat's uterus during post-partum involution. J Physiol. 1956 Jun 28;132(3):502–508. doi: 10.1113/jphysiol.1956.sp005543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI M. DISTRIBUTION OF BETA-GLUCURONIDASE ACTIVITY IN RAT TISSUES EMPLOYING THE NAPHTHOL AS-BI GLUCURONIDE HEXAZONIUM PARAROSANILIN METHOD. J Histochem Cytochem. 1964 Sep;12:659–669. doi: 10.1177/12.9.659. [DOI] [PubMed] [Google Scholar]
- KAO K. Y., HILKER D. M., MCGAVACK T. H. Connective tissue. IV. Synthesis and turnover of proteins in tissues of rats. Proc Soc Exp Biol Med. 1961 Jan;106:121–124. doi: 10.3181/00379727-106-26257. [DOI] [PubMed] [Google Scholar]
- KARMEN A., WROBLEWSKI F., LADUE J. S. Transaminase activity in human blood. J Clin Invest. 1955 Jan;34(1):126–131. doi: 10.1172/JCI103055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOBEL B. L., DEANE H. W. Enzymic activity associated with postpartum involution of the uterus and with its regression after hormone withdrawal in the rat. Endocrinology. 1962 Apr;70:567–578. doi: 10.1210/endo-70-4-567. [DOI] [PubMed] [Google Scholar]
- MAIBENCO H. G. Connective tissue changes in postpartum uterine involution in the albino rat. Anat Rec. 1960 Jan;136:59–71. doi: 10.1002/ar.1091360106. [DOI] [PubMed] [Google Scholar]
- MORRIONE T. G., SEIFTER S. Alteration in the collagen content of the human uterus during pregnancy and post partum involution. J Exp Med. 1962 Feb 1;115:357–365. doi: 10.1084/jem.115.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRESS E. M., PORTER R. R., CEBRA J. The isolation and properties of a proteolytic enzyme, cathepsin D, from bovine spleen. Biochem J. 1960 Mar;74:501–514. doi: 10.1042/bj0740501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SELLINGER O. Z., BEAUFAY H., JACQUES P., DOYEN A., DE DUVE C. Tissue fractionation studies. 15. Intracellular distribution and properties of beta-N-acetylglucosaminidase and beta-galactosidase in rat liver. Biochem J. 1960 Mar;74:450–456. doi: 10.1042/bj0740450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKID N. W., NEEDHAM D. M. Cytoplasmic fractions of rat myometrium. 2. Localization of some cellular constituents in the pregnant and ovariectomized states. Biochem J. 1960 Jul;76:95–102. doi: 10.1042/bj0760095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER R. On the biological function of cathepsin in tail tissue of Xenopus larvae. Experientia. 1957 Apr 15;13(4):153–155. doi: 10.1007/BF02158143. [DOI] [PubMed] [Google Scholar]
- WOESSNER J. F., Jr Catabolism of collagen and non-collagen protein in the rat uterus during post-partum involution. Biochem J. 1962 May;83:304–314. doi: 10.1042/bj0830304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakid N. W. Cytoplasmic fractions of rat myometrium. 1. General description and some enzymic properties. Biochem J. 1960 Jul;76(1):88–95. doi: 10.1042/bj0760088. [DOI] [PMC free article] [PubMed] [Google Scholar]


