Abstract
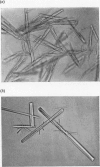
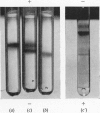
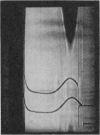
1. The toxic principles in the venom of the sea-snake Laticauda semifasciata were separated into two components by CM-cellulose chromatography and obtained in crystalline forms. They were named `erabutoxins a and b'. 2. The homogeneity of each toxin was shown by rechromatography, by disk electrophoresis, by ultracentrifuging, by toxicity measurements before and after repeated crystallizations and by N-terminal analysis. 3. They had molecular weights of about 7000. Both of them contained 61 (or 62) amino acid residues/molecule. The only difference between erabutoxins a and b was that one of the aspartic acid (or asparagine) residues in erabutoxin a was replaced by a histidine residue in erabutoxin b. 4. Both of the toxins had LD50 values of 0·15μg./g. body wt. for mice and 0·07μg./g. for rats. It was shown with frog-muscle preparations that they acted on postsynaptic membrane to block neuromuscular transmission.
Full text
PDF
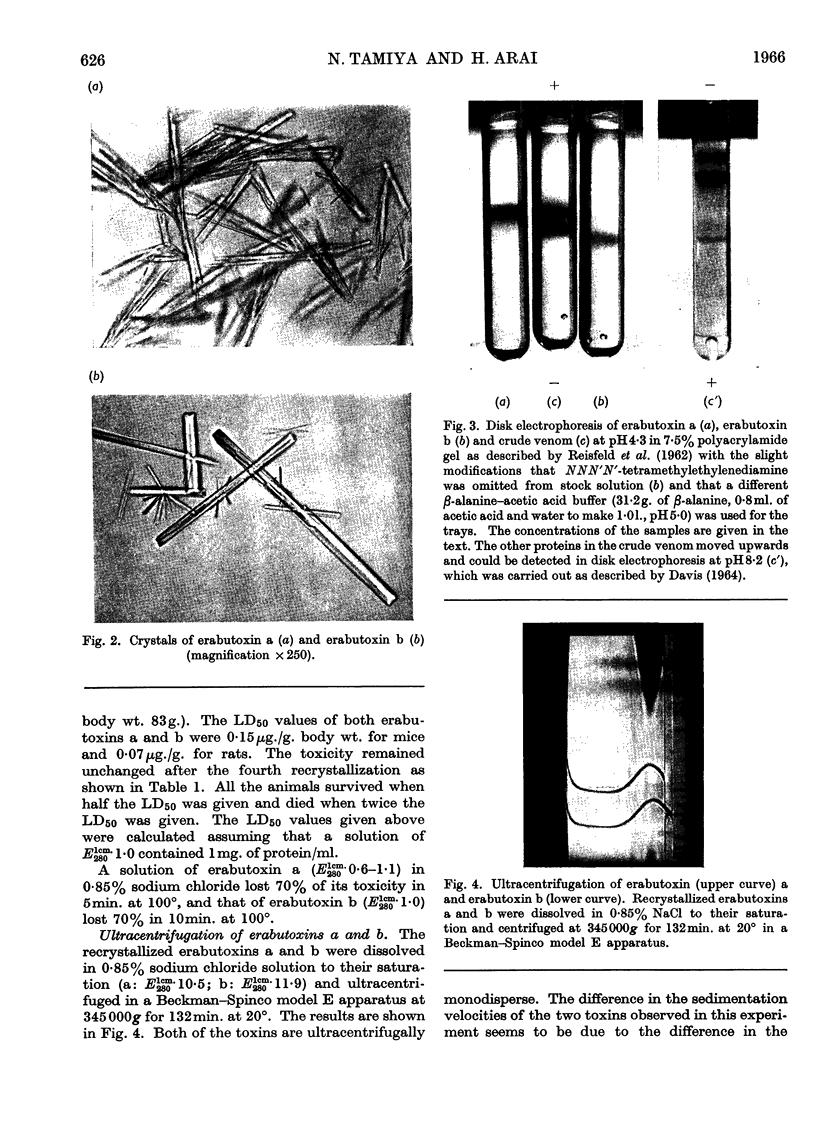


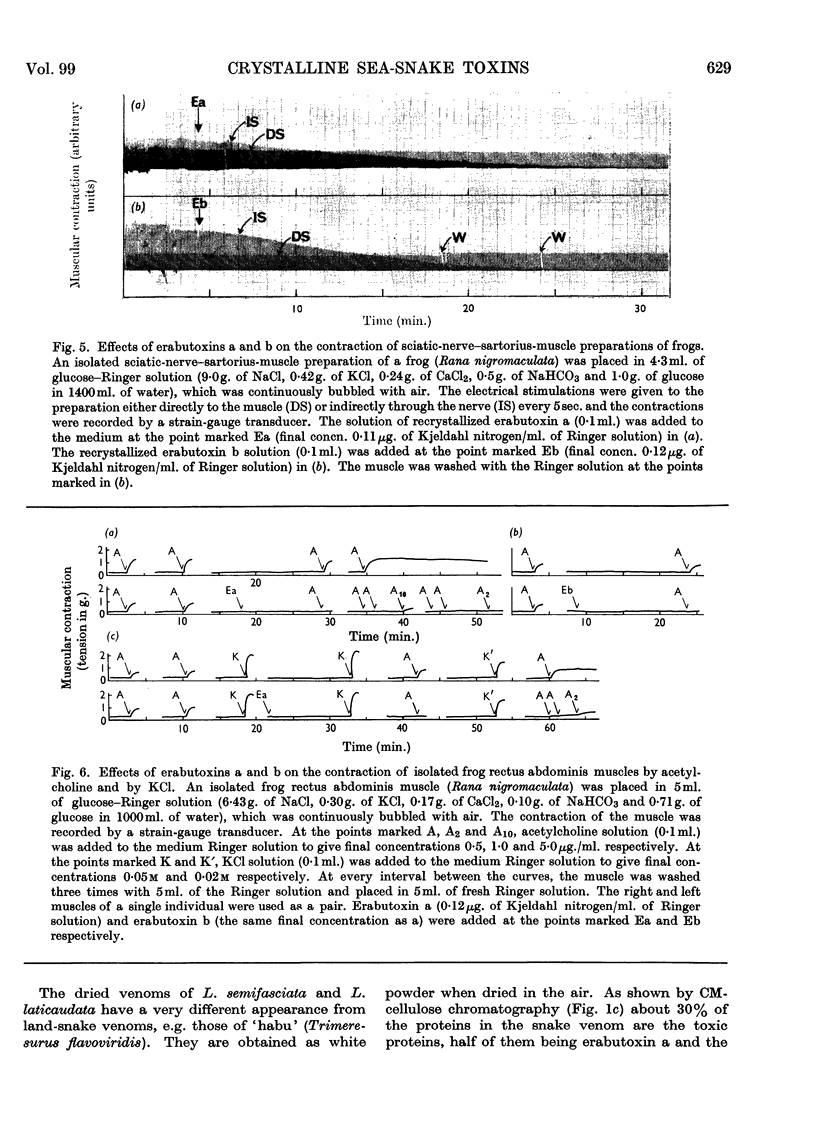

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARAI H., TAMIYA N., TOSHIOKA S. I., SHINONAGA S., KANO R. STUDIES ON SEA-SNAKE VENOMS. I. PROTEIN NATURE OF THE NEUROTOXIC COMPONENT. J Biochem. 1964 Dec;56:568–571. doi: 10.1093/oxfordjournals.jbchem.a128035. [DOI] [PubMed] [Google Scholar]
- CAREY J. E., WRIGHT E. A. Isolation of the neurotoxic component of the venom of the sea snake, Enhydrina schistosa. Nature. 1960 Jan 9;185:103–104. doi: 10.1038/185103b0. [DOI] [PubMed] [Google Scholar]
- CHANG C. C., LEE C. Y. ISOLATION OF NEUROTOXINS FROM THE VENOM OF BUNGARUS MULTICINCTUS AND THEIR MODES OF NEUROMUSCULAR BLOCKING ACTION. Arch Int Pharmacodyn Ther. 1963 Jul 1;144:241–257. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., HARRIS J. I., LEVY A. L. Recent developments in techniques for terminal and sequence studies in peptides and proteins. Methods Biochem Anal. 1955;2:359–425. doi: 10.1002/9780470110188.ch12. [DOI] [PubMed] [Google Scholar]
- Goodwin T. W., Morton R. A. The spectrophotometric determination of tyrosine and tryptophan in proteins. Biochem J. 1946;40(5-6):628–632. doi: 10.1042/bj0400628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVY A. L. A paper chromatographic method for the quantitative estimation of amino-acids. Nature. 1954 Jul 17;174(4420):126–127. doi: 10.1038/174126a0. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- SANGER F. The terminal peptides of insulin. Biochem J. 1949;45(5):563–574. doi: 10.1042/bj0450563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG C. C. CRYSTALLIZATION AND PROPERTIES OF COBROTOXIN FROM FORMOSAN COBRA VENOM. J Biol Chem. 1965 Apr;240:1616–1618. [PubMed] [Google Scholar]
- YPHANSTIS D. A. Rapid determination of molecular weights of peptides and preteins. Ann N Y Acad Sci. 1960 Aug 31;88:586–601. doi: 10.1111/j.1749-6632.1960.tb20055.x. [DOI] [PubMed] [Google Scholar]