Abstract
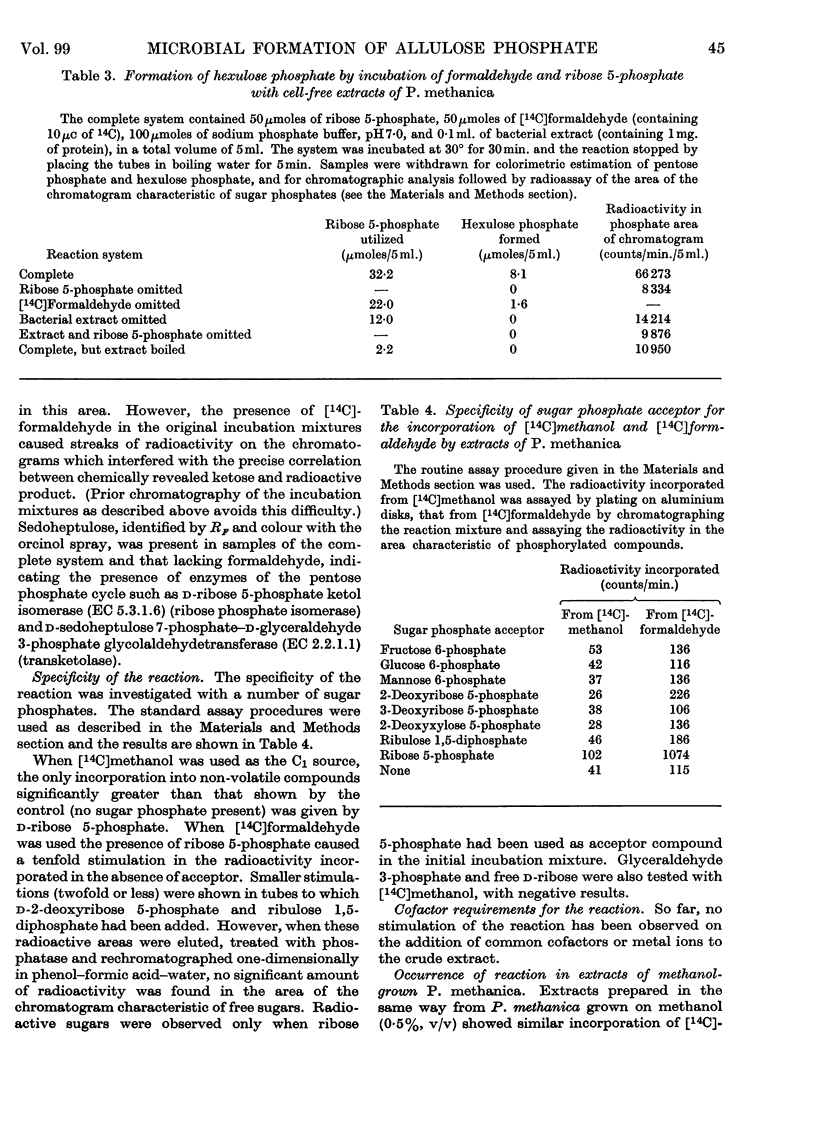
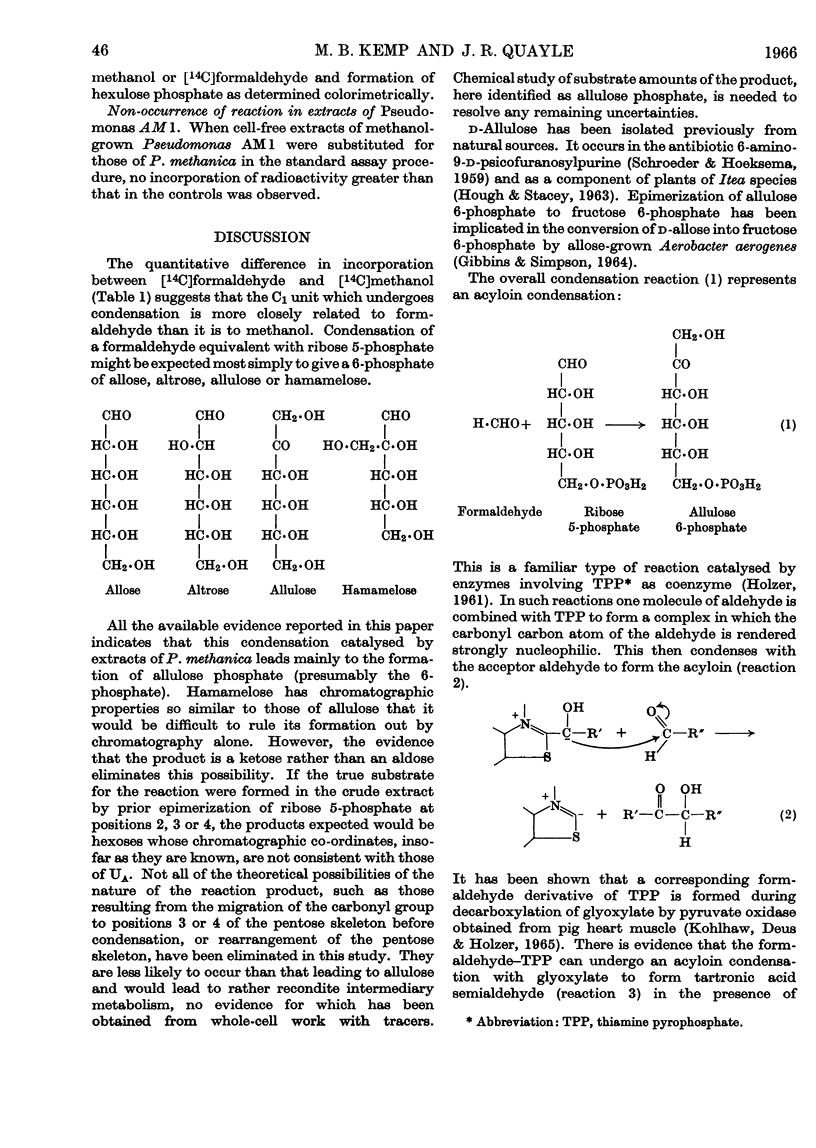
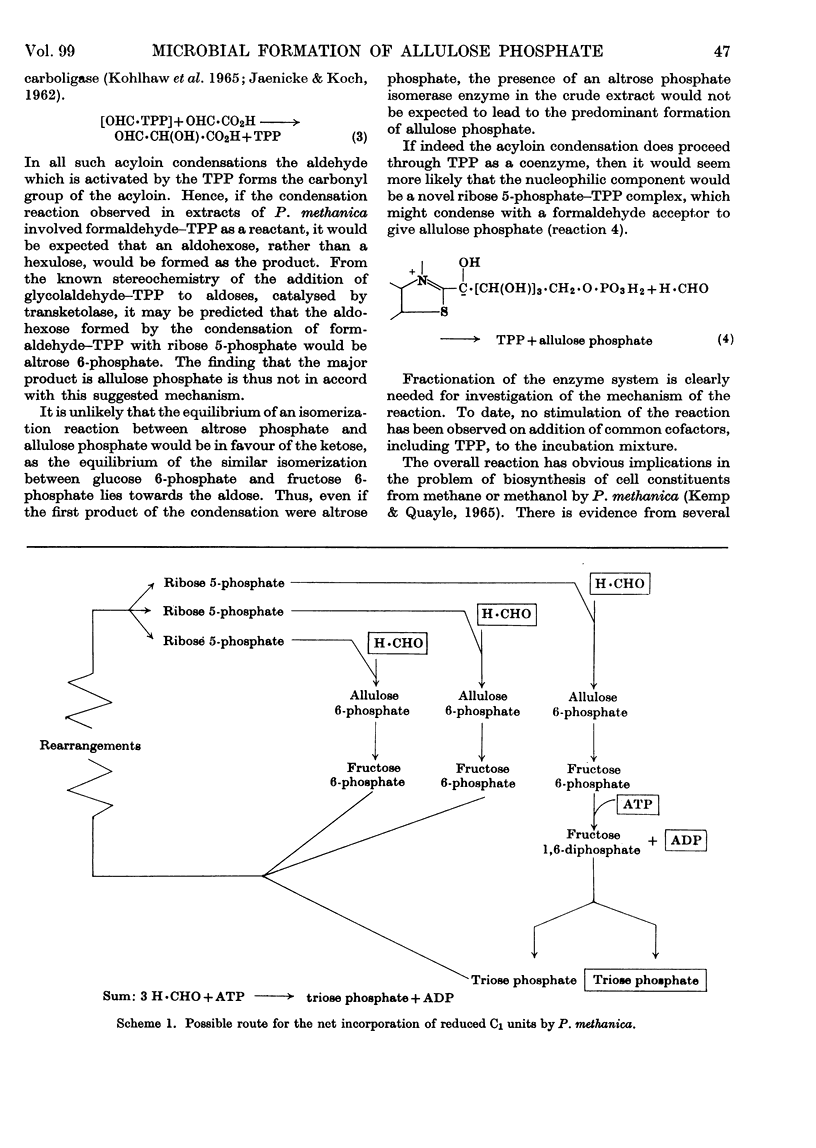
1. Incubation of cell-free extracts of methane- or methanol-grown Pseudomonas methanica with [14C]formaldehyde and d-ribose 5-phosphate leads to incorporation of radioactivity into a non-volatile product, which has the chromatographic properties of a phosphorylated compound. 2. Treatment of this reaction product with a phosphatase, followed by chromatography, shows the presence of two compounds whose chromatographic properties are consistent with their being free sugars. 3. The minor component of the dephosphorylated products has been identified as fructose. The major component has been identified as allulose (psicose) on the basis of co-chromatography, co-crystallization of the derived phenylosazone and dinitrophenylosazone with authentic derivatives of allulose and behaviour towards oxidation with bromine water. 4. It is suggested that the bacterial extracts catalyse the condensation of a C1 unit identical with, or derived from, formaldehyde with ribose 5-phosphate to give allulose 6-phosphate. 5. Testing of hexose phosphates and pentose phosphates as substrates has so far shown the reaction to be specific for ribose 5-phosphate. 6. The condensation reaction is not catalysed by extracts of methanol-grown Pseudomonas AM1. 7. A variant of the pentose phosphate cycle, involving this condensation reaction, is suggested as an explanation for the net synthesis of C3 compounds from C1 units by P. methanica.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN L. R., STRAWINSKI R. J., MCCLESKEY C. S. THE ISOLATION AND CHARACTERIZATION OF METHANOMONAS METHANOOXIDANS BROWN AND STRAWINSKI. Can J Microbiol. 1964 Oct;10:791–799. doi: 10.1139/m64-100. [DOI] [PubMed] [Google Scholar]
- COHEN S. Studies on D-ribulose and its enzymatic conversion to D-arabinose. J Biol Chem. 1953 Mar;201(1):71–84. [PubMed] [Google Scholar]
- DWORKIN M., FOSTER J. W. Studies on Pseudomonas methanica (Söhngen) nov. comb. J Bacteriol. 1956 Nov;72(5):646–659. doi: 10.1128/jb.72.5.646-659.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBINS L. N., SIMPSON F. J. THE INCORPORATION OF D-ALLOSE INTO THE GLYCOLYTIC PATHWAY BY AEROBACTER AEROGENES. Can J Microbiol. 1964 Dec;10:829–836. doi: 10.1139/m64-108. [DOI] [PubMed] [Google Scholar]
- HARRINGTON A. A., KALLIO R. E. Oxidation of methanol and formaldehyde by pseudomonas methanica. Can J Microbiol. 1960 Feb;6:1–7. doi: 10.1139/m60-001. [DOI] [PubMed] [Google Scholar]
- HORECKER B. L., SMYRNIOTIS P. Z., KLENOW H. The formation of sedoheptulose phosphate. J Biol Chem. 1953 Dec;205(2):661–682. [PubMed] [Google Scholar]
- HORECKER B. L., SMYRNIOTIS P. Z., SEEGMILLER J. E. The enzymatic conversion of 6-phosphogluconate to ribulose-5-phosphate and ribose-5-phosphate. J Biol Chem. 1951 Nov;193(1):383–396. [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- JAENICKE L., KOCH J. [On the mechanism of the carboligase reaction. Hydroxymethyl-thiamine pyrophosphate, a new active carbon fragment]. Biochem Z. 1962;336:432–443. [PubMed] [Google Scholar]
- JOHNSON P. A., QUAYLE J. R. MICROBIAL GROWTH ON C1 COMPOUNDS. SYNTHESIS OF CELL CONSTITUENTS BY METHANE- AND METHANOL-GROWN PSEUDOMONAS METHANICA. Biochem J. 1965 Jun;95:859–867. doi: 10.1042/bj0950859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. A., Quayle J. R. Microbial growth on C-1 compounds. 6. Oxidation of methanol, formaldehyde and formate by methanol-grown Pseudomonas AM-1. Biochem J. 1964 Nov;93(2):281–290. doi: 10.1042/bj0930281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KANEDA T., ROXBURGH J. M. Serine as an intermediate in the assimilation of methanol by a Pseudomonas. Biochim Biophys Acta. 1959 May;33(1):106–110. doi: 10.1016/0006-3002(59)90503-7. [DOI] [PubMed] [Google Scholar]
- KOHLHAW G., DEUS B., HOLZER H. ENZYMATIC PREPARATION, STRUCTURE, AND PROPERTIES OF THIAMINE PYROPHOSPHATE-ACTIVATED FORMALDEHYDE. J Biol Chem. 1965 May;240:2135–2141. [PubMed] [Google Scholar]
- KORNBERG H. L. The metabolism of C2 compounds in micro-organisms. I. The incorporation of [2-14C] acetate by Pseudomonas fluorescens, and by a Corynebacterium, grown on ammonium acetate. Biochem J. 1958 Mar;68(3):535–542. doi: 10.1042/bj0680535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp M. B., Quayle J. R. Incorporation of C1 units into allulose phosphate by methane-grown Pseudomonas methanica. Biochim Biophys Acta. 1965 Aug 24;107(1):174–176. doi: 10.1016/0304-4165(65)90415-0. [DOI] [PubMed] [Google Scholar]
- LARGE P. J., PEEL D., QUAYLE J. R. Microbial growth on C1 compounds. II. Synthesis of cell constituents by methanol- and formate-grown Pseudomonas AM 1, and methanol-grown Hyphomicrobium vulgare. Biochem J. 1961 Dec;81:470–480. doi: 10.1042/bj0810470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]


