Abstract
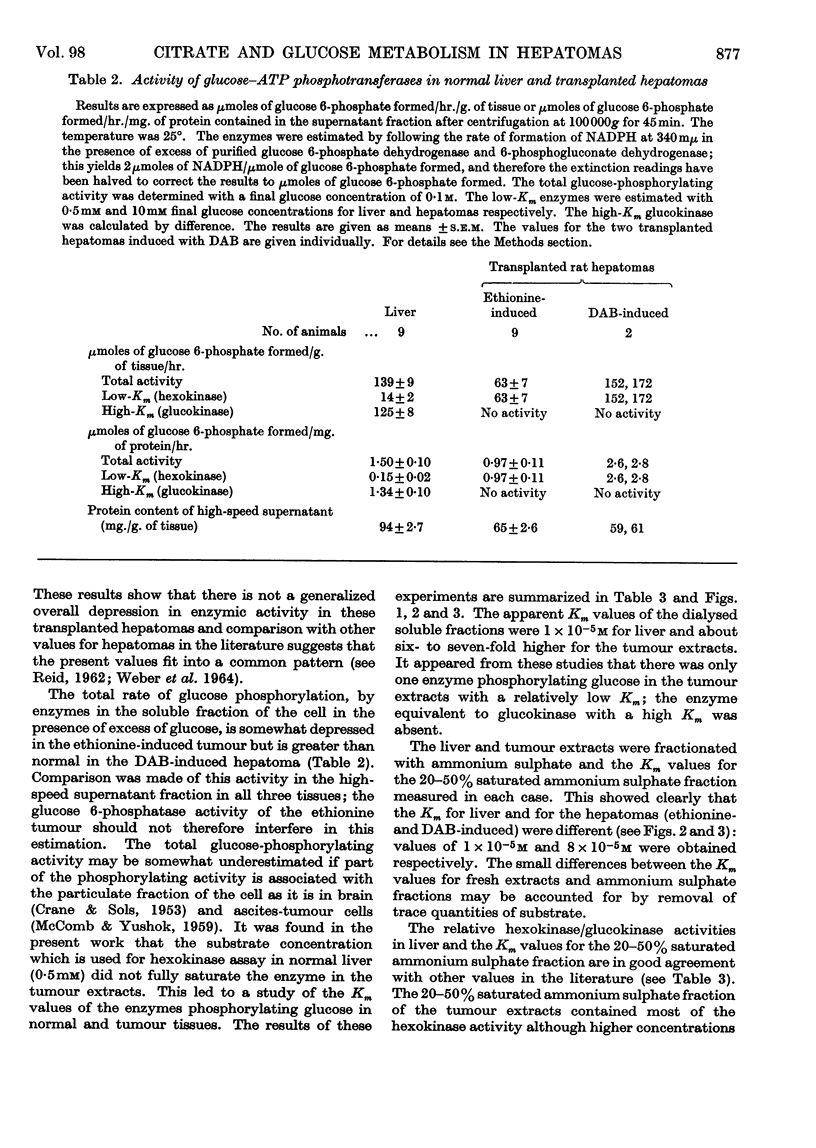
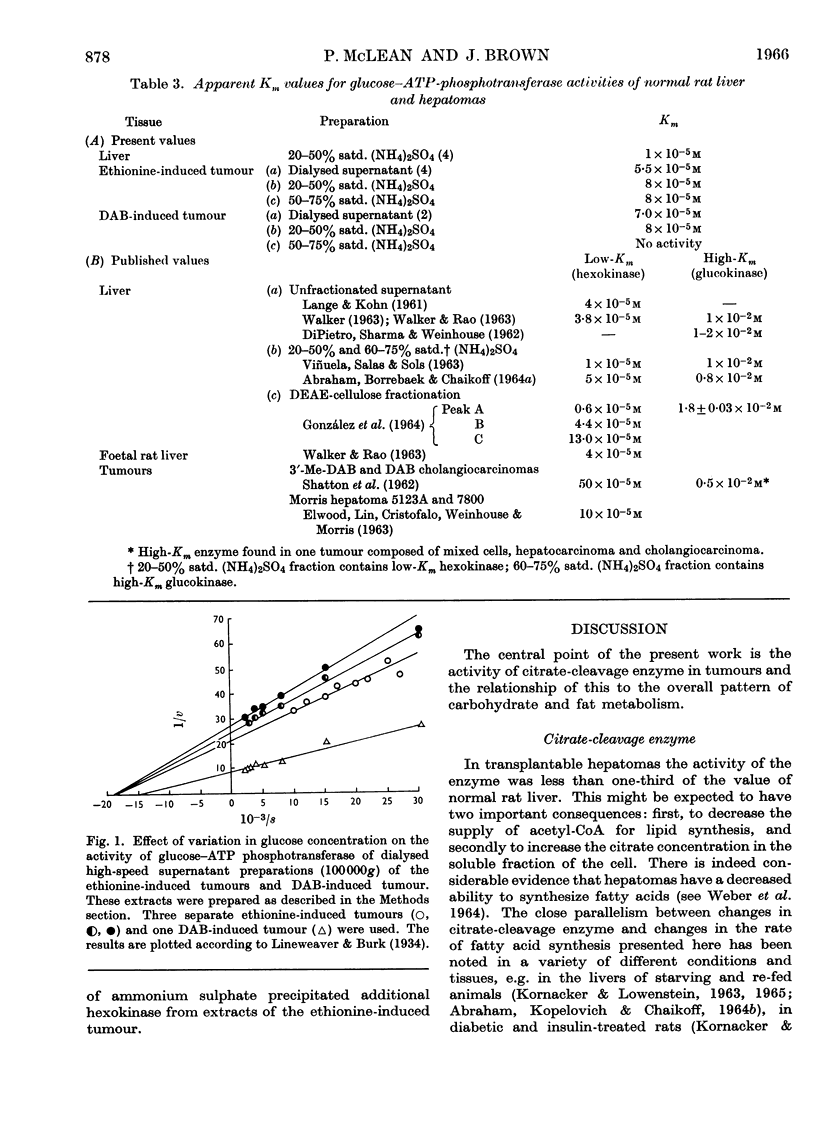
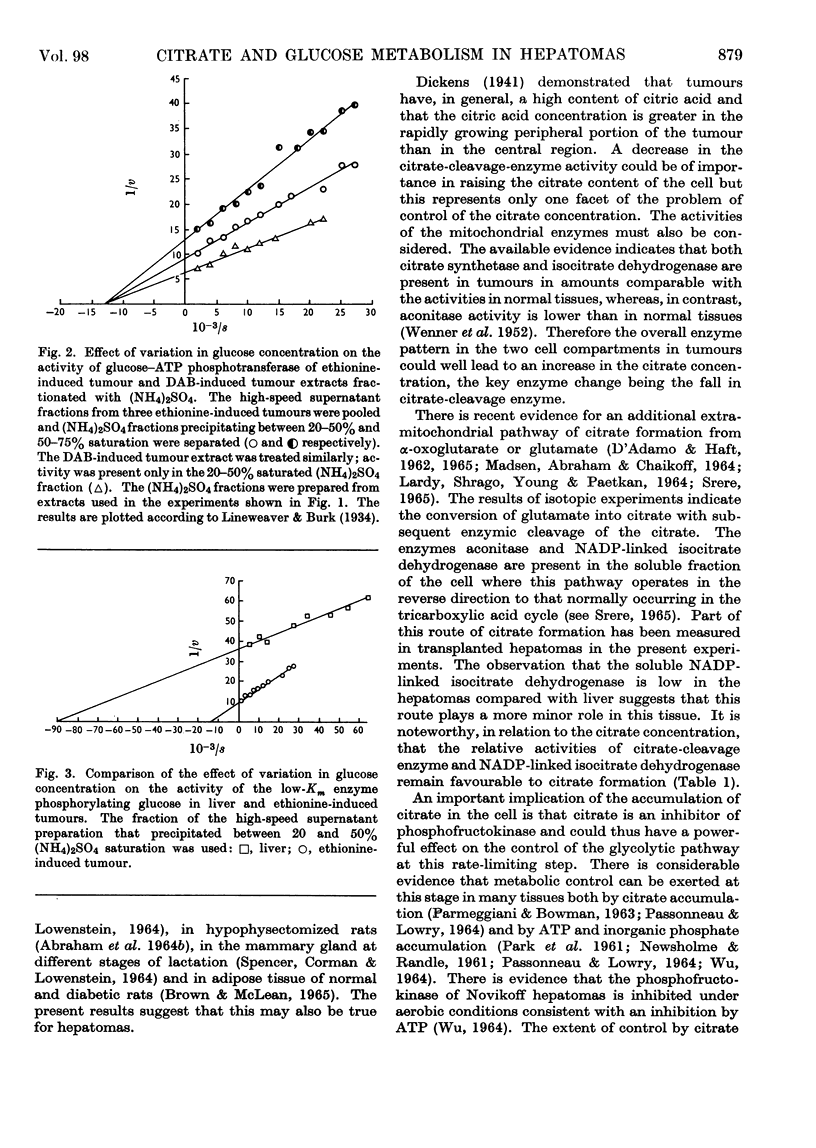
1. Certain enzymes concerned with citrate and glucose metabolism have been measured in two transplanted rat hepatomas, one induced with ethionine (minimal deviation type) and one induced with dimethylaminoazobenzene. In these hepatomas both citrate-cleavage enzyme and NADP-linked isocitrate dehydrogenase in the soluble fraction of the cell were approximately one-third of the values for normal rat liver. These changes have been discussed in relation to the increased citric acid content of tumours and depressed rate of fatty acid synthesis. 2. The glucose–ATP-phosphotransferase activity was below normal liver values in the ethionine-induced tumour but greater than normal in the dimethylaminoazobenzene-induced hepatoma. The apparent Km values for the glucose–ATP phosphotransferases of these hepatomas were approx. 8×10−5m; no evidence was found for an enzyme with a high Km for glucose equivalent to liver glucokinase. 3. Of the enzymes of the pentose phosphate pathway, glucose 6-phosphate-dehydrogenase activity was three to five times as great whereas 6-phosphogluconate-dehydrogenase activity was the same or lower than normal liver in the ethionine-and dimethylaminoazobenzene-induced tumours respectively.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM S., KOPELOVICH L., CHAIKOFF I. L. DIETARY AND HORMONAL REGULATION OF THE HEPATIC CITRATE-CLEAVAGE ENZYME. Biochim Biophys Acta. 1964 Oct 9;93:185–187. doi: 10.1016/0304-4165(64)90278-8. [DOI] [PubMed] [Google Scholar]
- BOTTOMLEY R. H., PITOT H. C., POTTER V. R., MORRIS H. P. Metabolic adaptations in rat hepatomas. V. Reciprocal relationship between threonine dehydrase and glucose-6-phosphate dehydrogenase. Cancer Res. 1963 Mar;23:400–409. [PubMed] [Google Scholar]
- Brown J., McLean P. Effect of alloxan-diabetes on the activity of citrate cleavage enzyme in adipose tissue. Nature. 1965 Jul 24;207(995):407–408. doi: 10.1038/207407a0. [DOI] [PubMed] [Google Scholar]
- CRANE R. K., SOLS A. The association of hexokinase with particulate fractions of brain and other tissue homogenates. J Biol Chem. 1953 Jul;203(1):273–292. [PubMed] [Google Scholar]
- D ADAMO A. F., Jr, HAFT D. E. AN ALTERNATE PATHWAY OF ALPHA-KETOGLUTARATE CATABOLISM IN THE ISOLATED, PERFUSED RAT LIVER. I. STUDIES WITH DL-GLUTAMATE-2- AND -5-14C. J Biol Chem. 1965 Feb;240:613–617. [PubMed] [Google Scholar]
- DIPIETRO D. L., SHARMA C., WEINHOUSE S. Studies on glucose phosphorylation in rat liver. Biochemistry. 1962 May 25;1:455–462. doi: 10.1021/bi00909a014. [DOI] [PubMed] [Google Scholar]
- Dickens F. The citric acid content of animal tissues, with reference to its occurrence in bone and tumour. Biochem J. 1941 Sep;35(8-9):1011–1023. doi: 10.1042/bj0351011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELWOOD J. C., LIN Y. C., CRISTOFALO V. J., WEINHOUSE S., MORRIS H. P. GLUCOSE UTILIZATION IN HOMOGENATES OF THE MORRIS HEPATOMA 5123 AND RELATED TUMORS. Cancer Res. 1963 Jul;23:906–913. [PubMed] [Google Scholar]
- GLOCK G. E., MCLEAN P. Levels of oxidized and reduced diphosphopyridine nucleotide and triphosphopyridine nucleotide in tumours. Biochem J. 1957 Feb;65(2):413–416. doi: 10.1042/bj0650413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLOCK G. E., McLEAN P. Further studies on the properties and assay of glucose 6-phosphate dehydrogenase and 6-phosphogluconate dehydrogenase of rat liver. Biochem J. 1953 Oct;55(3):400–408. doi: 10.1042/bj0550400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENBAUM A. L., CLARK J. B., MCLEAN P. THE ESTIMATION OF THE OXIDIZED AND REDUCED FORMS OF THE NICOTINAMIDE NUCLEOTIDES. Biochem J. 1965 Apr;95:161–166. doi: 10.1042/bj0950161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González C., Ureta T., Sánchez R., Niemeyer H. Multiple molecular forms of ATP:hexose 6-phosphotransferase from rat liver. Biochem Biophys Res Commun. 1964 Jul 1;16(4):347–352. doi: 10.1016/0006-291x(64)90038-5. [DOI] [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. M. CITRATE AND THE CONVERSION OF CARBOHYDRATE INTO FAT. THE ACTIVITIES OF CITRATE-CLEAVAGE ENZYME AND ACETATE THIOKINASE IN LIVERS OF STARVED AND RE-FED RATS. Biochem J. 1965 Jan;94:209–215. doi: 10.1042/bj0940209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNACKER M. S., LOWENSTEIN J. W. CITRATE CLEAVAGE AND ACETATE ACTIVATION IN LIVERS OF NORMAL AND DIABETIC RATS. Biochim Biophys Acta. 1964 Aug 5;84:490–492. doi: 10.1016/0926-6542(64)90021-6. [DOI] [PubMed] [Google Scholar]
- Kaplan N. O., Goodfriend T. L. Role of the two types of lactic dehydrogenase. Adv Enzyme Regul. 1964;2:203–212. doi: 10.1016/s0065-2571(64)80014-5. [DOI] [PubMed] [Google Scholar]
- LANGE C. F., Jr, KOHN P. Substrate specificity of hexokinases. J Biol Chem. 1961 Jan;236:1–5. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lardy H. A., Shrago E., Young J. W., Paetkau V. The Pathway of Gluconeogenesis in Liver. Science. 1964 May 1;144(3618):564–564. doi: 10.1126/science.144.3618.564-b. [DOI] [PubMed] [Google Scholar]
- MADSEN J., ABRAHAM S., CHAIKOFF I. L. THE CONVERSION OF GLUTAMATE CARBON TO FATTY ACID CARBON VIA CITRATE. I. THE INFLUENCE OF GLUCOSE IN LACTATING RAT MAMMARY GLAND SLICES. J Biol Chem. 1964 May;239:1305–1309. [PubMed] [Google Scholar]
- MATTHES K. J., ABRAHAM S., CHAIKOFF I. L. Hydrogen transfer in fatty acid synthesis by rat liver and mammarygland cell-free preparations studied with tritum-labeled pyridine nucleotides and glucose. Biochim Biophys Acta. 1963 Jun 18;70:242–259. doi: 10.1016/0006-3002(63)90749-2. [DOI] [PubMed] [Google Scholar]
- MEDES G., FRIEDMANN B., WEINHOUSE S. Fatty acid metabolism. VIII. Acetate metabolism in vitro during hepatocarcinogenesis by p-dimethylaminoazobenzene. Cancer Res. 1956 Jan;16(1):57–62. [PubMed] [Google Scholar]
- McLean P., Reid E., Gurney M. W. Effect of azo-dye carcinogenesis on enzymes concerned with urea synthesis in rat liver. Biochem J. 1964 Jun;91(3):464–473. doi: 10.1042/bj0910464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWSHOLME E. A., RANDLE P. J. Regulation of glucose uptake by muscle. 5. Effects of anoxia, insulin, adrenaline and prolonged starving on concentrations of hexose phosphates in isolated rat diaphragm and perfused isolated rat heart. Biochem J. 1961 Sep;80:655–662. doi: 10.1042/bj0800655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NODES J. T., REID E. AZO-DYE CARCINOGENESIS: RIBONUCLEOTIDES AND RIBONUCLEASES. Br J Cancer. 1963 Dec;17:745–774. doi: 10.1038/bjc.1963.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARK C. R., MORGAN H. E., HENDERSON M. J., REGEN D. M., CADENAS E., POST R. L. The regulation of glucose uptake in muscle as studied in the perfused rat heart. Recent Prog Horm Res. 1961;17:493–538. [PubMed] [Google Scholar]
- PARMEGGIANI A., BOWMAN R. H. REGULATION OF PHOSPHOFRUCTOKINASE ACTIVITY BY CITRATE IN NORMAL AND DIABETIC MUSCLE. Biochem Biophys Res Commun. 1963 Aug 1;12:268–273. doi: 10.1016/0006-291x(63)90294-8. [DOI] [PubMed] [Google Scholar]
- POTTER V. R., ONO T. Enzyme patterns in rat liver and Morris hepatoma 5123 during metabolic transitions. Cold Spring Harb Symp Quant Biol. 1961;26:355–362. doi: 10.1101/sqb.1961.026.01.043. [DOI] [PubMed] [Google Scholar]
- Passonneau J. V., Lowry O. H. The role of phosphofructokinase in metabolic regulation. Adv Enzyme Regul. 1964;2:265–274. doi: 10.1016/s0065-2571(64)80018-2. [DOI] [PubMed] [Google Scholar]
- Pitot H. C., Peraino C., Pries N., Kennan A. L. Glucose repression and induction of enzyme synthesis in rat liver. Adv Enzyme Regul. 1964;2:237–247. doi: 10.1016/s0065-2571(64)80016-9. [DOI] [PubMed] [Google Scholar]
- REID E. Significant biochemical effects of hepatocarcinogens in the rat: a review. Cancer Res. 1962 May;22:398–430. [PubMed] [Google Scholar]
- SHARMA C., MANJESHWAR R., WEINHOUSE S. EFFECTS OF DIET AND INSULIN ON GLUCOSE-ADENOSINE TRIPHOSPHATE PHOSPHOTRANSFERASES OF RAT LIVER. J Biol Chem. 1963 Dec;238:3840–3845. [PubMed] [Google Scholar]
- SHARMA R. M., SHARMA C., DONNELLY A. J., MORRIS H. P., WEINHOUSE S. GLUCOSE-ATP PHOSPHOTRANSFERASES DURING HEPATOCARCINOGENESIS. Cancer Res. 1965 Feb;25:193–199. [PubMed] [Google Scholar]
- SHATTON J. B., DONNELLY A. J., WEINHOUSE S. Metabolism of neoplastic tissues. XVI. Glucokinase activity and glycogen levels during hepatocarcinogenesis by azo dyes. Cancer Res. 1962 Dec;22:1372–1380. [PubMed] [Google Scholar]
- STEINER D. F., WILLIAMS R. H. Some observations concerning hepatic glucose 6-phosphate content in normal and diabetic rats. J Biol Chem. 1959 Jun;234(6):1342–1346. [PubMed] [Google Scholar]
- Siperstein M. D., Fagan V. M. Studies on the feed-back regulation of cholesterol synthesis. Adv Enzyme Regul. 1964;2:249–264. doi: 10.1016/s0065-2571(64)80017-0. [DOI] [PubMed] [Google Scholar]
- Spencer A., Corman L., Lowenstein J. M. Citrate and the conversion of carbohydrate into fat. A comparison of citrate and acetate incorporation into fatty acids. Biochem J. 1964 Nov;93(2):378–388. doi: 10.1042/bj0930378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEPPERMAN H. M., TEPPERMAN J. ON THE RESPONSE OF HEPATIC GLUCOSE-6-PHOSPHATE DEHYDROGENASE ACTIVITY TO CHANGES IN DIET COMPOSITION AND FOOD INTAKE PATTERN. Adv Enzyme Regul. 1963;1:121–136. doi: 10.1016/0065-2571(63)90013-x. [DOI] [PubMed] [Google Scholar]
- WALKER D. G. ON THE PRESENCE OF TWO SOLUBLE GLUCOSE-PHOSPHORYLATING ENZYMES IN ADULT LIVER AND THE DEVELOPMENT OF ONE OF THESE AFTER BIRTH. Biochim Biophys Acta. 1963 Oct 1;77:209–226. doi: 10.1016/0006-3002(63)90494-3. [DOI] [PubMed] [Google Scholar]
- WEIL-MALHERBE H., BONE A. D. Studies on hexokinase. 1. The hexokinase activity of rat-brain extracts. Biochem J. 1951 Aug;49(3):339–347. doi: 10.1042/bj0490339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WENNER C. E., SPIRTES M. A., WEINHOUSE S. Metabolism of neoplastic tissue. II. A survey of enzymes of the citric acid cycle in transplanted tumors. Cancer Res. 1952 Jan;12(1):44–49. [PubMed] [Google Scholar]
- Weber G., Henry M. C., Wagle S. R., Wagle D. S. Correlation of enzyme activities and metabolic pathways with growth rate of hepatomas. Adv Enzyme Regul. 1964;2:335–346. doi: 10.1016/s0065-2571(64)80024-8. [DOI] [PubMed] [Google Scholar]
- Wu R. Control of glycolysis by phosphofructokinase in slices of rat liver, Novikoff hepatoma, and adenocarcinomas. Biochem Biophys Res Commun. 1964;14:79–85. doi: 10.1016/0006-291x(63)90215-8. [DOI] [PubMed] [Google Scholar]


