Abstract
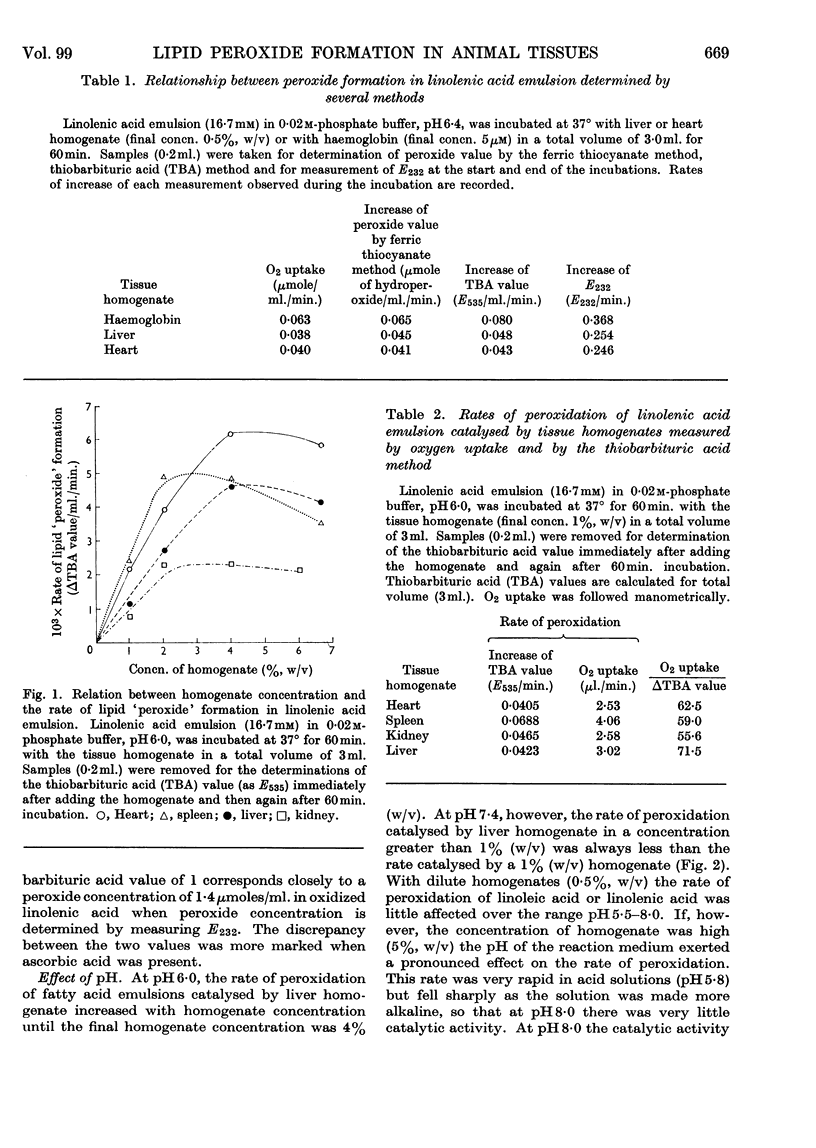
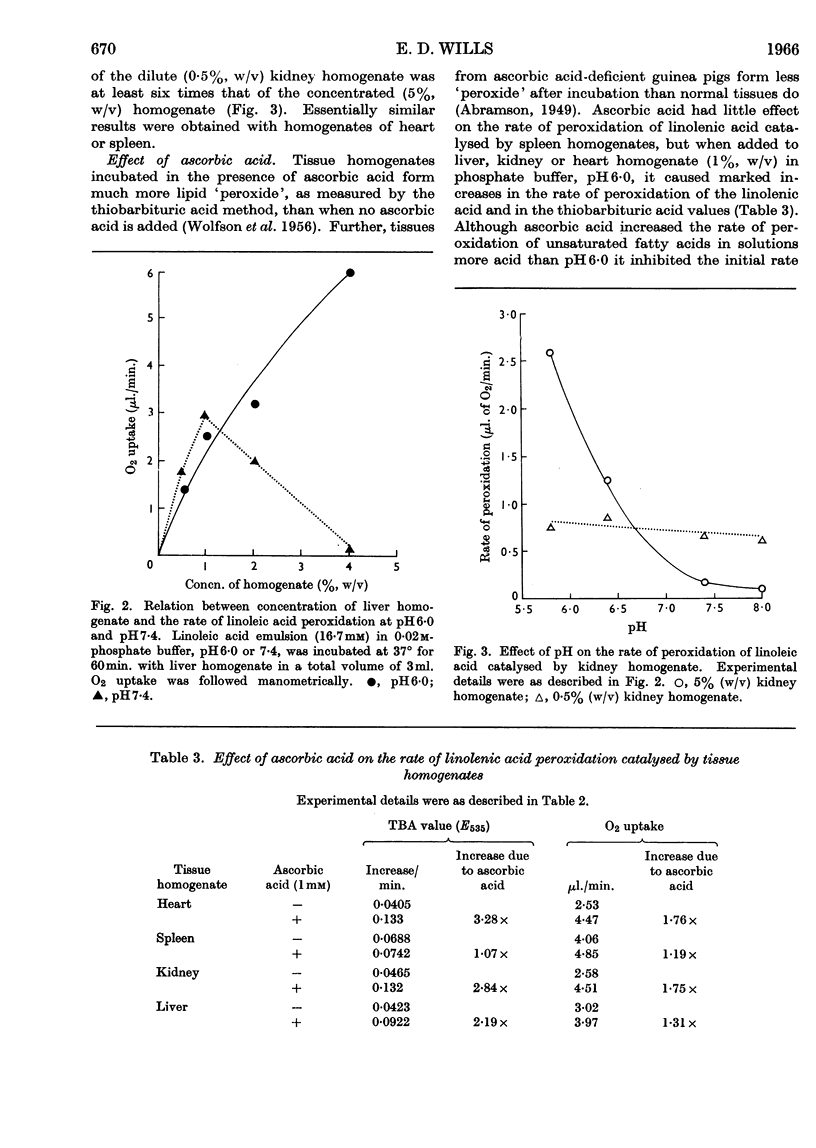
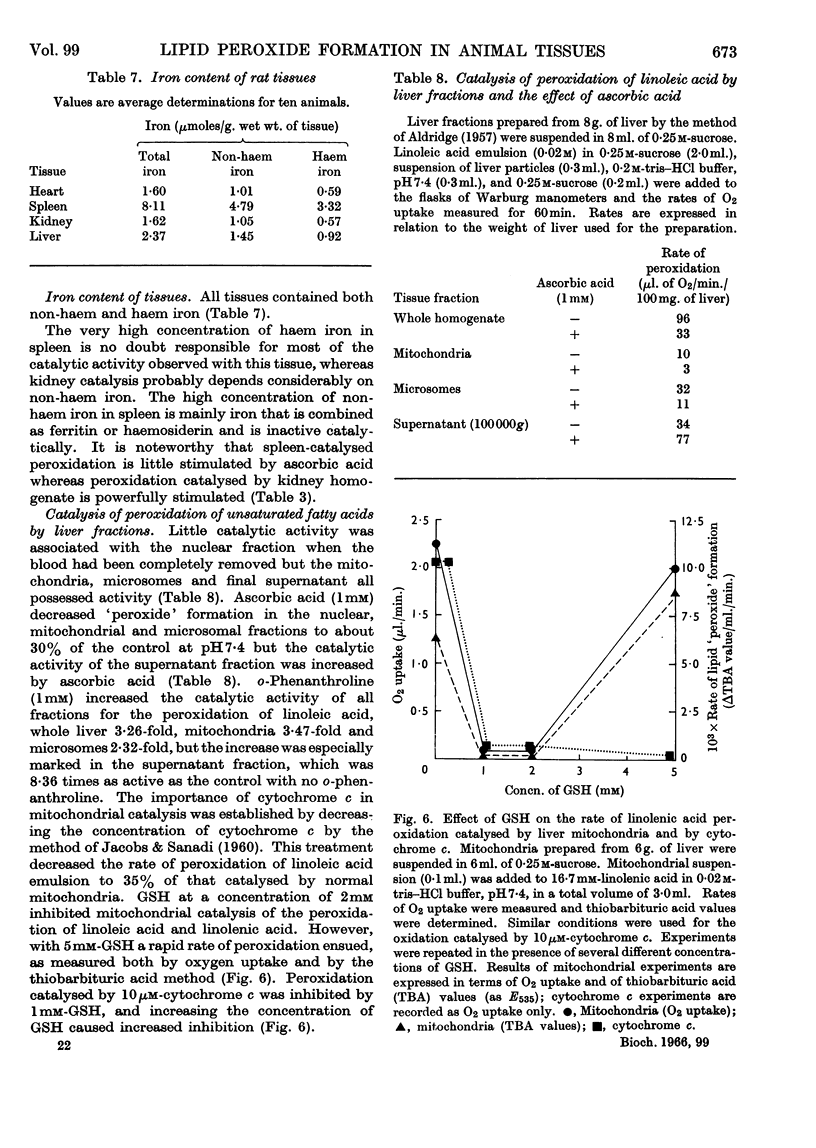
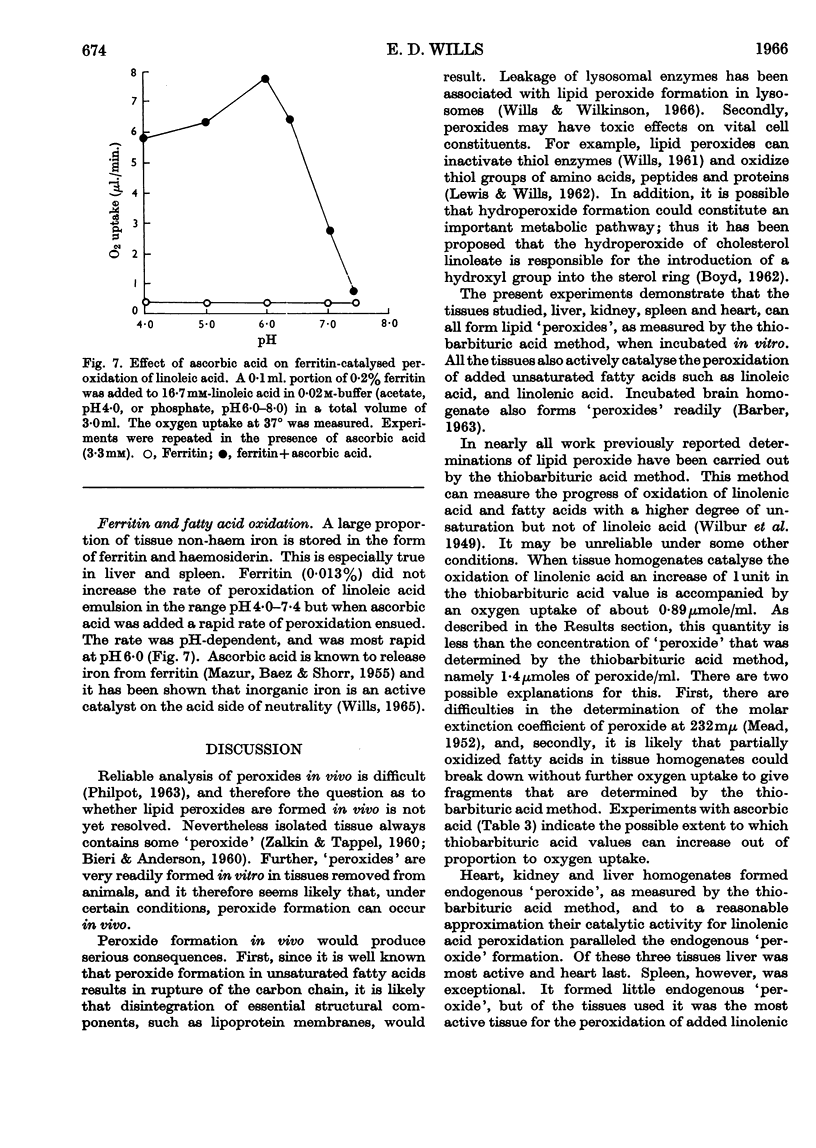
1. Homogenates of rat liver, spleen, heart and kidney form lipid peroxides when incubated in vitro and actively catalyse peroxide formation in emulsions of linoleic acid or linolenic acid. 2. In liver, catalytic activity is distributed throughout the nuclear, mitochondrial and microsomal fractions and is present in the 100000g supernatant. Activity is weak in the nuclear fraction. 3. Dilute (0·5%, w/v) homogenates catalyse peroxidation over the range pH5·0–8·0 but concentrated (5%, w/v) homogenates inhibit peroxidation and destroy peroxide if the solution is more alkaline than pH7·0. 4. Ascorbic acid increases the rate of peroxidation of unsaturated fatty acids catalysed by whole homogenates of liver, heart, kidney and spleen at pH6·0 but not at pH7·4. 5. Catalysis of peroxidation of unsaturated fatty acids by the mitochondrial and microsomal fractions of liver is inhibited by ascorbic acid at pH7·4 but the activity of the supernatant fraction is enhanced. 6. Inorganic iron or ferritin are active catalysts in the presence of ascorbic acid. 7. Lipid peroxide formation in linoleic acid or linolenic acid emulsions catalysed by tissue homogenates is partially inhibited by EDTA but stimulated by o-phenanthroline. 8. Cysteine or glutathione (1mm) inhibits peroxide formation catalysed by whole homogenates, mitochondria or haemoprotein. Inhibition increases with increase of pH.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N. Liver and brain mitochondria. Biochem J. 1957 Nov;67(3):423–431. doi: 10.1042/bj0670423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD G. S. Effect of linoleate and estrogen on cholesterol metabolism. Fed Proc. 1962 Jul-Aug;21(4):86–92. [PubMed] [Google Scholar]
- HORGAN V. J., PHILPOT J. S., PORTER B. W., ROODYN D. B. Toxicity of autoxidized squalene and linoleic acid, and of simpler peroxides, in relation to toxicity of radiation. Biochem J. 1957 Dec;67(4):551–558. doi: 10.1042/bj0670551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBS E. E., SANADI D. R. The reversible removal of cytochrome c from mitochondria. J Biol Chem. 1960 Feb;235:531–534. [PubMed] [Google Scholar]
- LEWIS S. E., WILLS E. D. Inhibition of the autoxidation of unsaturated fatty acids by haematin proteins. Biochim Biophys Acta. 1963 Jun 18;70:336–338. doi: 10.1016/0006-3002(63)90757-1. [DOI] [PubMed] [Google Scholar]
- MAZUR A., BAEZ S., SHORR E. The mechanism of iron release from ferritin as related to its biological properties. J Biol Chem. 1955 Mar;213(1):147–160. [PubMed] [Google Scholar]
- Mead J. F. The Irradiation-Induced Autoxidation of Linoleic Acid. Science. 1952 Apr 25;115(2991):470–472. doi: 10.1126/science.115.2991.470. [DOI] [PubMed] [Google Scholar]
- Proceedings of the Biochemical Society. Biochem J. 1954 Jul;57(3):xvii–xxv. [PMC free article] [PubMed] [Google Scholar]
- RAMSAY W. N. M. Iron metabolism and haemoglobin formation in the embryonated hen egg. 2. Some observations on the embryonic blood supply. Biochem J. 1951 Sep;49(4):494–499. doi: 10.1042/bj0490494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAPPEL A. L. Unsaturated lipide oxidation catalyzed by hematin compounds. J Biol Chem. 1955 Dec;217(2):721–733. [PubMed] [Google Scholar]
- TSEN C. C., COLLIER H. B. The protective action of tocopherol against hemolysis of rat erythrocytes by dialuric acid. Can J Biochem Physiol. 1960 Sep;38:957–964. [PubMed] [Google Scholar]
- WILBUR K. M., BERNHEIM F., SHAPIRO O. W. The thiobarbituric acid reagent as a test for the oxidation of unsaturated fatty acids by various agents. Arch Biochem. 1949 Dec;24(2):305–313. [PubMed] [Google Scholar]
- WILLS E. D. Effect of unsaturated fatty acids and their peroxides on enzymes. Biochem Pharmacol. 1961 Jul;7:7–16. doi: 10.1016/0006-2952(61)90119-8. [DOI] [PubMed] [Google Scholar]
- WILLS E. D. MECHANISMS OF LIPID PEROXIDE FORMATION IN TISSUES. ROLE OF METALS AND HAEMATIN PROTEINS IN THE CATALYSIS OF THE OXIDATION UNSATURATED FATTY ACIDS. Biochim Biophys Acta. 1965 Apr 5;98:238–251. doi: 10.1016/0005-2760(65)90118-9. [DOI] [PubMed] [Google Scholar]
- WILLS E. D., ROTBLAT J. THE FORMATION OF PEROXIDES IN TISSUE LIPIDS AND UNSATURATED FATTY ACIDS BY IRRADIATION. Int J Radiat Biol Relat Stud Phys Chem Med. 1964;8:551–567. doi: 10.1080/09553006414550701. [DOI] [PubMed] [Google Scholar]
- WILLS E. D. THE EFFECT OF INORGANIC IRON ON THE THIOBARBITURIC ACID METHOD FOR THE DETERMINATION OF LIPID PEROXIDES. Biochim Biophys Acta. 1964 Aug 5;84:475–477. doi: 10.1016/0926-6542(64)90016-2. [DOI] [PubMed] [Google Scholar]
- WOLFSON N., WILBUR K. M., BERNHEIM F. Lipid peroxide formation in regenerating rat liver. Exp Cell Res. 1956 Apr;10(2):556–558. doi: 10.1016/0014-4827(56)90031-3. [DOI] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. A procedure for the isolation of enzymically active rat-liver nuclei. Biochem J. 1964 Aug;92(2):313–317. doi: 10.1042/bj0920313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills E. D., Wilkinson A. E. Release of enzymes from lysosomes by irradiation and the relation of lipid peroxide formation to enzyme release. Biochem J. 1966 Jun;99(3):657–666. doi: 10.1042/bj0990657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZALKIN H., TAPPEL A. L. Studies of the mechanism of vitamin E action. IV. Lipide peroxidation in the vitamin E-deficient rabbit. Arch Biochem Biophys. 1960 May;88:113–117. doi: 10.1016/0003-9861(60)90205-8. [DOI] [PubMed] [Google Scholar]


