Abstract
In this study we find that the function of BRCA1 inhibits the microtubule nucleation function of centrosomes. In particular, cells in early S phase have quiescent centrosomes due to BRCA1 activity, which inhibits the association of γ-tubulin with centrosomes. We find that modification of either of two specific lysine residues (Lys-48 and Lys-344) of γ-tubulin, a known substrate for BRCA1-dependent ubiquitination activity, led to centrosome hyperactivity. Interestingly, mutation of γ-tubulin lysine 344 had a minimal effect on centrosome number but a profound effect on microtubule nucleation function, indicating that the processes regulating centrosome duplication and microtubule nucleation are distinct. Using an in vitro aster formation assay, we found that BRCA1-dependent ubiquitination activity directly inhibits microtubule nucleation by centrosomes. Mutant BRCA1 protein that was inactive as a ubiquitin ligase did not inhibit aster formation by the centrosome. Further, a BRCA1 carboxy-terminal truncation mutant that was an active ubiquitin ligase lacked domains critical for the inhibition of centrosome function. These experiments reveal an important new functional assay regulated by the BRCA1-dependent ubiquitin ligase, and the results suggest that the loss of this BRCA1 activity could cause the centrosome hypertrophy and subsequent aneuploidy typically found in breast cancers.
BRCA1 is a breast- and ovary-specific tumor suppressor, and mutations in this gene have been found in approximately 40% of familial breast cancer cases and most of combined familial breast and ovarian cancers (1, 8, 43). BRCA1 is a large phosphoprotein consisting of 1,863 amino acids in humans, with a number of domains that interact directly or indirectly with many proteins with diverse functions such as transcription control, cell cycle regulation, chromatin remodeling, and DNA repair (30, 40). BRCA1 has a RING domain at its amino terminus, and in association with BARD1, the heterodimer is an E3 ubiquitin ligase (16, 46). Identifying the critical role for the BRCA1-dependent ubiquitin ligase activity in breast cell biology has been a major focus of research. In this study, we find that the BRCA1-associated E3 ubiquitin ligase directly regulates centrosome function.
Centrosomes are the major microtubule (MT)-organizing centers of animal cells. Centrosomes control the number, polarity, and distribution of MTs, which are important in regulating cell polarity, shape, motility, intracellular transport, and cell division (13). In a normal cell, centrosomes start duplicating at early-S phase, and by M phase the cell has two mature centrosomes that form the bipolar spindle and ensure proper segregation of chromosomes to the two daughter cells. Currently more than 150 proteins have been shown to localize to centrosomes (3).
The cells in many tumor types, including breast cancer, display numerical and structural centrosome aberrations, which have been collectively termed centrosomal hypertrophy. Structural abnormalities include increased centrosomal volume, accumulation of pericentriolar matrix, supernumerary centrioles, and inappropriate phosphorylation of centrosomal proteins (10, 14, 23, 24, 32, 34). Breast tumor cells frequently have functionally abnormal centrosomes that exhibit increased nucleation of MTs (24).
BRCA1 plays a role in maintaining the centrosome number in breast cells. The first evidence that BRCA1 may have an extranuclear role came from its localization during M phase to the centrosomes, where it binds γ-tubulin (18, 19), a component of centrosomes that nucleates MTs as part of the γ-TuRC (γ-tubulin ring complex) (49). Also, murine cells deficient in BRCA1 accumulate extra centrosomes (47), and in a transient assay, inhibition of BRCA1 in several human breast cell lines caused centrosome amplification (36, 39).
We have shown that BRCA1/BARD1 ubiquitinate several centrosomal proteins in vitro and that one of the targets is γ-tubulin. A lysine on γ-tubulin (lysine 48) that is ubiquitinated by BRCA1/BARD1 was mutated and expressed in cells, resulting in amplification of the centrosome number. These results indicate that the ubiquitination of γ-tubulin is one of the mechanisms by which the centrosome number is regulated by BRCA1 (39).
While it is clear that BRCA1 regulates the centrosome number in breast cells, it is not known whether BRCA1 regulates the centrosome function, MT nucleation. Since centrosome hyperactivity is a hallmark of breast tumors, it might be anticipated that BRCA1 does regulate MT nucleation activity. We find that in living cells BRCA1 inhibits MT nucleation. Using purified components in a cell-free assay, we find that the ubiquitin ligase activity of BRCA1/BARD1 directly inhibits MT nucleation. These results link an activity of BRCA1, ubiquitination, with a phenotypic change, centrosomal hypertrophy, commonly observed in breast tumors.
MATERIALS AND METHODS
Plasmids.
The BRCA1 inhibitory fragment (BIF) of RNA helicase A (RHA) (amino acids 89 to 344) (36) was cloned into the pCDNA5/FRT/TO vector (Invitrogen). Hemagglutinin (HA)-tagged γ tubulin and the mutants were cloned as described previously (39). BRCA1-FLAG and BARD1 baculoviruses were constructed according to the FastBac system (Invitrogen). The BARD1 gene (45) was kindly provided by J. Chen and D. M. Livingston. GFP-centrin (33) was a kind gift from Michel Bornens. γ-Tubulin mutants were expressed as described previously (39). Green fluorescent protein (GFP)-γ-tubulin was kindly provided by K. Munger, and the K48R and K344R point mutants of γ-tubulin were subcloned into the pEgfp vector with a carboxy-terminal GFP tag.
Antibodies.
Anti-γ-tubulin and anti-α-tubulin (Sigma) were used at a 1:1,000 dilution for both immunoblots and immunofluorescence. Anti-BRCA1 was made as described previously (36) and used at a 1:3,000 dilution for immunoblots and a 1:2,500 dilution for immunofluorescence. An anti-BARD1 antibody was kindly provided by J. Harb (University of Nantes), and an antipericentrin antibody was kindly provided by S. Doxsey (University of Massachusetts) (12). An anticentrin antibody was kindly provided by J. Salisbury (Mayo Clinic Foundation).
Cell culture and transfection.
Hs578T (ATCC cell line HTB-1216), MCF7, and HeLa S3 cells were cultured according to ATCC recommendations. Cells were blocked at the S phase of the cell cycle by treatment with 20 mM hydroxyurea (HU) or 2 mM thymidine for 18 h. Fluorescence-activated cell sorter (FACS) analysis was carried out using standard protocols with a FACSCalibur instrument (BD Biosciences).
Transfections with various plasmid DNAs were carried out using Lipofectamine 2000 (Invitrogen), and protein expression and phenotypic changes were observed 2 days posttransfection. The GFP-centrin-encoding plasmid was cotransfected to mark transfected cell centrosomes. Short interfering RNA (siRNA) transfection was performed using Oligofectamine (Invitrogen), and further experiments were done 2 days posttransfection. The various oligonucleotides used were designed either from the coding region of BRCA1 (BRCA1-a, starting at nucleotide 205 [UCACAGUGUCCUUUAUGUA]; BRCA1-b, starting at nucleotide 2616 [AAGGUUUCAAAGCGCCAGUCA]) or from the 3′ untranslated region (3′ UTR) (BRCA1-c, starting at nucleotide 5787 [5′-AAGCUCCUCUCACUCUUCAGU-3′]) (15, 39). The control oligonucleotide targets luciferase mRNA (GL2 [AACGUACGCGGAAUACUUCGA]) (Dharmacon).
MT regrowth assay.
Cells were treated with 25 μM nocodazole containing cold medium for 40 min, and microtubules were allowed to regrow in warm medium without nocodazole for 2 to 15 min. At the indicated time points in each experiment, the medium was replaced with 200 μg/ml saponin in order to extract tubulin protomers, and the cells were subsequently stained for γ-tubulin (green) and for α-tubulin (red). We counted 100 to 150 cells per condition and scored for three different aster morphologies.
Immunofluorescence.
Cells were treated with 200 μg/ml saponin at room temperature for 2 min and fixed in cold methanol before staining with primary antibodies diluted 1:1,000 in 1× phosphate-buffered saline containing 3% bovine serum albumin and 0.1% Triton X-100.
For BRCA1 staining, the cells were fixed in cold acetone and the BRCA1 antibody was used at a 1:2,500 dilution. For BARD1 and pericentrin staining, cells were fixed in methanol and the primary antibodies were used at a 1:100 dilution. Cells were washed with phosphate-buffered saline plus 0.1% Triton X-100 and stained with secondary antibodies. Images were viewed using the 60× or 100× objective lens with a Nikon Eclipse TE2000-S microscope and captured using a model 2.3.1 SPOT digital camera. Images were processed using advanced SPOT software.
Protein purification.
The BRCA1 and BARD1 baculoviruses were coinfected in SF9 or Hi-Five insect cells using standard protocols and purified using M2-agarose (Sigma) as described previously (39). Protein concentrations were determined after sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis by comparing the intensities of Coomassie-stained polypeptides of BRCA1 and BARD1 with known protein standards.
Centrosome fractions were prepared from Hs578T and HeLa S3 cells according to a protocol previously described (5, 26). Xenopus extract was prepared as described previously (11, 29).
In vitro ubiquitination and MT nucleation assays.
Reaction mixtures contained 1× BRB80 [80 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (K-PIPES), 1 mM MgCl2, 1 mM EGTA], 2 mM ATP, 4 mM MgCl2, 50 μM MG132, 1 mM ubiquitin, 200 nM E1, 5 μM UbcH5c-his (the vector was a kind gift from R. Baer), and 30 to 40 nM of BRCA1-BARD1. The centrosome fraction (∼500 to 1,000 ng) was added to the reaction and incubated at 37°C for 30 min. The reaction mixtures were transferred to ice, and 10 μl of Xenopus extract (20 mg/ml) was added along with 1 mM GTP; then the reaction mixtures were incubated at 23°C for as long as 20 min to allow aster formation. The asters were fixed with 1% glutaraldehyde and spun through a 40% glycerol cushion onto glass coverslips precoated with poly-l-lysine. The asters were then fixed with cold methanol and visualized by immunostaining with anti-α-tubulin and anti-γ-tubulin antibodies as described under “Immunofluorescence” above.
For densitometric quantification of the MT content of asters, asters were photographed using a 60× objective lens, with the same exposure parameters. Using the histogram tool in Adobe Photoshop, the MT content of the asters was measured as the product of the mean intensity and the number of pixels. The average values (± standard errors of the means) were plotted for 20 asters from each condition.
For the experiment for which results are shown in Fig. 3Ca to e, the signal intensities for the different antibodies for 20 randomly picked cells, photographed using a 100× objective lens under the same exposure, were measured as described above. Plotted for each condition were the ratios of staining intensities for pairs of antibodies as shown in the figure, plus the standard errors of the means.
FIG.3.
BRCA1 inhibits centrosome function during early S phase. (A) Hs578T cells were transfected with a siRNA specific for BRCA1 (BRCA1a) or a control siRNA and 30 h posttransfection were either treated with either 20 mM HU or 2 mM thymidine or left untreated. The effects of these chemicals on the cell cycle were checked by FACS analysis (left panels). The positions indicating 2n and 4n DNA content are shown by arrowheads. Results of the MT regrowth assays (2-min time point) for these cells are shown at the right. Histograms are shown of the percentage of cells 48 h posttransfection with three different aster morphologies: no aster (white bars), small asters (gray bars), or large asters (black bars). (B) Hs578T cells were transfected with either BRCA1a or control siRNAs, followed by HU treatment as described for panel A, and subjected to the MT regrowth assay. Cells were fixed and stained for α- and γ-tubulin (left) or 4′,6′-diamidino-2-phenylindole (DAPI) (right). Representative fields are shown containing cells transfected with a siRNA specific for the control (top) or for BRCA1 (bottom), and these cells were assayed for MT regrowth for 2 min after removal of nocodazole. Bar, 10 μm. (C) (a) Hs578T cells were transfected with either a BRCA1-specific siRNA (BRCA1a) (bottom) or a control siRNA (top), and 30 h posttransfection, cells were treated with 20 mM HU. At 48 h posttransfection, the cells were treated with nocodazole for 40 min to depolymerize MTs, and then cells were fixed and stained for α- as well as γ-tubulin. Under these conditions, α-tubulin stains the centriole. (b) Signal intensities for α- and γ- tubulin staining, as in panel Ca, were measured for 20 randomly selected cells. The ratio of the mean intensity of γ-tubulin to that of α-tubulin was plotted for the cells transfected with a control siRNA or a siRNA specific for BRCA1. Under similar conditions the ratios of mean intensities for γ-tubulin to centrin (c), pericentrin to α-tubulin (d), and pericentrin to centrin (e) were plotted for 20 randomly selected cells.
RESULTS
Inhibition of BRCA1 leads to overactive centrosomes.
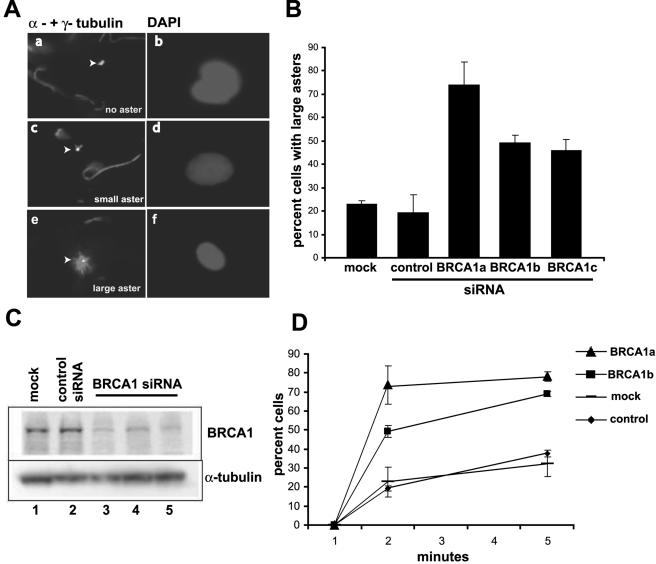
Centrosomes are the major MT nucleation centers. In order to test whether BRCA1 regulates the MT nucleation activity of the centrosome, we used the MT regrowth assay (9, 31). Hs578T breast cancer cells were utilized for these assays, since they are of breast origin and since they have high transfection efficiency. These cells were treated with nocodazole for 40 min in chilled medium to depolymerize microtubules, and then, when fresh prewarmed medium without nocodazole was added back, centrosomes initiated MT nucleation. Cells were fixed and stained for γ-tubulin and α-tubulin. Inactive centrosomes did not form asters (no associated MTs) (Fig. 1A, left panel). Small asters were visualized as positive for both γ- and α-tubulin, with extremely short and few (one or two) rays of MTs. Large asters had centrosomes positive for both γ- and α-tubulin, and the MTs were longer and had a greater number of rays (10 or more discernible fibers).
FIG. 1.
Inhibition of BRCA1 expression caused hyperactive MT regrowth in Hs578T cells. (A) Representative fields of the three different aster morphologies scored in the in vivo MT regrowth assay are shown. The left panels show α- and γ-tubulin staining, which mark MTs and centrosomes, respectively, and the right panels show 4′,6′-diamidino-2-phenylindole (DAPI) staining, which marks the position of the nucleus. All images were reproduced at the same magnification. Inactive centrosomes (Aa) and small asters (Ac) were obtained after transfection of Hs578T cells with the control siRNA. Large asters (Ae) were observed in cells transfected with the BRCA1-specific siRNA. Large asters have ∼10 microtubules of length, comparable to the nucleus, whereas small asters have 1 or 2 very short microtubules associated with the centrosome. Arrowheads indicate centrosomes and asters. (B) Hs578T cells were transfected with BRCA1-specific siRNAs, a control siRNA specific for luciferase, or no siRNA (mock). Forty-eight hours posttransfection, cells were subjected to the MT regrowth assay. The cells were fixed and stained after MT regrowth was allowed for 2 min. The percentages of cells with large asters, plus the standard errors of the means, from three independent experiments are graphed. (C) Western blot analysis of BRCA1 protein levels after cells were either transfected with siRNAs specific for the control (lane 2), BRCA1a (lane 4), BRCA1b (lane 3), or BRCA1c (lane 5) or mock transfected (lane 1). α-Tubulin levels were determined on the same blot as a control for equal loading. (D) Time course of the regrowth assay and the appearance of cells containing large asters after either no siRNA transfection (mock) or transfection with two different siRNAs specific for BRCA1 (BRCA1a and BRCA1b) or a control siRNA, as indicated. The data for the 2-min time point were averaged from three experiments, and the data for the 5-min time point were averaged from four experiments.
Transfection of Hs578T cells with siRNAs specific for BRCA1 dramatically affected centrosome function in this regrowth assay. Three different siRNAs each reduced BRCA1 protein levels to less than 50% of the control levels 48 h posttransfection (Fig. 1C). Two of these siRNAs were directed to the BRCA1 coding sequences, and one was specific to the 3′ UTR. Cells transfected with either the control or the BRCA1-specific siRNA were coimmunostained for BRCA1 and γ-tubulin. Cells transfected with the BRCA1 siRNA showed a significant decrease in BRCA1 staining (data not shown). Reduction of BRCA1 content was associated with a 2.5- to 3-fold increase in cells with large asters compared to the control siRNA or mock-transfected cells after MT repolymerization was allowed for 2 min (Fig. 1A and B). A time course, with scoring for the percentage of cells with large asters, showed that the BRCA1 knockdown caused a significantly higher rate of accumulation of cells with large asters within 5 minutes of removal of nocodazole compared to the control (Fig. 1D). The percentage of cells with large asters approached a plateau of about 100% for control cells after about 20 min of MT regrowth (data not shown), but inhibition of BRCA1 expression caused the cells to reach the plateau earlier. We tested whether the centrosome hyperactivity was reversed by overexpressing BRCA1 in siRNA-transfected cells. The BRCA1c siRNA was specific for the 3′ UTR of the BRCA1 gene and did not affect the cotransfected cytomegalovirus promoter-driven expression of the BRCA1 coding sequences. Cotransfection of both the BRCA1c siRNA and BRCA1 reversed the effect of the BRCA1-specific siRNA alone with regard to both the occurrence of supernumerary centrosomes and the hyperactivity of the MT regrowth assay (unpublished observations). These results suggest that BRCA1 is directly involved in controlling centrosome function and number.
To complement these observations, we tested whether functionally blocking BRCA1 results in a similar centrosome phenotype. A fragment of the RHA protein interacts with BRCA1 amino acid residues 1650 to 1800 (4). When this fragment of RHA (residues 89 to 344), called BIF, is expressed in cells, it inhibits BRCA1 function (36, 39). The BIF fragment was expressed in cells by transfection in a cytomegalovirus promoter-containing vector, and in order to mark centrosomes in transfected cells, a plasmid expressing GFP-centrin was cotransfected. In these experiments, the regrowth assay was fixed at the 5-min time point. Similar to the siRNA results, we observed that BIF expression also leads to overactive centrosomes (Fig. 2). By using these two techniques, siRNA against BRCA1 and BIF peptide inhibition of BRCA1, the data clearly indicate that BRCA1 negatively regulates the rate of MT nucleation by centrosomes.
FIG. 2.
Expression of a BRCA1-inhibiting peptide, BIF, caused hyperactive aster formation. (A) Schematic representation of BRCA1 showing the various domains. The RING domain (residues 1 to 110) is present at the amino terminus, residues 504 to 803 have been shown to bind γ-tubulin (18), and residues 1650 to 1800 bind to RHA. The BRCA1 binding domain of RHA (residues 89 to 344), named BIF, is shown. Also indicated is the BRCA1 carboxy-terminal domain (BRCT). (B) Hs578T cells cotransfected with either the vector or the BIF plasmid, along with the GFP-centrin plasmid. Forty-eight hours posttransfection, cells were subjected to the MT regrowth assay. The cells were fixed and stained after MT regrowth was allowed for 5 min. MTs were stained with an α-tubulin antibody, and the GFP-centrin marked the centrosomes of transfected cells (left panels). DAPI (4′,6′-diamidino-2-phenylindole) was used to stain the nuclei (right panels). Bar, 10 μm. (C) Histogram of the percentage of cells with the three different aster morphologies after transfection with the vector or the BIF plasmid as described for panel B. The results of two repeat experiments were averaged.
The BRCA1 effect on centrosomal microtubule nucleation is maximal at early-S phase in the cell cycle.
BRCA1 has been shown to localize to centrosomes during mitosis (19, 25). The expression of BRCA1 is cell cycle regulated, with maximal expression at S phase, when most of it localizes to discrete foci within the nucleus (37). To examine whether cells arrested in S phase were different from asynchronously growing cells with regard to the MT regrowth assay, Hs578T cells were blocked at early-S phase by treatment with HU or thymidine (Fig. 3A). Cells were transfected with siRNAs, as before, and 30 h posttransfection, 20 mM HU or 2 mM thymidine was added to the medium. At 48 h posttransfection, these cells were tested for cell cycle arrest by FACS analysis (Fig. 3) and for centrosome function by the regrowth assay. Both HU and thymidine cause an S-phase arrest due to depletion of deoxynucleotide triphosphates, but FACS analysis of the treated cells revealed that the HU block occurred at an earlier stage of S phase, with lower DNA content per cell, than the thymidine block (Fig. 3A). In these experiments, the transfection of the siRNA for BRCA1 or the control did not affect cell cycle progression (data not shown).
In cells blocked with HU and transfected with the control siRNA, the centrosomes were almost completely inactive. At the time point used in this regrowth assay, 86% of the cells had no asters and the remaining 14% had small asters. There were no cells with large asters (Fig. 3A). By contrast, over half of the cells transfected with a siRNA specific for BRCA1 had large asters. The effects were slightly different in the cells arrested at a later time point in S phase by treatment with thymidine. Among cells from the control transfection, most had no asters, but the cells transfected with a BRCA1-specific siRNA predominantly had small asters (Fig. 3A, bottom).
BRCA1 has been detected associated with centrosomes during mitosis (19, 25), but association of this tumor suppressor protein with centrosomes at other stages in the cell cycle has not been reported. We tested whether BRCA1 is also associated with the centrosome at early S phase, when BRCA1 strongly inhibited MT nucleation. Cells were treated with nocodazole for 40 min, as described above for the in vivo MT regrowth assay, to depolymerize MTs prior to staining for BRCA1 and γ-tubulin (Fig. 4). Thus, BRCA1 localization to centrosomes would not be dependent on MTs, and positive colocalization with the centrosome would indicate that BRCA1 was stably associated. We found that BRCA1 was detected at the centrosome in HU-blocked and aphidicolin-blocked cells (Fig. 4a to d). Centrosomes were stained with a γ-tubulin-specific antibody, and colocalization with BRCA1 was evaluated using a BRCA1-specific antibody. HU-blocked cells were found to be in early S phase by FACS analysis (Fig. 3A), and the cells contained two juxtaposed centrosomes (Fig. 4a). In these cells, significant amounts of BRCA1 were detected in the nucleus, and BRCA1 was also significantly present at the centrosome, which was clearly distinct from the nucleus in the cell shown in Fig. 4b. Aphidicolin-blocked cells were halted at a later stage of S phase, since these cells had two centrosomes, which were separated by about 2 to 8 μm (Fig. 4c). The BRCA1 content of these centrosomes was detectable (Fig. 4d), but at lower levels than observed in the HU-blocked cells.
FIG. 4.
BRCA1 localizes to centrosomes throughout the cell cycle. Hs578T cells were either blocked with 20 mM HU (a and b) or 1 μg/ml aphidicolin (c and d) for 18 h. MTs were depolymerized using 25 μM nocodazole for 40 min on ice. Nocodazole was washed off, and cells were extracted with saponin at room temperature, fixed with cold acetone, and stained for BRCA1 and γ-tubulin. Asynchronous cells were also stained for BRCA1 and γ-tubulin using the same protocol, and cells from different cell cycle stages were observed based on the stage of duplication of their centrosomes. Cells in G1 (e and f), early S (g and h), S (i and j), and M (prophase) (k and l) phases are shown. All cells are shown at the same magnification. White arrowheads indicate the positions of centrosomes.
These results in Fig. 4a to d were derived from cells in which the replication cycle had been arrested. Since normal centrosome maturation follows the cell cycle, we evaluated the BRCA1 content in centrosomes in an asynchronous population of cells. The stage of the cell cycle could be determined by the stage of duplication of centrosomes. Cells in the G1 phase of the cell cycle had no BRCA1 in the nucleus, and the only BRCA1 detected was at the single centrosome (Fig. 4f). In cells with two adjacent centrosomes (early S phase), we observed BRCA1 in the nucleus and also a higher level of BRCA1 associated with the centrosome (Fig. 4h) than was observed at this organelle during G1. Centrosomes separated by 2 to 8 μm, at a later stage of S phase, reproducibly had detectable BRCA1, but at slightly lower levels (Fig. 4j). Consistent with published results, the level of BRCA1 associated with the centrosome during mitosis was substantially higher (Fig. 4l). The colocalization of BRCA1 and γ-tubulin throughout the cell cycle was also observed in HeLa and MCF7 cells, and in other experiments BRCA1 colocalized with centriole markers, such as α-tubulin, in nocodazole-treated cells (data not shown).
Taken together, these results indicate that BRCA1 is required to maintain the centrosomes in a quiescent state in early-S phase in Hs578T cells and that BRCA1 is present at the centrosome at the appropriate time. We also found BARD1 to be stably associated with centrosomes throughout the cell cycle (unpublished observations), suggesting that the function of BARD1 was also important for centrosomal activity.
We tested for the γ-tubulin content of centrosomes in living cells that had been transfected with either BRCA1-specific or control siRNAs, and cells were blocked in early-S phase using HU. We completely depolymerized the MTs in cells by a 40-min incubation with nocodazole in order to ensure that α/β-tubulin did not mask any antigenic epitope recognized by the anti-γ-tubulin antibody. This can be done because γ-tubulin translocation from the cytosol to the centrosome is not dependent on the MTs (20). In cells in which BRCA1 protein levels were decreased by siRNA transfection, we detected an increase in the association of γ-tubulin with the centrosome (Fig. 3Ca, b, and c). We also tested the centrosomal levels of another protein, pericentrin, which, like γ-tubulin, is found in the pericentriolar matrix. We found that pericentrin did not show any significant difference in levels associated with centrosomes in the control and the BRCA1 siRNA-treated cells (Fig. 3Cd and e). These observations indicated that one of the effects of BRCA1 activity is to regulate the level of γ-tubulin at the centrosomes. Since γ-tubulin participates in the process that initiates MT polymerization by the centrosome, regulation of γ-tubulin levels at the centrosome by BRCA1 could in turn regulate the MT nucleation function of the organelle.
γ-Tubulin is an important target of BRCA1-mediated regulation of MT nucleation by centrosomes in cells.
We had found that BRCA1/BARD1 ubiquitinate several proteins in centrosome preparations, and we determined that γ-tubulin was ubiquitinated on lysine-48 and lysine-344 (39). Expression of γ-tubulin containing a mutation that changes lysine 48 to arginine led to centrosome amplification (39). We expressed the arginine substitution variants at lysine 48 and lysine 344, K48R and K344R, in Hs578T cells (Fig. 5A), and tested whether mutation of these γ-tubulin residues had any effect on MT regrowth of centrosomes 48 h posttransfection. Surprisingly, both mutants caused centrosome hyperactivity (Fig. 5B), suggesting that ubiquitination of both of these residues by BRCA1 was required to inhibit aster assembly. On the same slide we counted cells with extra centrosomes (>2 per cell), and we found that only expression of the γ-tubulin K48R mutant, not the γ-tubulin K344R mutant, caused centrosome amplification (Fig. 5C). Clearly, expression of the γ-tubulin K344R mutant was specific for MT assembly and not centrosome duplication, indicating that these two processes of the centrosome are separable. This result suggests that BRCA1-dependent ubiquitination activity has multiple effects on the biology of the centrosome and that some specific modifications affect duplication while other modifications affect MT nucleation. We conclude from this experiment that if BRCA1/BARD1 cannot ubiquitinate these lysines, then the centrosome-dependent MT nucleation activity in cells is not inhibited.
FIG. 5.
Expression of mutant γ-tubulin in cells recapitulates the effect on MT regrowth observed with BRCA1 inhibition. (A) Hs578T cells were transfected with a plasmid expressing wild-type γ-tubulin (wt), γ-tubulin with lysine 344 mutated to arginine (K344R), or γ-tubulin with lysine 48 mutated to arginine (K48R), or the vector control (mock), and the expression of these different constructs was tested by immunoblot analysis using an antibody specific for the HA tag present on these proteins (top panel) or an anti-γ-tubulin antibody for the endogenous protein (bottom panel). (B) Hs578T cells were transfected with the various γ-tubulin-expressing constructs described for panel A and cotransfected with a GFP-centrin-expressing plasmid. Forty-eight hours posttransfection, the MT regrowth assay was done on these cells, and α-tubulin was stained. Those cells on the coverslip that were positive for expression of transfected DNA had green-stained centrosomes due to GFP-centrin expression (not shown). The percentage of transfected cells (expressing GFP-centrin) containing large asters is shown for each condition. (C) In the same slide used for panel B, the number of centrosomes per cell was counted using GFP-centrin. The percentage of transfected cells containing more than two centrosomes is shown in the histogram for each condition.
We tested whether the mutant γ-tubulin proteins localized to centrosomes. GFP-tagged fusion proteins of the wild type and the K48R mutant were transfected into cells and assayed by immunofluorescence microscopy. These fusion proteins localized to the centrosome (data not shown). In this analysis, the K344R mutant fused to GFP could not be detected at the centrosome, but it was expressed at very low levels, and we interpreted this to indicate that this fusion protein used for microscopic detection was functionally different from the HA-tagged mutant protein used in Fig. 5.
BRCA1-dependent ubiquitination directly inhibits centrosome-mediated MT nucleation.
In the in vivo assays for which results are shown in Fig. 1 to 3, BRCA1 clearly inhibited the MT nucleation activity of centrosomes, but the mechanism was unclear. The effects of inhibiting BRCA1 could have been indirect, via regulation of transcription or DNA repair, or direct, via, perhaps, the BRCA1-dependent ubiquitination activity. The results of Fig. 5 clearly implicate γ-tubulin as critical for regulating centrosome number and function in cells, and the amino acid residues previously identified as ubiquitinated by BRCA1 are important in this phenotype. Since we have previously shown that BRCA1/BARD1 ubiquitinate centrosome proteins and thereby regulate centrosome duplication (39), we tested whether this ubiquitination activity affects MT nucleation. Centrosomes were purified from asynchronously growing cells and were pretreated with purified full-length BRCA1/BARD1, E1, E2 (UbcH5c), and ubiquitin prior to their use for in vitro MT nucleation assays.
Meiotic Xenopus laevis egg extracts are used for in vitro MT nucleation assays as a source of tubulin and other cofactors (41). We tested whether centrosomes ubiquitinated by BRCA1/BARD1 still nucleate MTs upon incubation with Xenopus extract. Centrosomes that had been preincubated with ubiquitination factors (E1, E2, and ubiquitin) and BRCA1/BARD1 formed significantly smaller asters than did those from control reactions (Fig. 6A). The MT content per aster was four- to fivefold lower (Fig. 6B) when BRCA1/BARD1 and ubiquitination factors were all present in the reaction than when either or both were excluded. A modest effect of BRCA1-dependent ubiquitination activity on aster number was also seen (decreased to 73% of the control [data not shown]), but the effect on aster size was clearly more significant. In published experiments, centrosomes that were rendered inactive by treatment with high salt or urea have been shown to reacquire MT nucleation activity by recruitment of γ-TuRC from the Xenopus extract (7, 21, 27). We conclude that BRCA1/BARD1, along with the ubiquitination factors, covalently modified the centrosomes, thus preventing the MT nucleation function of the organelle.
FIG. 6.
BRCA1-dependent ubiquitination activity inhibits aster formation in vitro. (A) Centrosomes purified from Hs578T cells were preincubated with ubiquitination factors comprising E1, E2, and ubiquitin (Ub) and with 30 nM BRCA1/BARD1 (B/B) as indicated and were then allowed to form asters in the presence of Xenopus extract. The centrosomes were then spun onto glass coverslips and visualized by double staining with antibodies against α-tubulin (red) and γ-tubulin (green). Representative fields are shown containing asters formed after treatments as labeled in the bottom right corner of each panel. Centrosomes were preincubated without ubiquitination factors or BRCA1/BARD1 (top left), with ubiquitination factors (top right), with both ubiquitination factors and BRCA1/BARD1 (bottom left), or with BRCA1/BARD1 but without ubiquitination factors (bottom right). Bar, 10 μm. (B) The mean of the product of fluorescence intensity and area (Mt content), expressed in arbitrary fluorescence units, was calculated for 40 asters from each condition explained above for panel A in two independent experiments. The average values were normalized with respect to the control reaction, which had no BRCA1/BARD1, E1, E2, or ubiquitin added (set at 100%), and values are shown as percentages with the standard errors of the mean. (C) Centrosomes purified from HeLa S3 cells were preincubated with ubiquitination factors as for panel A, except that in each reaction a single component was omitted. The MT content of asters was calculated as described for panel B, and the average values normalized to a control reaction (set at 100%) are shown with standard errors of the means.
We also tested centrosomes purified from a non-breast cell line, HeLa, and found a significant decrease in the MT content per aster for asters formed in vitro by centrosomes preincubated with BRCA1/BARD1 plus ubiquitination factors. Using HeLa centrosomes, we tested for the requirement of individual components of the ubiquitination reaction that may be important in the inhibition of MT nucleation by centrosomes under these in vitro conditions. Exclusion of single components, E1, E2, or ubiquitin, from the ubiquitination reaction did not significantly reduce the MT content of asters formed in vitro, whereas the complete reaction containing all of the ubiquitination factors resulted in a high level of inhibition (Fig. 6C). There was a modest level of inhibition of MT nucleation when E1 was omitted from reactions, but this factor was required at low concentration and was likely present in the Xenopus extract used for these assays. Taken together, these results clearly indicate that BRCA1/BARD1-mediated ubiquitination of centrosomes directly inhibits the centrosomal microtubule nucleation activity.
We titrated the amount of BRCA1/BARD1 in these ubiquitination reactions to determine whether the inhibition of aster formation activity was dependent on BRCA1 concentration. HeLa centrosomes were subjected to ubiquitination reactions with increasing quantities of BRCA1/BARD1 from 0 nM to 60 nM. At the highest concentration of BRCA1/BARD1 tested, 60 nM, the MT content of asters dropped sixfold (Fig. 7Aa to e; quantitated in Fig.7Af). There was also a direct correlation between the amount of γ-tubulin associated with the centrosomes, the amount of monoubiquitinated γ-tubulin detected by Western blot analysis (Fig.7Ag), and the amount of BRCA1/BARD1 in the reaction. Preincubation of centrosomes with BRCA1/BARD1 resulted in smaller asters, as noted above, but also the γ-tubulin content of these asters was decreased (Fig.7Aa′ to e′). We tested two different mutants of BRCA1 by the in vitro aster formation assay. BRCA1(1-500) contains the RING domain, and in complex with BARD1 it is an equally effective ubiquitin ligase as the full-length BRCA1/BARD1 in the modification of phosphorylated RNA polymerase II (38) and of NPM1 (data not shown). BRCA1(1-500)/BARD1 ubiquitinate γ-tubulin weakly compared to the full-length protein (Fig.7Ag). BRCA1(1-500)/BARD1 at the highest concentration showed a modest 40% decrease in the MT content of asters compared to a sixfold drop in MT nucleation with the full-length BRCA1/BARD1. BRCA1(I26A) has a point mutation in the RING domain that abolishes binding to the E2 UbcH5c without hindering its heterodimerization with BARD1 (6). When used at the same concentrations as the full-length BRCA1/BARD1, BRCA1(I26A)/BARD1 had a dominant-negative effect in the in vitro assay. At the highest concentration of BRCA1(I26A)BARD1, the MT content of asters was twofold higher than that of asters in the control reaction without any BRCA1/BARD1 (Fig.7Af), and this mutant did not ubiquitinate γ-tubulin (Fig.7Ah). We note that the level of ubiquitination of γ-tubulin by BRCA1/BARD1 was not high, but the effect on MT nucleation in vitro of the BRCA1/BARD1 ubiquitination activity was high. While it is clear that γ-tubulin mutated at residues ubiquitinated by BRCA1 affects centrosome activity in vivo, we cannot conclude that BRCA1/BARD1 ubiquitination of γ-tubulin regulates this in vitro reaction. Nevertheless, these data clearly implicate BRCA1/BARD1 ubiquitination activity in inhibition of centrosome MT nucleation activity, and ubiquitination of γ-tubulin is correlated with this activity of BRCA1/BARD1.
FIG. 7.
Mutation or inhibition of the BRCA1/BARD1 ubiquitin ligase causes a loss of regulation of the MT nucleation function of centrosomes. (A) Centrosomes purified from HeLa S3 cells were preincubated with the ubiquitination factors and the following concentrations of BRCA1/BARD1: 0 nM (panel Aa), 7.5 nM (panel Ab), 15 nM (panel Ac), 30 nM (panel Ad), and 60 nM (panel Ae). After these preincubation reactions, centrosomes were incubated with Xenopus extract and resultant asters were processed as for Fig. 6. Asters were stained for γ-tubulin (green) and α-tubulin (red). Representative asters from each reaction are shown. The γ-tubulin content of the same asters is shown in panels a′ to e′. (Af) The MT content of 20 randomly chosen asters from each reaction was measured for reactions containing different concentrations of full-length wild-type BRCA1/BARD1 (diamonds), BRCA1(1-500)/BARD1 (triangles), and full-length mutant BRCA1(I26A)/BARD1 (squares). All reactions were normalized to the reaction in the absence of any added BRCA1/BARD1 (set at 100%). Mean MT contents and standard errors of the means are shown. (Ag) Centrosomes purified from HeLa cells were incubated with ubiquitination factors and either no BRCA1/BARD1 (lane 1), full-length wild-type BRCA1/BARD1 (B/B) at 15 nM (lane 2), 30 nM (lane 3), or 45 nM (lane 4), or BRCA1(1-500)/BARD1 at 15 nM (lane 5), 30 nM (lane 6), or 45 nM (lane 7). Samples were subjected to immunoblot analysis using a monoclonal antibody specific for human γ-tubulin. Monoubiquitinated γ-tubulin is indicated by the arrowhead at the right. The positions of the 50- and 64-kDa markers are shown on the left. (Ah) HeLa centrosomes were incubated as for panel Ag above, with either no BRCA1/BARD1 (lane 1), 30 nM full-length wild-type BRCA1/BARD1 (lane 2), or full-length mutant BRCA1(I26A)/BARD1 at 30 nM (lane 3) or 45 nM (lane 4). The immunoblot was analyzed as for panel Ag. (B) Centrosomes purified from HeLa cells were preincubated with E1, E2, ubiquitin, 30 nM full-length wild-type BRCA1/BARD1, and either 0.45, 0.9, or 3.5 μM of GST-BIF peptide or 3.5 μM of the GST-control peptide. The BIF peptide contains RHA amino acid residues 89 to 344, and the control peptide contains RHA residues 1 to 250.
We then tested whether the BIF polypeptide, which inhibits the BRCA1-dependent ubiquitination of γ-tubulin (39), affected the centrosome MT nucleation activity. Inclusion of the BIF peptide in MT nucleation reactions resulted in the reversal of the inhibition by BRCA1 (Fig. 7B). In fact, at the highest concentrations of BIF used, the MT content of the asters was 1.4-fold higher than that of those in the control reactions in which no BRCA1/BARD1 was added (Fig. 7B). A control peptide at the highest concentration had no effect on the MT nucleation assay. The larger aster size in the presence of BIF was similar to the larger asters observed in the presence of catalytically inactive BRCA1. We infer from this observation that BRCA1/BARD1 bind to the centrosome and fail to ubiquitinate it but instead protect the substrate from ubiquitination by the Xenopus extract used in these experiments. From the results of this in vitro assay, it is clear that the inhibition of centrosomal MT nucleation activity correlates with ubiquitination of centrosomes by BRCA1/BARD1 and that in the absence of this BRCA1 enzymatic function, centrosomes exhibit hyperactivity. Since a key mechanism by which the Xenopus extract activates centrosomes is via recruitment of γ-tubulin from the extract to the centrosome (27), and since we observed a decreased γ-tubulin content of the centrosomes after ubiquitination by BRCA1/BARD1 and incubation with Xenopus extracts (Fig. 7a′ to e′), our data suggest that the BRCA1-dependent ubiquitination inhibits γ-tubulin recruitment from the extract to the centrosome. The decreased γ-tubulin content at the centrosomes after ubiquitination was not due to degradation of the protein, since these reactions included MG132, a proteasome inhibitor. Rather, BRCA1-dependent ubiquitination of some centrosomal protein results in diminished γ-tubulin content of the centrosome and smaller asters. Loss of this BRCA1/BARD1 activity by mutation or by inhibition resulted in hyperactive centrosomes in vitro and in vivo.
DISCUSSION
In order to understand how loss of BRCA1 results in breast cancer, it has been a high priority to identify the role of the ubiquitin ligase function in breast cell biology. In this study, we find that the BRCA1-dependent ubiquitin ligase inhibits MT nucleation by the centrosome in the Hs578T cell line. BRCA1-dependent inhibition of centrosome MT nucleation activity was most pronounced in early S phase, suggesting that the cell depends on BRCA1 to maintain the centrosome in a relatively quiescent state at this stage of the cell cycle. We found that BRCA1 regulates γ-tubulin function in this process in two ways. First, expression in cells of γ-tubulin that is mutated at residues ubiquitinated by BRCA1/BARD1 resulted in the same phenotype as loss of BRCA1: hyperactive MT nucleation. Since mutation of the substrate (γ-tubulin) resulted in the same phenotype as inhibition of the enzyme (BRCA1), we conclude that BRCA1-dependent ubiquitination activity directed at γ-tubulin inhibits centrosome function in cells. Second, we found that BRCA1 regulated the level of γ-tubulin associated with centrosomes.
In cells, the observed effect of BRCA1 on centrosome function could be indirect, for example, by regulating transcription, but the in vitro assays using purified proteins and centrosomes revealed that BRCA1 directly regulated this process. In the in vitro assays, effects of BRCA1 on transcription or DNA damage repair were impossible, since there was no DNA in the reaction. Instead, BRCA1/BARD1, E1, E2, and ubiquitin were required to inhibit MT nucleation in vitro. A mutant of BRCA1 [BRCA1(I26A)] that is incapable of ubiquitinating substrates failed to inhibit MT nucleation. Wild-type BRCA1/BARD1-mediated inhibition was reversed by addition of an inhibitor (BIF peptide) that blocks this enzymatic function of BRCA1. The in vitro assay was certainly inhibited by the BRCA1-dependent ubiquitin ligase activity, and this activity caused a decrease in the γ-tubulin content of centrosomes. It was not clear whether the in vitro reaction was dependent on the BRCA1 ubiquitination of γ-tubulin or of another protein. The amount of γ-tubulin ubiquitinated in these reactions did not appear to exceed about 10%, but all centrosomes had a decrease in activity due to BRCA1-dependent ubiquitination. For this reaction to be regulated in vitro via γ-tubulin modification, a low concentration of this ubiquitinated γ-tubulin would have to have a profound effect. While unlikely, such an effect is possible, since γ-tubulin binds as a multimer in the γ-TuRC complex. Thus, our results indicate that BRCA1-dependent ubiquitination directly inhibits MT nucleation, but it is not clear whether the identified substrate or another is regulating this process in vitro.
We have shown previously that BRCA1/BARD1 ubiquitinate a number of proteins in the centrosome, including γ-tubulin (39). It is striking that two lysine residues of γ-tubulin are required to be available for ubiquitination in order to inhibit MT nucleation in cells yet one of these residues affects only centrosome duplication (39). This result indicates the specificity of the effects of different ubiquitination modifications. The data suggest that the ubiquitination of γ-tubulin disrupts γ-tubulin association with the centrosome. The recent crystal structure of the human γ-tubulin suggests that residues 50 and 350 of this protein are involved in lateral interactions with adjacent γ-tubulin molecules (2). Modifications of the lysines close to these interaction sites could lead to steric hindrance, causing diminished association in the γ-TuRC complex. We propose that the activity of BRCA1/BARD1 inhibits the forward reaction of γ-tubulin binding to γ-TuRC, thus producing the net effect of driving the equilibrium toward dissociation of γ-tubulin. An earlier study analyzed human γ-tubulin by scanning mutagenesis and expression in a mutant yeast lacking endogenous γ-tubulin. In these yeast cells expressing γ-tubulin mutated at lysine 344 or lysine 48, cold-sensitive phenotypes with defects in chromosomal segregation and cytokinesis were observed (17). The expression of a mutant γ-tubulin with lysine 344 changed to alanine (and other charged residues adjacent to lysine 344 also mutated to alanine) resulted in a dominant-negative phenotype and caused abnormal chromosomal segregation, leading to aneuploidy (17). Thus, the effect of this mutant was similar to that of the lysine 344 mutation tested in this study, which had a dominant effect when expressed in cells containing wild-type γ-tubulin. Unlike the results of the current study, when the mutant γ-tubulin (containing alanine substitutions at residues 339 and 341 to 344) was expressed in yeast, fewer microtubules were observed than with wild-type γ-tubulin (17). In this study we found a higher level of MT nucleation. The difference in the amount of microtubule nucleation may be due to the nature of the mutation and the background of the cell expressing it, but in each case the same process is regulated by γ-tubulin dependent on Lys-344. The concordance between the results of the scanning mutagenesis, with the identification of lysine residues modified by the BRCA1/BARD1 ubiquitin ligase (39), and the effects of the mutation of these residues on centrosome number and MT nucleation function is striking.
The siRNA approach only modestly inhibited BRCA1 expression and yet had a profound effect on centrosome MT nucleation (this study) and on centrosome number (39). In nonfamilial breast cancer cases, BRCA1 function tends not to be lost via mutation; rather, its expression is reduced by epigenetic downregulation of the gene (42, 44). We suggest that this function of BRCA1 at the centrosome is very sensitive to changes in its concentration and thus may be important in breast cancer cases in which BRCA1 levels are downregulated. Consistent with this idea, breast tumor cells have demonstrated hyperactive MT nucleation (24).
The results of the current study reveal an important aspect of the biology of cells that is regulated by BRCA1-dependent ubiquitination activity. While ubiquitination of γ-tubulin by BRCA1/BARD1 has a significant role in regulating MT nucleation, we cannot rule out contributions by other proteins. Another centrosomal protein that has been shown to be a target for BRCA1/BARD1-mediated ubiquitination is nucleophosmin (35), but this protein has been shown not to have a role in MT nucleation (48).
The centrosome organizes the MT network throughout the cell cycle. In mammalian cells, 80% of γ-tubulin is cytoplasmic and not associated with the centrosomes (28). In a study with live cells expressing GFP-tagged γ-tubulin, the protein was found to form a dynamic cytoplasmic pool that was constantly exchanged between the cytoplasm and the centrosome. During mitosis there is a sudden >3-fold increase of the GFP-tagged γ-tubulin content in the centrosome (20). This finding correlated with the previous observation that mitotic centrosomes exhibit enhanced MT nucleation capacity (as much as 5- to 10-fold) (22). Thus, γ-tubulin content in the centrosome regulates MT nucleation function. During early-S phase, when the centrosome is relatively quiescent with regard to MT nucleation, this low activity level of centrosomes depends on BRCA1. The presence of increased BRCA1 at the centrosomes at early-S phase and the resulting quiescence of centrosomes can be attributed to a BRCA1-dependent decrease in γ-tubulin recruitment to centrosomes (Fig. 3).
In summary, in these experiments we found that BRCA1/BARD1-dependent ubiquitination activity inhibits the MT nucleation function of centrosomes. Cancer cells frequently have centrosome hypertrophy, and we suggest that in some breast cancers, this phenotype may be due to loss of BRCA1 function. Taking our results together with published data indicating that BRCA1 regulates centrosome duplication, it is possible that disruption of BRCA1 causes centrosome abnormalities that result in aneuploidy.
Acknowledgments
We thank J. Harb, J. Salisbury, K. Munger, and S. Doxsey for plasmids and antibodies used in these experiments.
This work was supported by a Komen Foundation fellowship (S.S.), a Department of Defense Breast Cancer Research Program fellowship (L.M.S.), a National Science Foundation Graduate Research Fellowship (A.C.G.), a BK21 Research Fellowship from the Ministry of Education, Republic of Korea (M.J.K.), and grant CA90281 from the National Cancer Institute (J.D.P.).
REFERENCES
- 1.Alberg, A. J., and K. J. Helzlsouer. 1997. Epidemiology, prevention, and early detection of breast cancer. Curr. Opin. Oncol. 9:505-511. [DOI] [PubMed] [Google Scholar]
- 2.Aldaz, H., L. M. Rice, T. Stearns, and D. A. Agard. 2005. Insights into microtubule nucleation from the crystal structure of human gamma-tubulin. Nature 435:523-527. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, J. S., C. J. Wilkinson, T. Mayor, P. Mortensen, E. A. Nigg, and M. Mann. 2003. Proteomic characterization of the human centrosome by protein correlation profiling. Nature 426:570-574. [DOI] [PubMed] [Google Scholar]
- 4.Anderson, S. F., B. P. Schlegel, T. Nakajima, E. S. Wolpin, and J. D. Parvin. 1998. BRCA1 protein is linked to the RNA polymerase II holoenzyme complex via RNA helicase A. Nat. Genet. 19:254-256. [DOI] [PubMed] [Google Scholar]
- 5.Bornens, M., M. Paintrand, J. Berges, M. C. Marty, and E. Karsenti. 1987. Structural and chemical characterization of isolated centrosomes. Cell Motil. Cytoskel. 8:238-249. [DOI] [PubMed] [Google Scholar]
- 6.Brzovic, P. S., J. R. Keeffe, H. Nishikawa, K. Miyamoto, D. Fox III, M. Fukuda, T. Ohta, and R. Klevit. 2003. Binding and recognition in the assembly of an active BRCA1/BARD1 ubiquitin-ligase complex. Proc. Natl. Acad. Sci. USA 100:5646-5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buendia, B., G. Draetta, and E. Karsenti. 1992. Regulation of the microtubule nucleating activity of centrosomes in Xenopus egg extracts: role of cyclin A-associated protein kinase. J. Cell Biol. 116:1431-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couch, F. J. 2004. Genetic epidemiology of BRCA1. Cancer Biol. Ther. 3:509-514. [DOI] [PubMed] [Google Scholar]
- 9.Dammermann, A., and A. Merdes. 2002. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. J. Cell Biol. 159:255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Assoro, A. B., S. L. Barrett, C. Folk, V. C. Negron, K. Boeneman, R. Busby, C. Whitehead, F. Stivala, W. L. Lingle, and J. L. Salisbury. 2002. Amplified centrosomes in breast cancer: a potential indicator of tumor aggressiveness. Breast Cancer Res. Treat. 75:25-34. [DOI] [PubMed] [Google Scholar]
- 11.Desai, A., A. Murray, T. J. Mitchison, and C. E. Walczak. 1999. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 61:385-412. [DOI] [PubMed] [Google Scholar]
- 12.Dictenberg, J. B., W. Zimmerman, C. A. Sparks, A. Young, C. Vidair, Y. Zheng, W. Carrington, F. S. Fay, and S. J. Doxsey. 1998. Pericentrin and gamma-tubulin form a protein complex and are organized into a novel lattice at the centrosome. J. Cell Biol. 141:163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doxsey, S. 2001. Re-evaluating centrosome function. Nat. Rev. Mol. Cell Biol. 2:688-698. [DOI] [PubMed] [Google Scholar]
- 14.Fry, A. M., T. Mayor, and E. A. Nigg. 2000. Regulating centrosomes by protein phosphorylation. Curr. Top. Dev. Biol. 49:291-312. [DOI] [PubMed] [Google Scholar]
- 15.Ganesan, S., D. P. Silver, R. A. Greenberg, D. Avni, R. Drapkin, A. Miron, S. C. Mok, V. Randrianarison, S. Brodie, J. Salstrom, T. P. Rasmussen, A. Klimke, C. Marrese, Y. Marahrens, C. X. Deng, J. Feunteun, and D. M. Livingston. 2002. BRCA1 supports XIST RNA concentration on the inactive X chromosome. Cell 111:393-405. [DOI] [PubMed] [Google Scholar]
- 16.Hashizume, R., M. Fukuda, I. Maeda, H. Nishikawa, D. Oyake, Y. Yabuki, H. Ogata, and T. Ohta. 2001. The RING heterodimer BRCA1-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J. Biol. Chem. 276:14537-14540. [DOI] [PubMed] [Google Scholar]
- 17.Hendrickson, T. W., J. Yao, S. Bhadury, A. H. Corbett, and H. C. Joshi. 2001. Conditional mutations in gamma-tubulin reveal its involvement in chromosome segregation and cytokinesis. Mol. Biol. Cell 12:2469-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu, L. C., T. P. Doan, and R. L. White. 2001. Identification of a gamma-tubulin-binding domain in BRCA1. Cancer Res. 61:7713-7718. [PubMed] [Google Scholar]
- 19.Hsu, L. C., and R. L. White. 1998. BRCA1 is associated with the centrosome during mitosis. Proc. Natl. Acad. Sci. USA 95:12983-12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khodjakov, A., and C. L. Rieder. 1999. The sudden recruitment of gamma-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J. Cell Biol. 146:585-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klotz, C., M. C. Dabauvalle, M. Paintrand, T. Weber, M. Bornens, and E. Karsenti. 1990. Parthenogenesis in Xenopus eggs requires centrosomal integrity. J. Cell Biol. 110:405-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuriyama, R., and G. G. Borisy. 1981. Microtubule-nucleating activity of centrosomes in Chinese hamster ovary cells is independent of the centriole cycle but coupled to the mitotic cycle. J. Cell Biol. 91:822-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lingle, W. L., S. L. Barrett, V. C. Negron, A. B. D'Assoro, K. Boeneman, W. Liu, C. M. Whitehead, C. Reynolds, and J. L. Salisbury. 2002. Centrosome amplification drives chromosomal instability in breast tumor development. Proc. Natl. Acad. Sci. USA 99:1978-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lingle, W. L., W. H. Lutz, J. N. Ingle, N. J. Maihle, and J. L. Salisbury. 1998. Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity. Proc. Natl. Acad. Sci. USA 95:2950-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lotti, L. V., L. Ottini, C. D'Amico, R. Gradini, A. Cama, F. Belleudi, L. Frati, M. R. Torrisi, and R. Mariani-Costantini. 2002. Subcellular localization of the BRCA1 gene product in mitotic cells. Genes Chromosomes Cancer 35:193-203. [DOI] [PubMed] [Google Scholar]
- 26.Mitchison, T. J., and M. W. Kirschner. 1986. Isolation of mammalian centrosomes. Methods Enzymol. 134:261-268. [DOI] [PubMed] [Google Scholar]
- 27.Moritz, M., Y. Zheng, B. M. Alberts, and K. Oegema. 1998. Recruitment of the gamma-tubulin ring complex to Drosophila salt-stripped centrosome scaffolds. J. Cell Biol. 142:775-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moudjou, M., N. Bordes, M. Paintrand, and M. Bornens. 1996. γ-Tubulin in mammalian cells: the centrosomal and the cytosolic forms. J. Cell Sci. 109:875-887. [DOI] [PubMed] [Google Scholar]
- 29.Murray, A. W. 1991. Cell cycle extracts. Methods Cell Biol. 36:581-605. [PubMed] [Google Scholar]
- 30.Narod, S. A., and W. D. Foulkes. 2004. BRCA1 and BRCA2: 1994 and beyond. Nat. Rev. Cancer 4:665-676. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen, H. L., D. Gruber, and J. C. Bulinski. 1999. Microtubule-associated protein 4 (MAP4) regulates assembly, protomer-polymer partitioning and synthesis of tubulin in cultured cells. J. Cell Sci. 112:1813-1824. [DOI] [PubMed] [Google Scholar]
- 32.Nigg, E. A. 2002. Centrosome aberrations: cause or consequence of cancer progression? Nat. Rev. Cancer 2:815-825. [DOI] [PubMed] [Google Scholar]
- 33.Piel, M., J. Nordberg, U. Euteneuer, and M. Bornens. 2001. Centrosome-dependent exit of cytokinesis in animal cells. Science 291:1550-1553. [DOI] [PubMed] [Google Scholar]
- 34.Salisbury, J. L., W. L. Lingle, R. A. White, L. E. Cordes, and S. Barrett. 1999. Microtubule nucleating capacity of centrosomes in tissue sections. J. Histochem. Cytochem. 47:1265-1274. [DOI] [PubMed] [Google Scholar]
- 35.Sato, K., R. Hayami, W. Wu, T. Nishikawa, H. Nishikawa, Y. Okuda, H. Ogata, M. Fukuda, and T. Ohta. 2004. Nucleophosmin/B23 is a candidate substrate for the BRCA1-BARD1 ubiquitin ligase. J. Biol. Chem. 279:30919-30922. [DOI] [PubMed] [Google Scholar]
- 36.Schlegel, B. P., L. M. Starita, and J. D. Parvin. 2003. Overexpression of a protein fragment of RNA helicase A causes inhibition of endogenous BRCA1 function and defects in ploidy and cytokinesis in mammary epithelial cells. Oncogene 22:983-991. [DOI] [PubMed] [Google Scholar]
- 37.Scully, R., J. Chen, R. L. Ochs, K. Keegan, M. Hoekstra, J. Feunteun, and D. M. Livingston. 1997. Dynamic changes of BRCA1 subnuclear location and phosphorylation state are initiated by DNA damage. Cell 90:425-435. [DOI] [PubMed] [Google Scholar]
- 38.Starita, L. M., A. A. Horwitz, M. C. Keogh, C. Ishioka, J. D. Parvin, and N. Chiba. 2005. BRCA1/BARD1 ubiquitinate phosphorylated RNA polymerase II. J. Biol. Chem. 280:24498-24505. [DOI] [PubMed] [Google Scholar]
- 39.Starita, L. M., Y. Machida, S. Sankaran, J. E. Elias, K. Griffin, B. P. Schlegel, S. P. Gygi, and J. D. Parvin. 2004. BRCA1-dependent ubiquitination of γ-tubulin regulates centrosome number. Mol. Cell. Biol. 24:8457-8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Starita, L. M., and J. D. Parvin. 2003. The multiple nuclear functions of BRCA1: transcription, ubiquitination and DNA repair. Curr. Opin. Cell Biol. 15:345-350. [DOI] [PubMed] [Google Scholar]
- 41.Stearns, T., and M. Kirschner. 1994. In vitro reconstitution of centrosome assembly and function: the central role of gamma-tubulin. Cell 76:623-637. [DOI] [PubMed] [Google Scholar]
- 42.Taylor, J., M. Lymboura, P. E. Pace, R. P. A'hern, A. J. Desai, S. Shousha, R. C. Coombes, and S. Ali. 1998. An important role for BRCA1 in breast cancer progression is indicated by its loss in a large proportion of non-familial breast cancers. Int. J. Cancer 79:334-342. [DOI] [PubMed] [Google Scholar]
- 43.Venkitaraman, A. R. 2004. Tracing the network connecting BRCA and Fanconi anaemia proteins. Nat. Rev. Cancer 4:266-276. [DOI] [PubMed] [Google Scholar]
- 44.Wilson, C. A., L. Ramos, M. R. Villasenor, K. H. Anders, M. F. Press, K. Clarke, B. Karlan, J. J. Chen, R. Scully, D. Livingston, R. H. Zuch, M. H. Kanter, S. Cohen, F. J. Calzone, and D. J. Slamon. 1999. Localization of human BRCA1 and its loss in high-grade, non-inherited breast carcinomas. Nat. Genet. 21:236-240. [DOI] [PubMed] [Google Scholar]
- 45.Wu, L. C., Z. W. Wang, J. T. Tsan, M. A. Spillman, A. Phung, X. L. Xu, M. C. Yang, L. Y. Hwang, A. M. Bowcock, and R. Baer. 1996. Identification of a RING protein that can interact in vivo with the BRCA1 gene product. Nat. Genet. 14:430-440. [DOI] [PubMed] [Google Scholar]
- 46.Xia, Y., G. M. Pao, H. W. Chen, I. M. Verma, and T. Hunter. 2003. Enhancement of BRCA1 E3 ubiquitin ligase activity through direct interaction with the BARD1 protein. J. Biol. Chem. 278:5255-5263. [DOI] [PubMed] [Google Scholar]
- 47.Xu, X., Z. Weaver, S. P. Linke, C. Li, J. Gotay, X. W. Wang, C. C. Harris, T. Ried, and C. X. Deng. 1999. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol. Cell 3:389-395. [DOI] [PubMed] [Google Scholar]
- 48.Zatsepina, O. V., A. Rousselet, P. K. Chan, M. O. Olson, E. G. Jordan, and M. Bornens. 1999. The nucleolar phosphoprotein B23 redistributes in part to the spindle poles during mitosis. J. Cell Sci. 112:455-466. [DOI] [PubMed] [Google Scholar]
- 49.Zheng, Y., M. L. Wong, B. Alberts, and T. Mitchison. 1995. Nucleation of microtubule assembly by a gamma-tubulin-containing ring complex. Nature 378:578-583. [DOI] [PubMed] [Google Scholar]