Abstract
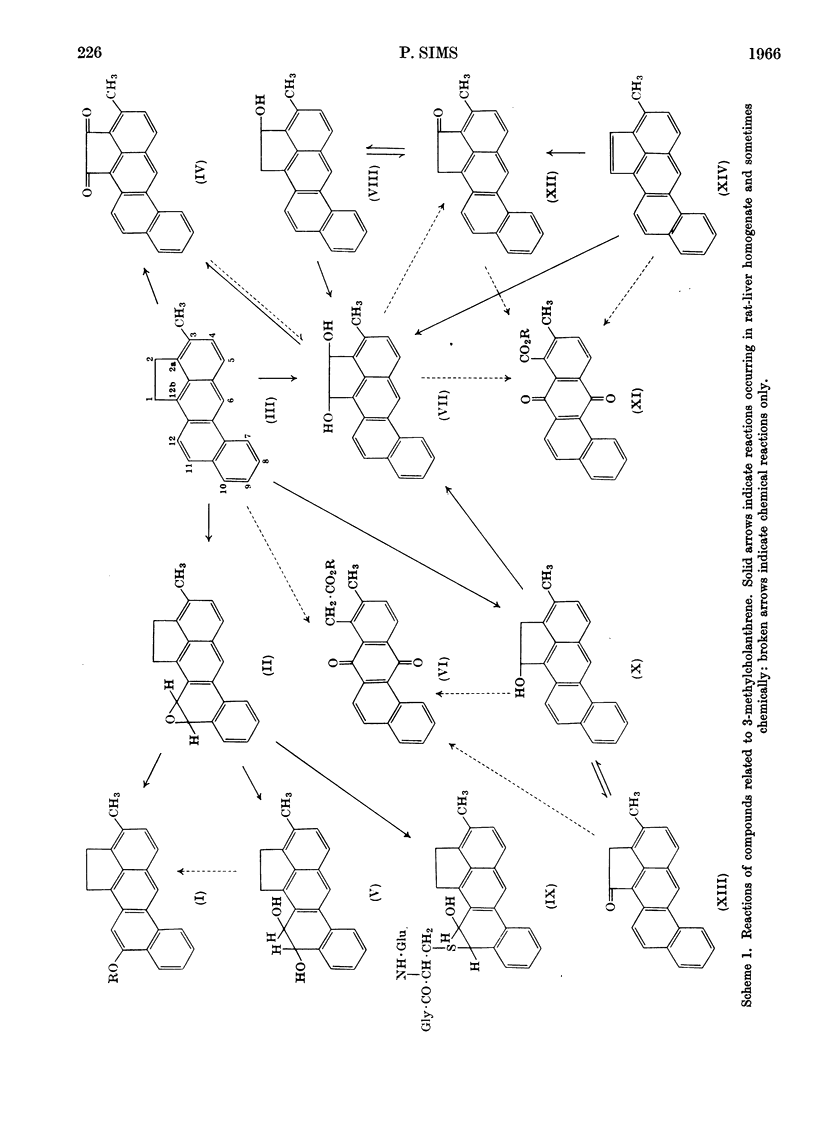
1. A chromatographic investigation of the products of the metabolism of 3-methylcholanthrene by rat-liver homogenates showed the formation of compounds with the properties of 1- and 2-hydroxy-3-methylcholanthrene, cis- and trans-1,2-dihydroxy-3-methylcholanthrene and 11,12-dihydro-11,12-dihydroxy-3-methylcholanthrene. A glutathione conjugate that is probably S-(11,12-dihydro-12-hydroxy-3-methyl-11-cholanthrenyl)glutathione was also detected. 3-Methylcholanthrene-1- and -2-one and -1,2-quinone were also present, but these products may have arisen by the chemical oxidation of the corresponding hydroxy compounds. 2. Other metabolic products were tentatively identified as 9- and 10-hydroxy-3-methylcholanthrene, 4,5-dihydro-4,5-dihydroxy-3-methylcholanthrene and 3-hydroxymethylcholanthrene. 3. 1- and 2-Hydroxy-3-methylcholanthrene were converted by homogenates into the related ketones and into products with the properties of cis- and trans-1,2-dihydroxy-3-methylcholanthrene: 3-methylcholanthren-1- and -2-one were converted into their related hydroxy compounds and into the isomeric 1,2-dihydroxy compounds. The isomeric 1,2-dihydroxy compounds were each partly converted into the other isomer by these homogenates. All the above substrates also yielded products that appeared to be derivatives of 3-hydroxymethylcholanthrene. 4. 3-Methylcholanthrylene was converted by rat-liver homogenates into products with the properties of trans-1,2-dihydroxy-3-methylcholanthrene, 2-hydroxy-3-methylcholanthrene and 3-methylcholanthren-2-one. A small amount of the cis-1,2-dihydroxy compound was also formed, together with a glutathione conjugate that is possibly S-(2-hydroxy-3-methyl-1-cholanthrenyl)glutathione or its positional isomer. 5. An unidentified product was detected in the metabolism of 3-methylcholanthrene, the monohydroxy compounds, the ketones and the dihydroxy compounds, the formation of which appeared to involve metabolism at the 1,2-bond. 6. 11,12-Epoxy-11,12-dihydro-3-methylcholanthrene was converted by rat-liver homogenates into products with the properties of 11-hydroxy-3-methylcholanthrene (or, less likely, the 12-isomer), 11,12-dihydro-11,12-dihydroxy-3-methylcholanthrene and the glutathione conjugate described above. Products with the properties of these compounds were formed when the epoxide was allowed to react with glutathione in an aqueous medium. 7. Mouse-liver homogenate converted 3-methylcholanthrene into products with the chromatographic properties of 1- and 2-hydroxy-3-methylcholanthrene, cis- and trans-1,2-dihydroxy-3-methylcholanthrene, 11,12-dihydro-11,12-dihydroxy-3-methylcholanthrene, 3-methylcholanthrene-1- and -2-one and -1,2-quinone and the unidentified hydroxy-3-methylcholanthrenes. 8. The syntheses of cis- and trans-1,2-dihydroxy-3-methylcholanthrene, 3-methylcholanthren-2-one, 2-hydroxy-3-methylcholanthrene, 3-methylcholanthrylene, 11,12-epoxy-11,12-dihydro-3-methylcholanthrene and trans-11,12-dihydro-11,12-dihydroxy-3-methylcholanthrene are described.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOYLAND E., SIMS P. METABOLISM OF POLYCYCLIC COMPOUNDS. THE METABOLISM OF 7,12-DIMETHYLBENZ(ALPHA)ANTHRACENE BY RAT-LIVER HOMOGENATES. Biochem J. 1965 Jun;95:780–787. doi: 10.1042/bj0950780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKS C. J., YOUNG L. Biochemical studies of toxic agents. 9. The metabolic conversion of indene into cis- and trans- indane-1: 2-diol. Biochem J. 1956 Jun;63(2):264–269. doi: 10.1042/bj0630264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyland E., Sims P. Metabolism of polycyclic compounds. 24. The metabolism of benz[alpha]anthracene. Biochem J. 1964 Jun;91(3):493–506. doi: 10.1042/bj0910493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyland E., Sims P. The metabolism of benz[a]anthracene and dibenz[a,h]anthracene and their 5,6-epoxy-5,6-dihydro derivatives by rat-liver homogenates. Biochem J. 1965 Oct;97(1):7–16. doi: 10.1042/bj0970007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARPER K. H. The polycyclic hydrocarbons: metabolism, cellular binding and carcinogenesis. Br J Cancer. 1959 Dec;13:732–745. doi: 10.1038/bjc.1959.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOPKINS R. P., BROOKS C. J., YOUNG L. Biochemical studies of toxic agents. 13. The metabolism of acenaphthylene. Biochem J. 1962 Mar;82:457–466. doi: 10.1042/bj0820457. [DOI] [PMC free article] [PubMed] [Google Scholar]


