Abstract
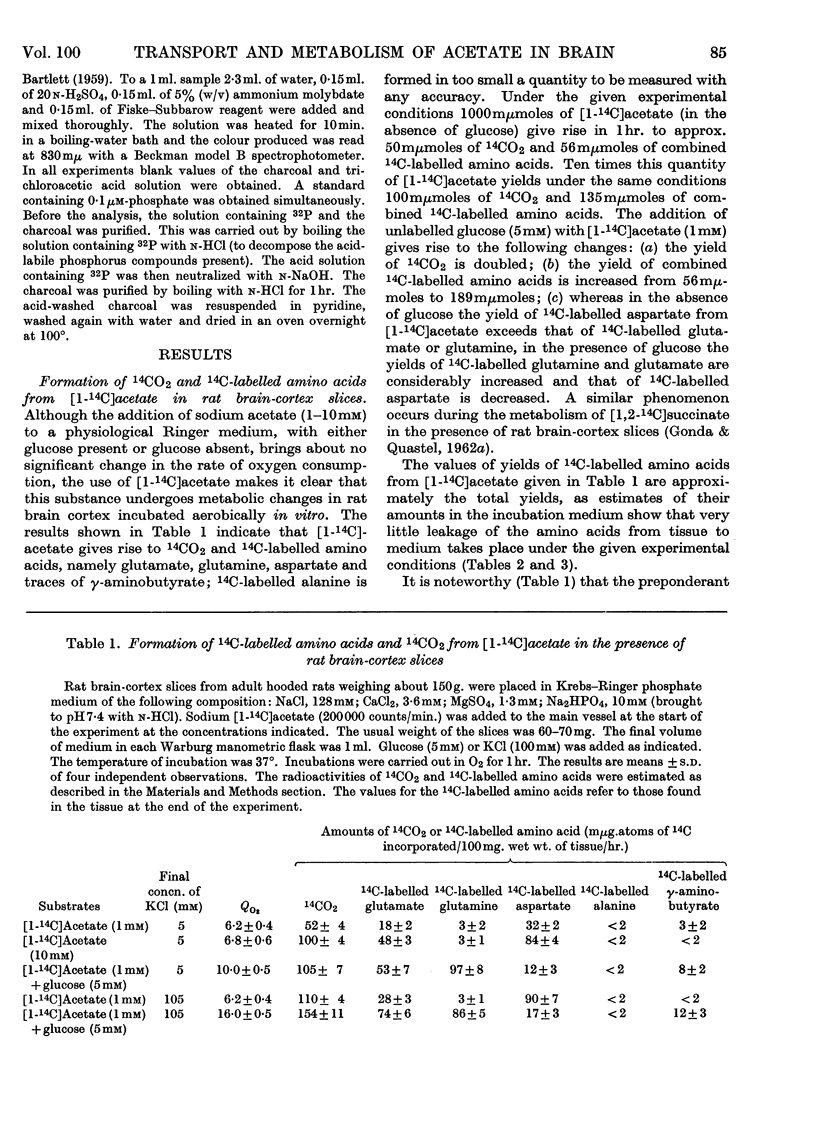
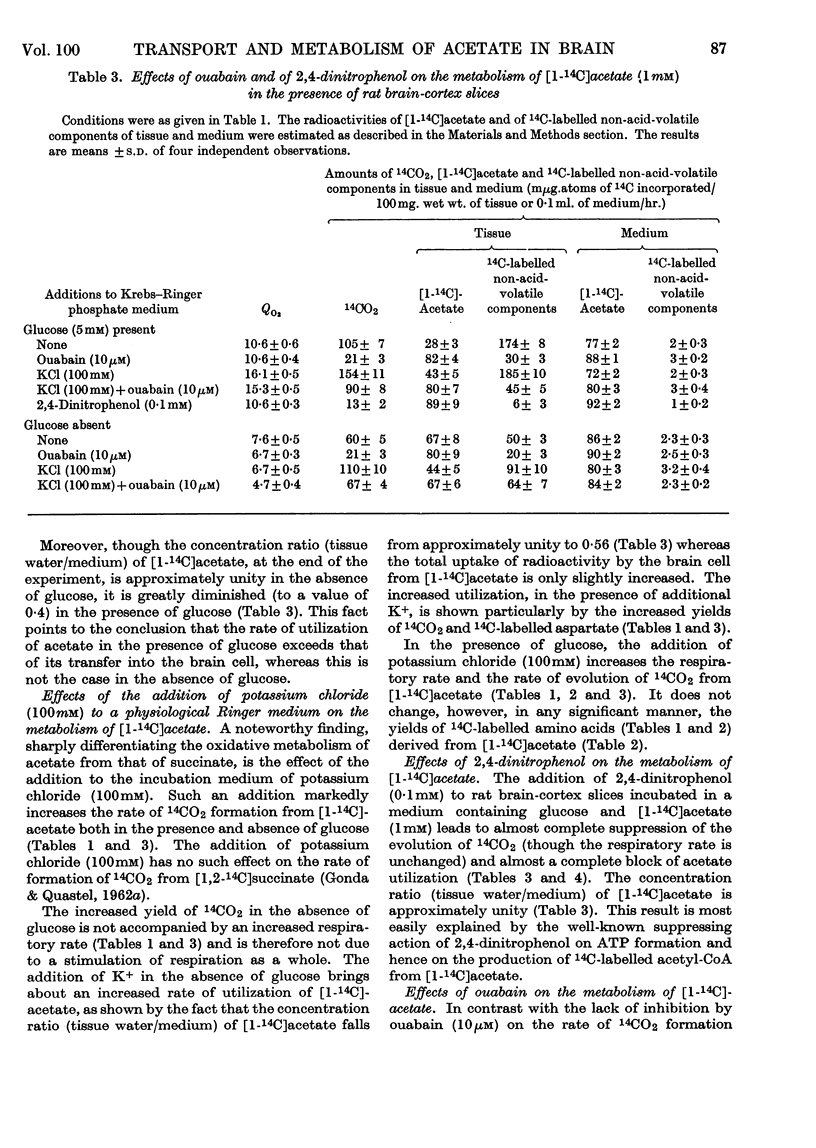
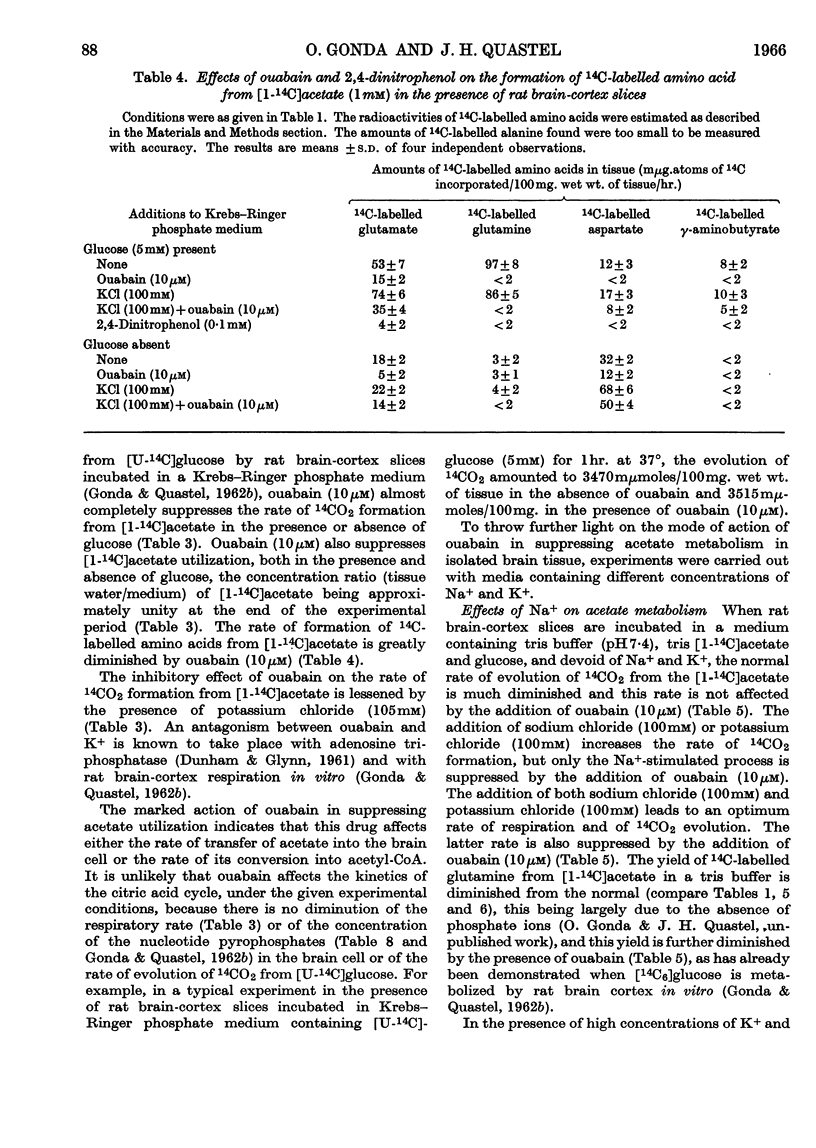
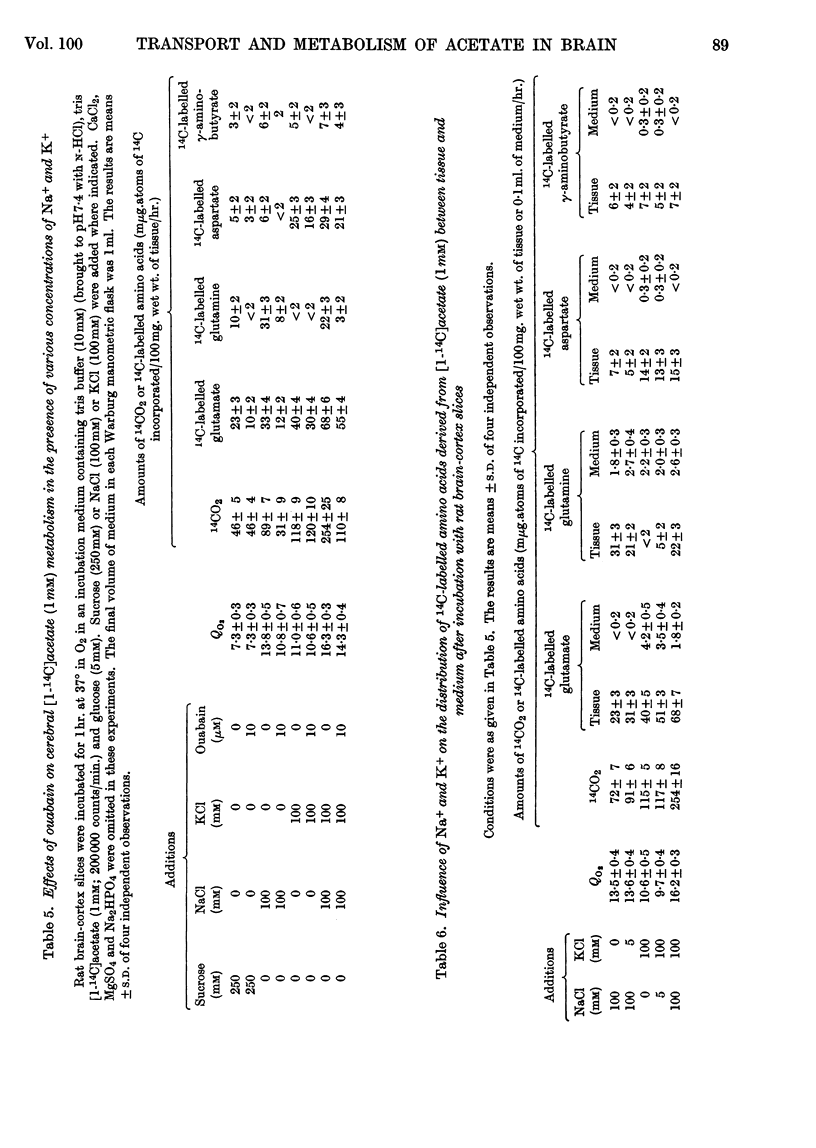
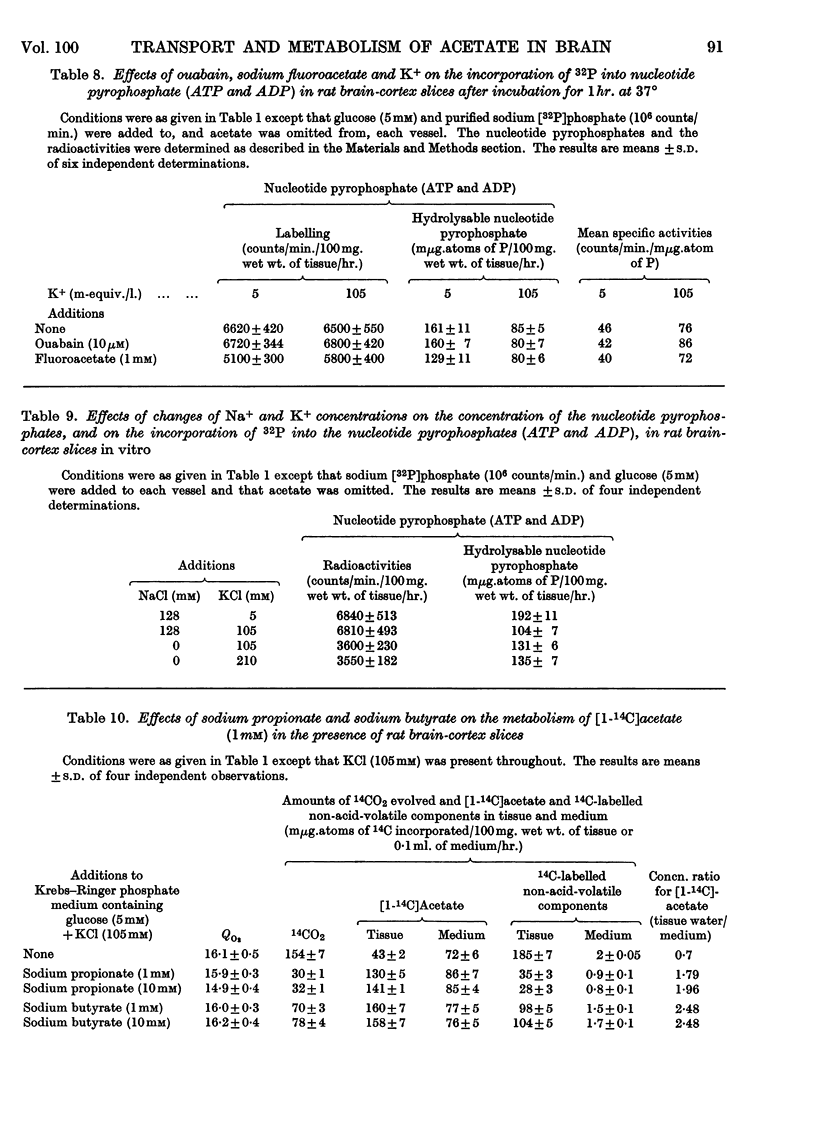
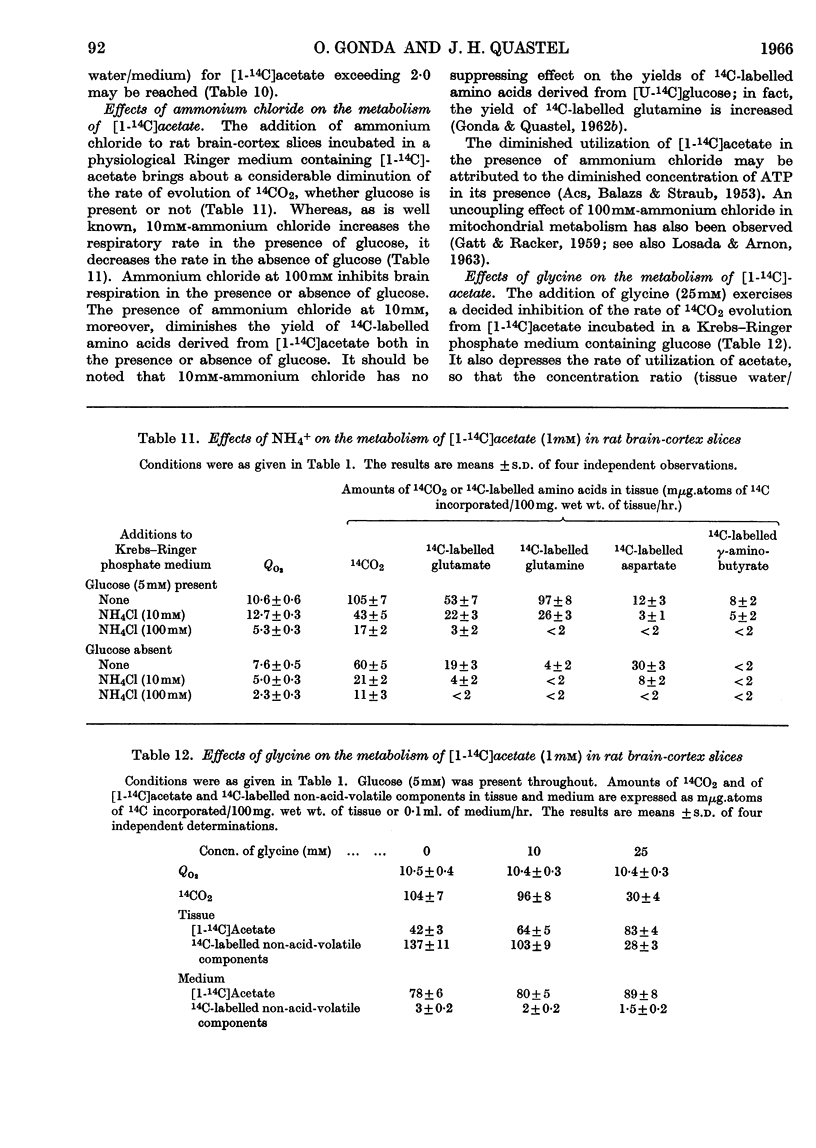
1. [1-14C]Acetate undergoes metabolism when incubated aerobically at 37° in the presence of rat brain-cortex slices, forming 14CO2 and 14C-labelled amino acids (glutamate, glutamine, aspartate and relatively small quantities of γ-aminobutyrate). In the absence of glucose the yield of 14C-labelled aspartate exceeds that of 14C-labelled glutamate and glutamine. The addition of glucose brings about a doubling of the rate of formation of 14CO2 and a greatly increased yield of 14C-labelled glutamate or glutamine, whereas that of 14C-labelled aspartate is diminished. 2. The addition of potassium chloride (100mm) to the incubation medium causes an increased rate of 14CO2 formation in the presence or absence of glucose and an increased rate of utilization of acetate. 3. The addition of 2,4-dinitrophenol (0·1mm) suppresses the rate of utilization of [1-14C]acetate. 4. The presence of ouabain (10μm) suppresses the rate of formation of 14CO2 from [1-14C]acetate and the rate of acetate utilization. Acetate conversion into carbon dioxide in the rat brain cortex is both Na+- and K+-dependent and controlled by operation of the active sodium-transport process. Only the Na+-stimulated rate is suppressed by ouabain. 5. Sodium fluoroacetate (1mm) decreases the rate of 14CO2 evolution from [1-14C]acetate in the presence of rat brain cortex without affecting the respiratory rate. The results are consistent with the conclusion that fluoroacetate competes with, or blocks, a transport carrier for acetate, so that in its presence only the passive diffusion rate of acetate takes place. 6. The presence of sodium propionate or sodium butyrate suppresses the utilization of [1-14C]acetate in rat brain cortex and leads to a concentration ratio (tissue/medium) of [1-14C]-acetate greater than unity. 7. The presence of NH4+ diminishes acetate utilization, this being attributed to a diminished ATP concentration. Glycine is also inhibitory. It is concluded that acetate transport into the brain is carrier-mediated and dependent on the operation of the sodium pump.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACS G. Y., BALAZS R., STRAUB F. B. Adenozintrifoszfát szintézis agykéregszeletekben. Kiserl Orvostud. 1953 Nov;5(6):466–472. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BILINSKI E., MCCONNELL W. B. Studies on wheat plants using C14 compounds. IV. Distribution of C14 in glutamic acid, aspartic acid, and threonine arising from acetate-1-C14 and -2-C14. Can J Biochem Physiol. 1957 Jun;35(6):365–371. [PubMed] [Google Scholar]
- ELLIS D. B., SCHOLEFIELD P. G. Studies of fatty acid oxidation. IX. The effects of uncoupling agents on the oxidation of fatty acids by transplantable tumors. Cancer Res. 1962 Apr;22:305–313. [PubMed] [Google Scholar]
- ELLIS D. B., SCHOLEFIELD P. G. The effects of adenine and glucose on synthesis of nucleotides by Ehrlich ascites carcinoma cells in vitro. Can J Biochem Physiol. 1962 Mar;40:343–352. [PubMed] [Google Scholar]
- GATT S., RACKER E. Regulatory mechanisms in carbohydrate metabolism. I. Crabtree effect in reconstructed systems. J Biol Chem. 1959 May;234(5):1015–1023. [PubMed] [Google Scholar]
- GONDA O., QUASTEL J. H. Effects of ouabain on cerebral metabolism and transport mechanisms in vitro. Biochem J. 1962 Aug;84:394–406. doi: 10.1042/bj0840394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONDA O., QUASTEL J. H. Succinate/amino-acid interrelations in rat brain cortex in vitro. Nature. 1962 Jan 13;193:138–140. doi: 10.1038/193138a0. [DOI] [PubMed] [Google Scholar]
- Quastel J. H. Molecular transport at cell membranes. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):169–196. doi: 10.1098/rspb.1965.0065. [DOI] [PubMed] [Google Scholar]
- SKOU J. C. ENZYMATIC BASIS FOR ACTIVE TRANSPORT OF NA+ AND K+ ACROSS CELL MEMBRANE. Physiol Rev. 1965 Jul;45:596–617. doi: 10.1152/physrev.1965.45.3.596. [DOI] [PubMed] [Google Scholar]
- VON KORFF R. W. The effects of alkali metal ions on the acetate activating enzyme system. J Biol Chem. 1953 Jul;203(1):265–271. [PubMed] [Google Scholar]


