Abstract
PDK-1 is a protein kinase that is critical for the activation of many downstream protein kinases in the AGC superfamily, through phosphorylation of the activation loop site on these substrates. Cells lacking PDK-1 show decreased activity of these protein kinases, including protein kinase B (PKB) and p70S6K, whereas mTOR activity remains largely unaffected. Here we show, by assessing both association of cellular RNAs with polysomes and by metabolic labeling, that PDK-1−/− embryonic stem (ES) cells exhibit defects in mRNA translation. We identify which mRNAs are most dramatically translationally regulated in cells lacking PDK-1 expression by performing microarray analysis of total and polysomal RNA in these cells. In addition to the decreased translation of many RNAs, a smaller number of RNAs show increased association with polyribosomes in PDK-1−/− ES cells relative to PDK-1+/+ ES cells. We show that PKB activity is a critical downstream component of PDK-1 in mediating translation of cystatin C, RANKL, and Rab11a, whereas mTOR activity is less important for effective translation of these targets.
Phosphoinositide-dependent kinase-1 (PDK-1) was identified by its ability to activate protein kinase B (PKB; also known as Akt) in the presence of the phospholipid products of phosphoinositide 3-kinase (1). Activation of PKB by PDK-1 is accompanied by phosphorylation on the activation loop residue (Thr-308) of PKB. The active conformation of PKB is stabilized by phosphorylation of Ser-473 at the C-terminal of this protein in a conserved hydrophobic motif (40), most likely by the mammalian target of rapamycin (mTOR) complexed to Rictor (29), although phosphorylation by DNA-PK may also occur under some circumstances (9). Subsequent to the discovery of PDK-1 as a PKB kinase, PDK-1 has been shown to phosphorylate many other protein kinases in the AGC family, including p70 ribosomal S6 Kinase (p70S6K), p90 ribosomal S6 kinase (p90rsk), different isoforms of protein kinase C (PKCs), and serum- and glucocorticoid-stimulated protein kinase (SGK) (reviewed in reference 35). In contrast to PKB, activation of other PDK-1 substrates does not require PtdIns(3,4,5)P3 binding since they lack PH domains (p70S6K, p90rsk, SGK, and PKCs). Instead, phosphorylation of the hydrophobic motif by distinct protein kinases creates a “docking” site for PDK-1, thereby triggering a PDK-1-mediated phosphorylation of a T-loop residue and activation of these enzymes (5, 11). For p70S6K, the protein kinase responsible for the phosphorylation of the hydrophobic motif site (Thr-389) is mTOR complexed to Raptor (7).
The regulation of mTOR is complex and still not fully understood. mTOR activity depends on signals generated by both extracellular growth factors and by intracellular nutrients (10). mTOR activity is restrained by a complex of two proteins hamartin and tuberin, the products of the TSC1 and TSC2 genes that are mutated in the inherited cancer predisposition syndrome tuberous sclerosis. This is achieved through the GTPase activating protein (GAP) domain of tuberin, which hydrolyzes GTP associated with the small GTPase Rheb, thereby inhibiting its ability to activate mTOR (13, 17). A possible mechanism whereby growth factors induce activation of mTOR is through phosphorylation of tuberin by PKB, which antagonizes the inhibitory effect of the hamartin/tuberin complex on Rheb and mTOR (23). However, there is also evidence that regulation of tuberin can be independent of direct phosphorylation by PKB (8). The regulation of mTOR by nutrients is less understood, but recent evidence also points to the importance of the hamartin/tuberin complex (12, 18), although this is also controversial (30).
The major effect of mTOR activity within the cell is to regulate the initiation step of mRNA translation (14). mTOR phosphorylates p70S6K, thereby creating a docking site for PDK-1, which can then phosphorylate Thr-229 resulting in its activation. p70S6K phosphorylates a component of the 40S ribosome, S6, which has been suggested to increase the translation of a subset of mRNAs containing a stretch of pyrimidines at their 5′ termini (TOP mRNAs) (19). However, this notion has been challenged (31), and recent studies examining cells lacking both isoforms of p70S6K show defective S6 phosphorylation, yet translation of 5′ TOP containing mRNAs is not affected (24). Therefore, the role of p70S6K in mRNA translation needs to be reevaluated. The other known substrates of mTOR are three isoforms of small proteins that bind eukaryotic initiation factor eIF4E, 4EBP1, 4EBP2, and 4EBP3, of which regulation of 4EBP1 is the best understood. All nuclear transcribed mRNAs contain a 5′ methyl GTP Cap, which bind to eIF4E. eIF4E also binds eIF4G, a scaffold protein that recruits the RNA helicases eIF4A and eIF4B, which are required to unwind the secondary structure of 5′ untranslated regions (5′UTRs). 4EBP1 competes with eIF4G in binding eIF4E, thereby inhibiting translation initiation. Phosphorylation of 4EBP1 by mTOR prevents binding to eIF4E, thereby increasing translation, especially of mRNAs containing a high degree of secondary structure in their 5′UTRs (20). Rapamycin, a drug that modulates the ability of mTOR to phosphorylate its substrates, has been shown to have inhibitory effects on the translation of both 5′ TOP RNAs (32), and 5′ capped mRNAs (4). The identification of specific mRNAs that are translationally regulated by specific sign transduction pathways should help in unraveling how this is achieved.
Mouse embryonic stem (ES) cells lacking PDK-1 expression have been generated, in which the activity of many protein kinases in the AGC family, including PKB and p70S6K is compromised (39). Since both of these protein kinases have been implicated in the regulation of mRNA translation, through both mTOR and S6 phosphorylation, we investigated whether there was any defect in mRNA translation in ES cells lacking PDK-1 expression. We show that PDK-1−/− ES cells show a defect in mRNA translation, which is most apparent in the presence of nutrients but absence of growth factors. This defect manifests both as a decrease in translation of many mRNAs, as well as increased translation of a smaller number of specific mRNAs, as identified by microarray analysis of polysomal RNAs. Analysis of specific translational targets of PDK-1 demonstrates that these require PKB activity but are less dependent on mTOR and p70S6K. These results reveal a previously unanticipated pathway downstream of PDK-1 and PKB that regulates translation of specific mRNAs.
MATERIALS AND METHODS
Cell culture.
PDK-1+/+ and PDK-1−/− ES cells were grown on gelatinized 100-mm-diameter dishes in KnockOut Dulbecco modified Eagle medium (DMEM; Invitrogen) supplemented with 15% KnockOut serum replacement (SR; consisting of albumin, transferrin, and insulin) (Invitrogen), 0.1 mM nonessential amino acids, antibiotics (penicillin G and streptomycin), 2 mM l-glutamine, 0.1 mM 2-mercaptoethanol, and 1,000 U of ESGRO/ml (Chemicon). When indicated, cells were incubated with 100 nM rapamycin for 15 h.
Protein extraction, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), Western blot analysis, and assay of protein kinases.
PDK-1+/+ and PDK-1−/− ES cells (3 × 106 cells) were seeded into 100-mm-diameter dishes and grown in KnockOut DMEM supplemented with 15% SR for 12 h. Cells were cultured in KnockOut DMEM with or without SR for 13 h, and then cultured in KnockOut DMEM with or without 15% SR, or phosphate-buffered saline (PBS) with or without 15% dialyzed SR for 2 h. Cells were rinsed twice with PBS, and lysed in 0.5 ml of lysis buffer (50 mM Tris-HCl [pH 7.5], 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM dithiothreitol, protease inhibitor cocktail [Roche]). The homogenate was centrifuged at 12,000 × g using a microcentrifuge for 10 min at 4°C. Protein concentrations were determined by DC protein assay (Bio-Rad) with bovine serum albumin as a standard. Proteins (10 μg) were loaded onto a 4 to 20% polyacrylamide gel, separated, and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore). After transfer, membranes were incubated for 1 h in blocking buffer (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.1%[vol/vol] Tween 20, 5% nonfat milk). The membranes were incubated overnight at 4°C with anti-phospho-PKB (Thr308) antibody (Cell Signaling), anti-phospho-PKB (Ser473) antibody, anti-PKB antibody (Upstate), anti-phospho-p70S6K (Thr389) antibody (Cell Signaling), anti-p70S6K antibody (Cell Signaling), anti-phospho-S6 (Ser235/236) antibody (Cell Signaling), anti-S6 antibody (Cell Signaling), anti-phospho-4EBP1 (Ser65) antibody (Cell Signaling), anti-phospho-4EBP1 (Thr37/46) antibody (Cell Signaling), anti-4EBP1 antibody (Cell Signaling), anti-β-actin monoclonal antibody (Sigma), anti-Cystatin C antibody (R&D Systems), anti-Rab11a antibody (Zymed), and anti-RANKL monoclonal antibody (Oncogene). Immunoblot detection was carried out with a horseradish peroxidase-conjugated anti-rabbit, anti-mouse, or anti-goat immunoglobulin antibody (Amersham Biosciences) at a 1:2,000 dilution, and realized by ECL (Amersham Biosciences). Endogenous p70S6K was immunoprecipitated from 150 μg of soluble protein. Activity was assessed by using 50 μM short S6 peptide (RRRLSS), 5 mM MgCl2, 20 μM ATP, and 2.5 μCi of [γ-32P]ATP (Perkin-Elmer, Boston, MA) for 15 min. The reaction was terminated by adding sample buffer, and the phosphorylated peptide was resolved by SDS-PAGE. PKB was assayed in the same way, except with a rabbit polyclonal antibody generated against full-length PKB and a 30 μM concentration of the peptide Crosstide (GRPRTSSFAEG) as the substrate.
RNA isolation and polysomal fractionation.
A total of 90 μg of cycloheximide/ml was added to PDK-1+/+ and PDK-1−/− ES cells 10 min before harvesting. Cells were washed twice with PBS, treated with trypsin, and pelleted at 1,000 × g. Cell pellets were washed twice with PBS, resuspended in 150 μl of RSB (10 mM Tris-HCl [pH 7.4], 10 mM NaCl, 15 mM MgCl2) containing 100 μg of heparin/ml, and lysed in 1.2% Triton X-100-1.2% deoxycholate by brief mixing before and after 3 min of incubation on ice. Nuclei and cell debris were pelleted by centrifugation at 12,000 × g for 3 min in a microcentrifuge at 4°C. The supernatant was diluted with an equal volume of polysomal buffer (25 mM Tris-HCl [pH 7.4], 25 mM NaCl, 25 mM MgCl2, 0.05% Triton X-100, 0.14 M sucrose, 500 μg of heparin/ml). This was layered over 12 ml of a 5 to 56% (wt/wt) sucrose gradient. The gradients were centrifuged at 50,000 × g for 150 min at 4°C in an SW40 Ti rotor (Beckman). After centrifugation, the gradients were divided into 12 fractions. RNA was isolated from each fraction by using an RNeasy minikit (QIAGEN) and quantified by absorbance at 260 nm, and fractions 4 to 7 were pooled for cRNA synthesis. RNA quality, as well as the assembly of intact ribosomes across the sucrose gradient, was monitored by using an Agilent 2100 Bioanalyzer and is shown in Fig. S1 in the supplemental material.
Affymetrix GeneChip analysis.
Double-stranded cDNA was synthesized from 7 μg of total or polysomal RNA from three independent cell cultures. Preparation of cRNA, hybridization, and scanning of the arrays were performed according to the manufacturer's protocol (Affymetrix). The biotin-labeled cRNA was purified by using RNeasy minikit, fragmented, and hybridized to the GeneChip Mouse Expression Array 430A (Affymetrix). The data generated from the scan were analyzed by using the robust multichip analysis (RMA). Significance analysis of microarrays (SAM) was used to examine differential expression patterns between the different groups (34). This method uses a moderated t-statistic as a gene score. The false discovery rate (FDR) or expected proportion of false calls among genes identified as differentially expressed is estimated by using a permutation approach, based on a user-defined threshold. A 10-nearest-neighbor imputation engine was applied to estimate missing data, and 1,000 permutations were carried out to estimate the FDR.
Quantitative real-time PCR of total and polysomal RNA.
Total RNA was purified from PDK-1+/+ and PDK-1−/− ES cells by using the Agilent RNA purification kit (catalog no. 5185-600) according to the manufacturer's instructions. Polysomal RNA was purified as described above and precipitated with LiCl (2 M final concentration) overnight on ice. The pellet was washed twice with 70% ethanol and resuspended in RNase-free H2O. Then, 500 ng of polysomal RNA and 500 ng of total RNA were used for first-strand cDNA synthesis, and gene-specific primers were used to amplify each target cDNA. Real-time formation of product was monitored in an ABI 4700 analyzer through the hybridization of specific internal fluorescent probes (primers and internal fluorescent probes purchased from Applied Biosystems, Inc., and the sequences are available on their website). Ratios were calculated by subtracting the cycle threshold (CT) of the polysome target gene from the CT of either hGUS present in the polysomes or the CT of the target mRNA amplified from total mRNA. The resulting difference in cycle number (ΔCT) is the exponent of the base 2 (because of the doubling function of PCR), representing the fold difference of the template for these two genes.
Protein half-life determinations.
PDK-1+/+ and PDK-1−/− ES cells were grown in KnockOut DMEM with 15% SR for 30 h and then serum starved for 15 h. Cells were then exposed to 20 μg of cycloheximide/ml, and lysates were prepared after 15, 30, 60, 120, and 180 min. Western blot analysis was performed to determine the levels of cystatin C, Rab 11a, and RANKL.
Metabolic labeling.
PDK-1+/+ and PDK-1−/− ES cells (3 × 106 cells) were seeded into 100-mm dishes, grown in KnockOut DMEM with 15% serum-replacement for 12 h, and then serum starved for 13 h. Cells were preincubated at 37°C for 2 h in methionine/cysteine-free DMEM (Invitrogen). [35S]Methionine-[35S]cysteine mixture (Perkin-Elmer Life Sciences) was added for 10, 20, and 40 min, and cells were washed twice with PBS. The cells were lysed in 0.4 ml of lysis buffer (50 mM Tris-HCl [pH 7.5], 1% Triton X-100, 1 mM EDTA, 1 mM EGTA, 50 mM sodium fluoride, 1 mM sodium orthovanadate, 1 mM DTT, protease inhibitor cocktail). The supernatant was recovered after centrifugation for 10 min at 4°C. Cell extracts (5 μg) were loaded onto a 4 to 20% polyacrylamide gel, separated by SDS-PAGE, and stained with Coomassie brilliant blue. After Coomassie brilliant blue staining, the gel was dried onto 3MM paper and subjected to autoradiography. Radioactivity associated with cellular proteins was quantified by using ImageQuant software. For the immunoprecipitation analysis, the lysates (150 μg of each) were incubated 2 h at 4°C with the antibodies against cystatin C, Rab11a, and β-actin. The immunoprecipitates were collected by using protein G-Sepharose 4 fast flow (Amersham Biosciences), resolved by SDS-PAGE as described above, and exposed to film for autoradiography. For trichloroacetic acid (TCA) precipitation, 100 μl of 20% TCA was mixed with 40 μg of soluble protein, followed by incubation on ice for 1 h. Then, 10 μl was spotted onto 0.22-μm-pore-size GS Millipore membranes, and the membranes were vacuum filtered and washed twice with 10% TCA and once with 100% ethanol. The membranes were dried, and precipitated 35S-labeled material was measured in the presence of liquid scintillant. Cellular uptake of [35S]methionine-[35S]cysteine was measured by counting the radioactivity associated with normalized (by cell number) amounts of cell lysate in the presence of liquid scintillant.
RESULTS
To analyze PKB and mTOR-dependent signaling in PDK-1−/− ES cells in response to growth factors and nutrients, we assayed PKB and p70S6K phosphorylation and activation, as well as 4EBP1 phosphorylation. As previously demonstrated (39), phosphorylation of PKB on Thr-308 and PKB activity were both severely restricted in PDK-1−/− ES cells under all growth conditions (Fig. 1A). Phosphorylation of PKB on Ser-473 in PDK-1−/− cells was identical to PDK-1+/+ cells under all conditions examined, supporting the notion of an independent protein kinase phosphorylating this site, putatively Rictor/mTOR (29), but inconsistent with this being mediated by PDK-1 (2) or PKB itself (33). Activation of p70S6K and its phosphorylation on Thr-389 was abolished in the PDK-1−/− ES cells (Fig. 1B), similar to previous findings (39). PKB and p70S6K phosphorylation and activity in PDK-1+/+ ES cells were stimulated by the presence of growth factors (ES cell SR), as expected. Unexpectedly, when cells were grown in the absence of nutrients (Knockout DMEM) and growth factors, PKB phosphorylation on both sites, and its activity, was abolished not only in PDK-1−/− ES cells but also PDK-1+/+ ES cells (Fig. 1A). This is in contrast to the regulation of mTOR activity, which required only the loss of nutrients to inhibit the phosphorylation of its targets p70S6K and 4EBP1 (Fig. 1B and C). Unlike PKB and p70S6K, 4EBP1 phosphorylation was largely unaffected in PDK-1−/− ES cells, as judged by both phospho-specific antibodies directed against Ser-65, and the mobility of total 4EBP1 (Fig. 1C). This suggests that the contribution of PDK-1 and PKB to mTOR activity in ES cells growing under these conditions is not significant. Although the kinase(s) that mediates Thr-389 phosphorylation of p70S6K is not fully characterized, the finding that Thr-389 phosphorylation is severely reduced in the face of normal 4EBP1 phosphorylation suggests that mTOR is not the sole mediator of this event. Whether PDK-1 phosphorylates Thr-389 directly, as proposed by one group (3), or in combination with PKCζ, or partially through autophosphorylation (27) in ES cells has not yet been determined.
FIG. 1.
PDK-1−/− ES cells show defective PKB and p70S6K activation, but 4EBP1 phosphorylation is unaffected. (A) PDK-1+/+ and PDK-1−/− ES cells were cultured in the presence of DMEM and 15% knockout SR (lanes 1 and 2). SR was withdrawn for 13 h (lanes 3 and 4), and cells were transferred to PBS for 2 h either in the presence (lanes 5 and 6) or absence (lanes 7 and 8) of SR. Cells were lysed, and 10 μg of total soluble proteins was separated by SDS-PAGE, transferred to PVDF membranes, and Western blotted with phospho-antibody and total PKB antibody (upper panels). Then, 150 μg of soluble protein was used to immunoprecipitate endogenous PKB, which was subjected to a kinase assay using the peptide substrate Crosstide (lower panel). (B) Lysates were prepared in the same way as in panel A, except that PVDF membranes were probed with phospho-antibody and total p70S6K antibody and with phospho-antibody and total S6 antibody (upper panels). Then, 150 μg of soluble protein was used toimmunoprecipitate endogenous p70S6K, which was subjected to a kinase assay using the peptide substrate RRRLSS (lower panel). (C) Lysates were prepared in the same way as in panel A, except that PVDF membranes were probed with phospho-antibody, total 4EBP1 antibody, and β-actin antibody.
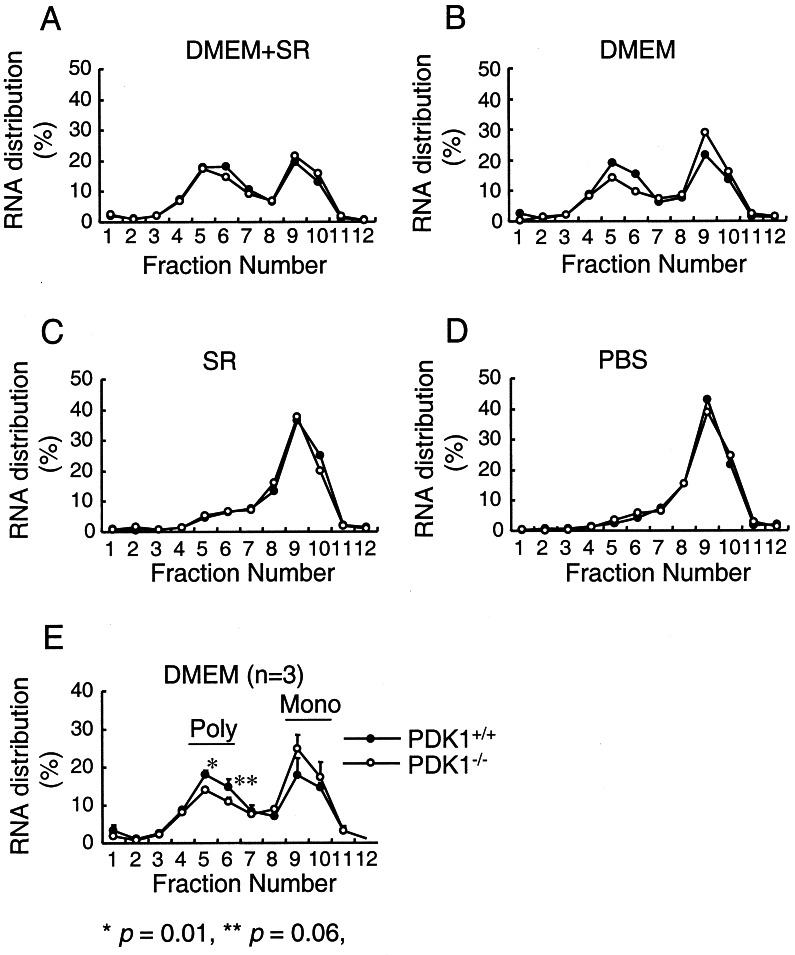
To examine whether PDK-1 contributes to the regulation of mRNA translation, we analyzed the accumulation of polyribosomes onto mRNAs (polysomes) in PDK-1+/+ and PDK-1−/− ES cells under the conditions of nutrient and growth factor depletion described above. When cultured in medium containing both nutrients and growth factors, approximately equal amounts of cellular RNA were distributed between polysomes and subpolysomes (containing monosomes and free RNA) in both cell types (Fig. 2A). Depletion of nutrients has a strong effect on the proportion of RNA associated with polysomes in both PDK-1+/+ and PDK-1−/− ES cells (compare Fig. 2A and C). Similarly, further depletion of growth factors from nutrient-deprived cells leads to an additional decrease in the polysome content from both cell types (Fig. 2D). Interestingly, when cells were cultured in the presence of nutrients but in the absence of growth factors, there was a difference between these two cell types in the amount of RNAs associated with polysomes, with PDK-1−/− cells showing a decrease relative to PDK-1+/+ ES cells (Fig. 2B). We repeated this experiment three times and demonstrated a statistically significant decrease of 25% in the amount of RNA associated with polysomes in the PDK-1−/− ES cells (Fig. 2E).
FIG. 2.
Association of RNAs with polysomes in PDK-1+/+ and PDK-1−/− ES cells. Lysates from PDK-1+/+ and PDK-1−/− ES cells cultured in the presence or absence of DMEM and SR were layered on top of 5 to 56% (wt/wt) sucrose gradients. After centrifugation, 1-ml fractions were collected (fraction 1 = 56% sucrose, fraction 12 = 5% sucrose) and used to purify RNA, which was measured by absorbance at 260 nm. Fractions 5 and 6 represent polysomal RNA, and fractions 9 and 10 represent free or monosomal RNA.
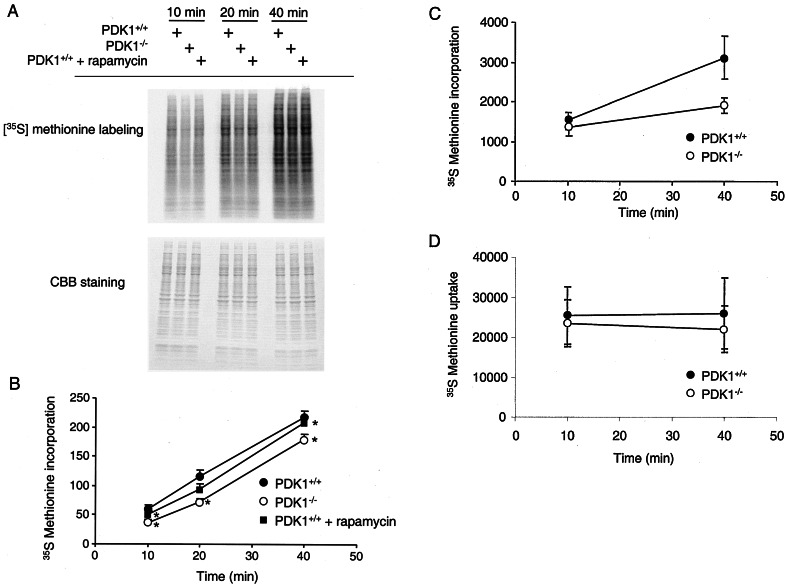
This small difference in polysomes in the PDK-1−/− ES cells could therefore be due to either a global decrease in translation of all RNAs in these cells, a strong decrease in specific RNAs, or a combination of both. To initially test these hypotheses, we incubated PDK-1+/+ and PDK-1−/− ES cells with medium containing 35S-labeled methionine and cysteine for increasing times to monitor the accumulation of all newly synthesized proteins. Figure 3A shows that, despite equal amounts of protein loaded from these two cell types, PDK-1−/− ES cells showed a decrease in the global translation of many proteins. Translation of all proteins detected by SDS-PAGE was quantitated, and the results are shown in Fig. 3B, giving an average decrease in global translation in PDK-1−/− ES cells of 33%. Global translation was also measured by precipitating cellular proteins by using TCA. This method also confirmed an average decrease in the rate of translation in PDK-1−/− ES cells of ∼24% (Fig. 3C). This decrease in translation was not due to differences in cellular uptake of 35S-labeled methionine and cysteine between these two cell lines (Fig. 3D). Rapamycin is a small molecule that inhibits the ability of mTOR to phosphorylate its substrates p70S6K and 4EBP1 (see Fig. 6B). Rapamycin inhibited mRNA translation in this assay, although to a lesser extent than loss of PDK-1 (Fig. 3A and B). Rapamycin also inhibited the accumulation of polyribosomes, in both PDK-1+/+ and PDK-1−/− ES cells (see Fig. S2 in the supplemental material).
FIG. 3.
PDK-1−/− ES cells display defective mRNA translation. (A) PDK-1+/+ or PDK-1−/− ES cells were transferred to methionine- and cysteine-free DMEM for 2 h. Then, 35S-labeled methionine and cysteine were added for the indicated times before the cells were harvested. A total of 10 μg of soluble proteins was separated by SDS-PAGE and stained with Coomassie brilliant blue (lower panel). The gel was then dried onto 3MM paper and subjected to autoradiography (upper panel). Where indicated, cells were treated with 100 nM rapamycin for 15 h prior to and after transfer into methionine- and cysteine-free DMEM. (B) Incorporated radioactivity from an experiment similar to panel A performed in triplicate was quantitated by using ImageQuant software. Asterisks indicate differences between the triplicates with a P value of <0.05, by using a paired two-tailed t test. (C) PDK-1+/+ and PDK-1−/− ES cells were treated as in panel A, except 40 μg of soluble proteins was precipitated by adding 20% TCA. Precipitated proteins were collected on 0.22-μm-pore-size filters and washed sequentially with 10% TCA and 100% ethanol. Dried filters were counted for 35S radioactivity in the presence of liquid scintillant. The experiment was performed in quadruplicate, and the standard deviations are indicated. (D) Cell lysate (normalized by cell number) was added to liquid scintillant and counted for 35S radioactivity. The experiment was performed in quadruplicate, and the standard deviations are indicated.
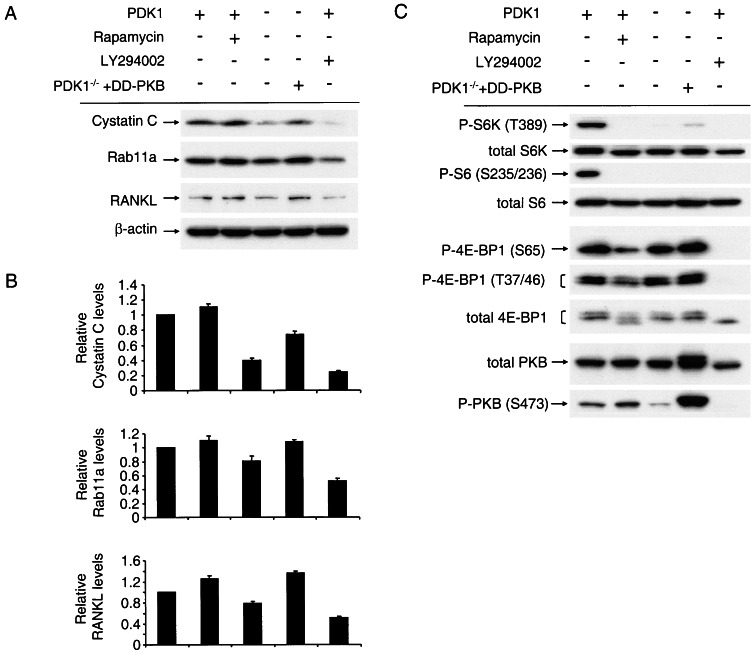
FIG. 6.
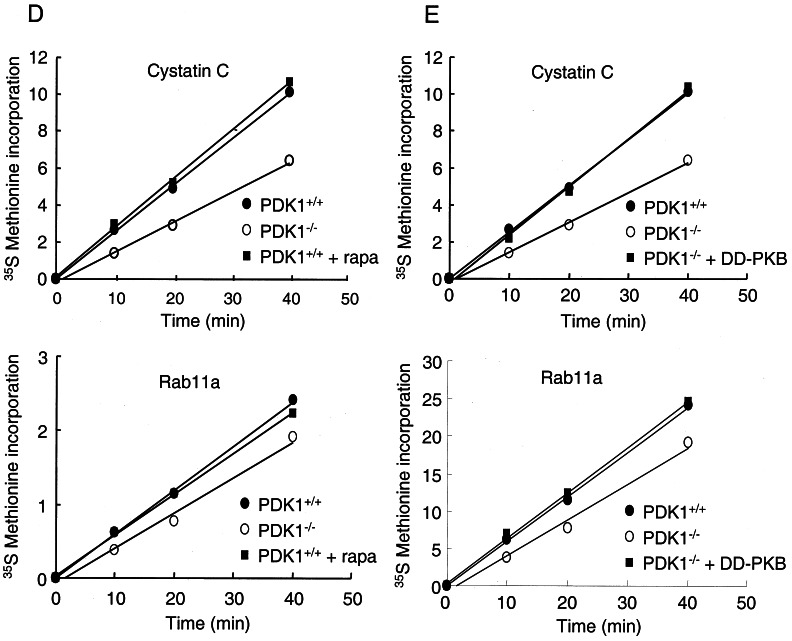
PDK-1 regulates translation of cystatin C, RANKL, and Rab11a through an mTOR-independent, PKB-dependent pathway. (A) PDK-1+/+ ES cells, PDK-1−/− ES cells, or PDK-1−/− ES cells stably expressing DD-PKB were cultured in DMEM, and soluble proteins were separated by SDS-PAGE. After transfer to PVDF membranes, antibodies against cystatin C, Rab11a, and RANKL were used to visualize the levels of these proteins. Where indicated, PDK-1+/+ ES cells were incubated with 100 nM rapamycin or 20 μM LY294002 for 15 h prior to cell lysis. (B) The levels of the proteins in panel A were quantitated from two experiments by using Odyssey software, and the differences form the mean values shown as error bars. (C) The same lysates from panel A were used for Western blotting with antibodies against phospho-p70S6K and total p70S6K, phospho-S6 and total S6, phospho-4EBP1 and total 4EBP1, phospho-PKB, and total PKB. (D and E) PDK-1+/+ ES cells, PDK-1−/− ES cells, or PDK-1−/− ES cells stably expressing DD-PKB were transferred to methionine- and cysteine-free DMEM for 2 h prior to the addition of 35S-labeled methionine-cysteine. Cells were harvested after the indicated times, and lysates used to immunoprecipitate cystatin C or Rab11a. Where indicated, rapamycin was added to the medium 15 h prior to and following transfer to methionine- and cysteine-free DMEM.
To determine which mRNAs were most dramatically translationally regulated in a PDK-1-dependent manner, we purified the RNAs present in the polysome fractions from PDK-1+/+ and PDK-1−/− ES cells grown in the presence of DMEM and absence of SR and made biotinylated cRNA for hybridization onto Affymetrix microarrays. For comparison, we also purified total RNA from both cell types and made biotinylated cRNA in the same manner. Triplicate samples for each condition were then hybridized to Affymetrix mouse 430A arrays representing ∼20,000 mouse genes. The SAM method was used to assess for statistically significant differential expression between the polysomal RNA pools of PDK-1−/− versus PDK-1+/+ ES cells. There were a total of 331 probes differentially expressed between these two pools. From these, 110 probes were highly over-represented and 221 were highly under-represented in the PDK-1−/− polysomal RNA pool, with an FDR of 0.26% (i.e., we expect less than 1 false discovery among the 331 differentially expressed probes). Analysis of total RNA purified from PDK-1+/+ and PDK-1−/− ES cells showed that many genes were highly differentially expressed between these two cell types. Using the same FDR, a total of 4,838 probes were identified as differentially expressed. From these, 2,410 were highly overexpressed and 2,428 were highly underexpressed in the PDK-1−/− ES cells. Of the 110 over-represented probes in the PDK-1−/− ES cell polysomes 80 were also increased in the total RNA pool, and of the 221 under-represented probes in the PDK-1−/− ES cell polysomes 138 were also decreased in the total RNA pool. Therefore, this cursory analysis indicates that 30 probes (representing 27 distinct RNAs/genes) were identified as translationally upregulated in the PDK-1−/− ES cells, while 78 probes (representing 72 distinct genes) were identified as translationally downregulated in the PDK-1−/− ES cells. These genes, and their fold regulation, are listed in Tables S1 and S2 in the supplemental material.
We first validated the differential association of six of these mRNAs with the polysomes of PDK-1+/+ and PDK-1−/− ES cells: three that showed increased association with polysomes and three that showed decreased association with polysomes. We applied quantitative real-time PCR to polysomal and total RNA isolated from these cells. This analysis demonstrated that all three of the mRNAs that showed increased association with polysomes in the PDK-1−/− ES cells (ATF4, BMP4, and Id4), as measured by microarray analysis, also showed increased polysome association by using Q-PCR (see Fig. S3 in the supplemental material). Of the three mRNAs identified by Affymetrix analysis as being downregulated in the polysomes of PDK-1−/− ES cells (cystatin C, RANKL, and IGF1), two also showed downregulation when normalized to an internal standard gene hGUS and two showed downregulation when normalized to total mRNA levels. The lack of apparent polysomal downregulation of cystatin C in this analysis is likely due to the fact that the hGUS standard is also downregulated in the polysomes of PDK-1−/− ES cells (data not shown). The lack of apparent polysomal downregulation of IGF1 in PDK-1−/− ES cells when normalized to total mRNA is due to the fact that IGF1 was strongly transcriptionally downregulated in PDK-1−/− ES cells, something that was not apparent from the microarray analysis. The reason for this discrepancy is unknown, but in general the Q-PCR results validate the microarray data quite well.
We next validated the translational regulation of some of these RNAs more directly based on the availability of relevant antibodies and their ability to detect the respective protein by immunoprecipitation or Western blotting in ES cells. Figure 4A, C, and E show the levels of cystatin C, Rab11a, and RANKL RNAs in both the total RNA fraction and the polysomal RNA fractions in PDK-1+/+ and PDK-1−/− ES cells. Figure 4B and D indicate the translation rate of these proteins as measured by immunoprecipitation from metabolically labeled cells at the indicated time points. Figure 4F confirms that the rate of actin translation is identical between PDK-1+/+ and PDK-1−/− ES cells, as expected from the equal distribution of actin RNA from the polysomes of PDK-1+/+ and PDK-1−/− ES cells (data not shown). The total steady-state levels of cystatin C, Rab11a, and RANKL were decreased in PDK-1−/− ES cells relative to PDK-1+/+ ES cells, a finding consistent with the decreased translation rates of these proteins in PDK-1−/− ES cells (Fig. 4G). To confirm that the different levels of these proteins were not due to changes in their stability, we also analyzed their stability after addition of cycloheximide. Figure 5 shows that cystatin C has an almost identical half-life of 33 min in both PDK-1+/+ and PDK-1−/− ES cells. Although Rab11a and RANKL had longer half-lives, making quantitation difficult under the time course performed, they did not show obvious differences in their stabilities in PDK-1+/+ and PDK-1−/− ES cells. Although cystatin C, Rab11a, and RANKL showed decreased protein levels consistent with their downregulation in polysomes of PDK-1−/− ES cells, two other proteins (IGFBP5 and Dikkopf-1), whose mRNAs were also downregulated did not show a consistent decrease. Potential reasons for this are described in the Discussion. The levels of the two proteins we examined whose mRNAs showed in increased association with polysomes (ATF4 and Id4) showed only a small, but reproducible increase in total protein levels. IGF1 protein levels are also decreased in PDK-1−/− ES cells, as expected from their strong transcriptional downregulation (see Fig. S4 in the supplemental material).
FIG. 4.
Validation of mRNAs translationally regulated by PDK-1. (A, C, and E) Graphical depiction of the levels of total and polysomal RNAs from the Affymetrix 430A arrays, with the standard deviations of the three independent hybridizations indicated by lines. Levels of cystatin C (A), Rab11a (C), and RANKL (E) in PDK-1+/+ (▪) and PDK-1−/− ( ) ES cells. (B, D, and F) PDK-1+/+ and PDK-1−/− ES cells were transferred to cysteine- and methionine-free DMEM for 2 h prior to addition of 35S-labeled methionine-cysteine. Cells were harvested after the indicated times, and lysates were used to immunoprecipitate cystatin C (B), Rab11a (D), or β-actin (F). The immunoprecipitates were then separated by SDS-PAGE and dried on 3MM paper; the incorporated radioactivity was measured by phosphorimager analysis, followed by quantitation by ImageQuant software. (G) PDK-1+/+ and PDK-1−/− ES cells were cultured in DMEM in the presence or absence of SR. Then, 10 μg of soluble proteins was separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with antibodies against cystatin C, Rab11a, RANKL, and β-actin.
) ES cells. (B, D, and F) PDK-1+/+ and PDK-1−/− ES cells were transferred to cysteine- and methionine-free DMEM for 2 h prior to addition of 35S-labeled methionine-cysteine. Cells were harvested after the indicated times, and lysates were used to immunoprecipitate cystatin C (B), Rab11a (D), or β-actin (F). The immunoprecipitates were then separated by SDS-PAGE and dried on 3MM paper; the incorporated radioactivity was measured by phosphorimager analysis, followed by quantitation by ImageQuant software. (G) PDK-1+/+ and PDK-1−/− ES cells were cultured in DMEM in the presence or absence of SR. Then, 10 μg of soluble proteins was separated by SDS-PAGE, transferred to PVDF membranes, and immunoblotted with antibodies against cystatin C, Rab11a, RANKL, and β-actin.
FIG. 5.
Stability of cystatin C, Rab11a, or RANKL is unaltered in PDK-1+/+ and PDK-1−/− ES cells. (A) PDK-1+/+ and PDK-1−/− ES cells were treated with 20 μg of cycloheximide (CHX)/ml for the indicated times. After harvesting the cells, 10 μg of soluble proteins were separated by SDS-PAGE and transferred to PVDF membranes. Immunoblotting with antibodies against cystatin C, Rab11a, and RANKL was performed. (B) The protein levels of cystatin C after the cycloheximide treatment in panel A were quantitated and are shown graphically.
Next, we addressed the pathways downstream of PDK-1 that could account for the observed regulation of mRNA translation. We used rapamycin to inhibit mTOR activity to determine whether this would mimic the effects seen in the PDK-1−/− ES cells. Surprisingly, rapamycin treatment of PDK-1+/+ ES cells caused no decrease in the steady-state levels of cystatin C, Rab11a, or RANKL and in fact resulted in small increases in these proteins (Fig. 6A and B). As expected, rapamycin effectively inhibited the phosphorylation of p70S6K, ribosomal protein S6, and the translational repressor 4EBP1 (Fig. 6C). We hypothesized that PKB was an important mediator of PDK-1 activity in regulating the observed effects on mRNA translation. Therefore, we examined the effect of stably expressing a form of PKB that does not require PDK-1 for its activity (Thr308Asp/Ser473Asp, termed DD-PKB) in the PDK-1−/− ES cells. Expression of DD-PKB at about the same levels as endogenous PKB increased the protein levels of cystatin C, Rab11a, and RANKL (Fig. 6A). DD-PKB expression slightly and variably increased the phosphorylation of p70S6K and 4EBP1 but had no effect on the phosphorylation of ribosomal protein S6 (Fig. 6B), as expected based on the absolute dependence of p70S6K phosphorylation on Thr-229 by PDK-1 for activation. Given the involvement of PKB in regulating the levels of cystatin C, Rab11a, and RANKL, the increase in these proteins seen after treatment with rapamycin may be due to the inhibition of PKB activity caused by p70S6K activity (22). To further validate a role for PKB in mediating the translational regulation in PDK-1+/+ ES cells, we treated these cells with LY294002. This compound inhibits phosphoinositide 3-kinase activity (36), resulting in inhibition of PKB phosphorylation (Fig. 6C). LY294002 is also an equipotent inhibitor of mTOR (6), resulting in dephosphorylation of its targets 4EBP1 and p70S6K (Fig. 6C). LY294002 also inhibited the expression of cystatin C, RANKL, and Rab11a in PDK-1+/+ ES cells (Fig. 6A). To confirm that the effects observed on the total protein levels of these PDK-1 targets were mediated through regulation of their translation, we again performed an analysis of [35S]methionine incorporation. Figure 6D demonstrates that rapamycin treatment has no effect on the [35S]methionine incorporation of cystatin C or Rab11a. However, the defective translation of cystatin C and Rab11a observed in the PDK-1−/− ES cells are reversed upon stable expression of DD-PKB (Fig. 6E; see also Fig. S5 in the supplemental material).
DISCUSSION
In this study, we examined the properties of ES cells lacking PDK-1 expression with respect to mRNA translation. These experiments were prompted by the previously published defects in signal transduction pathways in PDK-1−/− ES cells, many of which are thought to be important in regulating translation initiation. These include the lack of PKB activity in these cells (39), which could inhibit mTOR activity, due to a failure to inactivate the tuberin/hamartin complex (23). For example, in mouse embryo fibroblasts lacking PKBα and PKBβ, both basal and serum stimulated 4EBP1 phosphorylation was reduced, and basal (though not serum stimulated) p70S6K phosphorylation was also reduced (25). In addition, p90rsk, which is inactive in the PDK-1−/− ES cells (39), has also been shown to phosphorylate tuberin and contribute to its inactivation by mitogens (28). Surprisingly, however, there is little difference in mTOR activity in PDK-1−/− ES cells compared to PDK-1+/+ ES cells, as judged by the phosphorylation of 4EBP1 using both mobility and phospho-specific antibodies. Under conditions of nutrient deprivation in the presence of growth factors, a slight difference in the phosphorylation of 4EBP1 was detectable between these two cell types (Fig. 1C), but under all other growth conditions this difference was not apparent. A lack of effect on 4EBP1 phosphorylation in PDK-1−/− ES cells was also noted by Proud and coworkers (37). This suggests that the contribution of PKB activity to mTOR activity is different between various cell types and under distinct culture conditions. The other major target of PDK-1 that would be predicted to affect mRNA translation is p70S6K. Phosphorylation of ribosomal protein S6 was undetectable in PDK-1−/− ES cells, most likely due to the complete absence of p70S6K activity. Phosphorylation of S6 by p70S6K has been suggested to play a critical role in promoting the translation of a subset of mRNAs containing a stretch of pyrimidines at their 5′ termini (19). These mRNAs encode many components of the protein synthesis machinery and are translationally upregulated in response to growth factors. However, more recent evidence has questioned the importance of S6 phosphorylation in the translation of these mRNAs (31), making the current role of p70S6K in mRNA translation, if any, unknown. Nevertheless, PDK-1−/− ES cells show a clear defect in mRNA translation, as assessed by incorporation of radiolabeled methionine/cysteine, by precipitation of radioactive proteins with TCA, and by association of RNAs with polysomes. This latter defect was only apparent in cells grown in the presence of nutrients but the absence of growth factors. Whether this suggests that PDK-1 plays an important role in the signaling pathways initiated by nutrients or that growth factors can instigate alternative pathways that bypass the requirement for PDK-1 in mRNA translation remains to be elucidated.
As well as a global defect in the translation of many mRNAs, we also showed that a smaller number of mRNAs were more dramatically dissociated from polysomes in PDK-1−/− ES cells. Also some mRNAs actually showed an increased association with polysomes. The altered association of specific mRNAs with polysomes identified by microarray analysis was generally validated by using quantitative PCR. We were only able to validate a small proportion of the targets identified at the protein level due to limited availability of commercial antibodies appropriate for Western blotting and immunoprecipitation. Nevertheless, of five proteins we examined, whose mRNAs were decreased in polysomes of PDK-1−/− ES cells, three (cystatin C, Rab11a, and RANKL) showed consistently lower expression. In contrast, Dikkopf and IGFBP5 (shown in Fig. S2 in the supplemental material) did not show consistently decreased expression levels. Upon closer inspection of the data we noticed that Dikkopf is actually identified by SAM analysis as an RNA that is increased in the total RNA pool. Therefore, conflicting transcriptional and translational regulation may prevent reproducible changes in protein levels. In addition, the levels of IGFBP5 RNA present in the polysomal fraction are extremely low in the polysomes from both PDK-1+/+ and PDK-1−/− ES cells, suggesting that the differences may not be significant. Although the RANKL antibody generated acceptable Western blotting data, it was unusable for immunoprecipitation. However, we demonstrated that the decreased expression of cystatin C and Rab11a was indeed due to decreased translation rates, and not due to changes in mRNA levels or protein stability.
We are currently trying to understand the mechanism(s) underlying the decreased translation rates of these mRNAs in PDK-1−/− ES cells. Examination of the 5′UTRs of the 20 mRNAs that showed the most dramatic downregulation in the polysomal fraction of PDK-1−/− ES cells did not show any obvious conserved elements. The lengths of the 5′UTRs were highly heterogeneous, ranging from no apparent 5′UTR (C80829) to 383 nucleotides with a ΔG of −164 (AK089060) (5′UTRs defined by the UCSC genome browser). Significantly, none of the PDK-1 regulated translational targets possessed a polypyrimidine tract at their 5′ terminus. This is in contrast to the large number of 5′ TOP mRNAs that were identified from Jurkat cells treated with rapamycin (15), and consistent with TOP RNA translation being rapamycin sensitive, but independent of p70S6K activity. Rapamycin also did not affect the expression levels of cystatin C, Rab11a, and RANKL in PDK-1+/+ ES cells, despite inhibiting S6 phosphorylation and 4EBP1 phosphorylation (Fig. 6B). Rapamycin also did not affect the translation rate of cystatin C or Rab11a (Fig. 6C). Consistent with this, cystatin C was also noted as an RNA that was translationally unaffected in rapamycin-treated Jurkat T cells (15). Therefore, our evidence suggests that neither mTOR nor p70S6K account for the deregulated translation of these proteins in PDK-1−/− ES cells. Instead, it appears that PKB is the relevant downstream effector of PDK-1 in this process. When a form of PKB that does not require PDK-1 for activity (Thr-308Asp/Ser-473Asp) was stably expressed in PDK-1−/− ES cells, the protein levels of cystatin C, Rab11a, and RANKL were restored, which appears be to be due to a restoration in the rate of translation, at least for cystatin C (Fig. 6). Consistent with this, LY294002, which inhibits PKB phosphorylation and activity in ES cells, also decreases the expression levels of these proteins. We are currently examining which downstream substrates of PKB are important for this process.
In addition to mRNAs showing decreased translation, a small number of mRNAs showed an increased association with polysomes in PDK-1−/− ES cells. One of these, ATF4, has previously been shown to be regulated at the level of translation (16). Murine ATF4 contains a relatively long 5′UTR (594 bp), with three upstream AUGs. It has been proposed that these upstream AUGs maintain a low basal rate of ATF4 translation, which is derepressed under conditions that cause phosphorylation of eIF2α (16). Phosphorylation of eIF2α causes a global inhibition of protein synthesis by blocking the ability of eIF2B to initiate GDP/GTP exchange on eIF2γ. The decreased activity of eIF2 may facilitate translation of specific mRNAs containing upstream open reading frames (ORFs) by allowing inefficient ribosome scanning to bypass the inhibitory upstream ORFs. Interestingly, an isoform of eIF2γ was highly dissociated from polysomes in the PDK-1−/− ES cells, potentially explaining why translation of ATF4, and maybe other mRNAs possessing upstream ORFs, preferentially occurs in these cells. Another explanation for the increased translation of ATF4 seen in the PDK-1−/− ES cells could be through activation of GSK3 in these cells due to the lack of inhibitory phosphorylation by PKB (39). GSK3 has previously been shown to regulate translation initiation through phosphorylating and inhibiting the ɛ subunit of eIF2B (38). Therefore, GSK3 represents an attractive target of PKB in mediating these effects, which will be explored in future studies.
In contrast to the lack of overlap between the mRNAs regulated translationally in PDK-1−/− ES cells and in rapamycin-treated Jurkat cells, there were several mRNAs that were commonly regulated between PDK-1−/− ES cells and Ras/PKB-expressing glial cells. For example, BMP4 was decreased in the polysomes of Ras/PKB expressing glial cells relative to nonexpressing cells and increased when these cells were treated with LY294002 and rapamycin (26). BMP4 was the most upregulated mRNA in polysomes of PDK-1−/− cells (see Table S2 in the supplemental material). Similarly, IFGBP5 translation was decreased when Ras/PKB cells were treated with LY294002 and rapamycin (26), and IGFBP5 was downregulated in PDK-1−/− ES cell polysomes (Table S1 in the supplemental material).
Although translation is considered an important mechanism for the regulation of protein expression, the identification of the mRNAs affected and sequences responsible for such regulation have been more difficult to identify. The advent of microarray technology combined with polysomal purification of actively transcribed RNAs provides an exciting opportunity to study this phenomenon in more detail. The emerging evidence for the importance of deregulated translation in human disease will likely spur future such studies. The tuberous sclerosis complex (TSC) syndrome is likely to be a disease driven by deregulated translation due to the increased activity of mTOR in these patients (21). An identification of the mRNAs that are increased translationally in response to this pathway is likely to generate novel targets that could be inhibited to prevent tumorigenesis. Although mTOR can currently be inhibited by rapamycin and its analogues, these agents will be ineffective in tumors that show deregulation downstream of this protein, for example, overexpression of eIF4G seen in breast cancers (1). In contrast, understanding the translational consequences of deregulated mTOR activity will provide a better framework for the understanding and treatment of a larger number of human tumors.
Supplementary Material
Acknowledgments
We thank Dario Alessi for providing PDK-1+/+ and PDK-1−/− ES cells and Ekaterina Blaveri for help and advice in the analysis of microarray data. We thank Clodagh O'Shea for critical reading of the manuscript. We also thank the UCSF cancer center genome analysis core facility for help with RNA analysis.
This study was carried out in part in The J. David Gladstone Genomics Core and in the General Clinical Research Center at San Francisco General Hospital and was supported by grant 5-MO1-RR00083 from the Division of Research Resources, National Institutes of Health. This study was funded by Daiichi Pharmaceuticals and by grants from the NCI (P50 CA97257) and DOD (TS030017) to D.S.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Alessi, D. R., S. R. James, C. P. Downes, A. B. Holmes, P. R. J. Gaffney, C. B. Reese, and P. Cohen. 1997. Characterisation of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7:261-269. [DOI] [PubMed] [Google Scholar]
- 2.Balendran, A., A. Casamayor, M. Deak, A. Paterson, P. Gaffney, R. Currie, C. P. Downes, and D. R. Alessi. 1999. PDK1 acquires PDK2 activity in the presence of a synthetic peptide derived from the carboxyl terminus of PRK2. Curr. Biol. 9:393-404. [DOI] [PubMed] [Google Scholar]
- 3.Balendran, A., R. Currie, C. G. Armstrong, J. Avruch, and D. R. Alessi. 1999. Evidence that 3-phosphoinositide-dependent protein kinase-1 mediates phosphorylation of p70 S6 kinase in vivo at Thr-412 as well as Thr-252. J. Biol. Chem. 274:37400-37406. [DOI] [PubMed] [Google Scholar]
- 4.Beretta, L., A. C. Gingras, Y. V. Svitkin, M. N. Hall, and N. Sonenberg. 1996. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J. 15:658-664. [PMC free article] [PubMed] [Google Scholar]
- 5.Biondi, R. M., A. Kieloch, R. A. Currie, M. Deak, and D. R. Alessi. 2001. The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. EMBO J. 20:4380-4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunn, G. J., J. Williams, C. Sabers, G. Wiederrecht, J. C. Lawrence, Jr., and R. T. Abraham. 1996. Direct inhibition of the signaling functions of the mammalian target of rapamycin by the phosphoinositide 3-kinase inhibitors, wortmannin, and LY294002. EMBO J. 15:5256-5267. [PMC free article] [PubMed] [Google Scholar]
- 7.Burnett, P. E., R. K. Barrow, N. A. Cohen, S. H. Snyder, and D. M. Sabatini. 1998. RAFT1 phosphorylation of the translational regulators p70 S6 kinase and 4E-BP1. Proc. Natl. Acad. Sci. USA 95:1432-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong, J., and D. Pan. 2004. Tsc2 is not a critical target of Akt during normal Drosophila development. Genes Dev. 18:2479-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng, J., J. Park, P. Cron, D. Hess, and B. A. Hemmings. 2004. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J. Biol. Chem. 279:41189-41196. [DOI] [PubMed] [Google Scholar]
- 10.Fingar, D. C., and J. Blenis. 2004. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23:3151-3171. [DOI] [PubMed] [Google Scholar]
- 11.Frodin, M., T. L. Antal, B. A. Dummler, C. J. Jensen, M. Deak, S. Gammeltoft, and R. M. Biondi. 2002. A phosphoserine/threonine-binding pocket in AGC kinases and PDK1 mediates activation by hydrophobic motif phosphorylation. EMBO J. 21:5396-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao, X., Y. Zhang, P. Arrazola, O. Hino, T. Kobayashi, R. S. Yeung, B. Ru, and D. Pan. 2002. Tsc tumour suppressor proteins antagonize amino-acid-TOR signalling. Nat. Cell Biol. 4:699-704. [DOI] [PubMed] [Google Scholar]
- 13.Garami, A., F. J. T. Zwartkruis, T. Nobukuni, M. Joaquin, M. Roccio, H. Stocker, S. C. Kozma, E. Hafen, J. L. Bos, and G. Thomas. 2003. Insulin activation of RheB, a Mediator of mTOR/S6K/4EBP Signaling, Is Inhibited by TSC1 and 2. Mol. Cell 11:1457-1466. [DOI] [PubMed] [Google Scholar]
- 14.Gingras, A. C., B. Raught, and N. Sonenberg. 2001. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 15:807-826. [DOI] [PubMed] [Google Scholar]
- 15.Grolleau, A., J. Bowman, B. Pradet-Balade, E. Puravs, S. Hanash, J. A. Garcia-Sanz, and L. Beretta. 2002. Global and specific translational control by rapamycin in T cells uncovered by microarrays and proteomics. J. Biol. Chem. 277:22175-22184. [DOI] [PubMed] [Google Scholar]
- 16.Harding, H. P., I. Novoa, Y. Zhang, H. Zeng, R. Wek, M. Schapira, and D. Ron. 2000. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell 6:1099-1108. [DOI] [PubMed] [Google Scholar]
- 17.Inoki, K., Y. Li, T. Xu, and K. L. Guan. 2003. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 17:1829-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoki, K., T. Zhu, and K. L. Guan. 2003. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577-590. [DOI] [PubMed] [Google Scholar]
- 19.Jefferies, H. B., S. Fumagalli, P. B. Dennis, C. Reinhard, R. B. Pearson, and G. Thomas. 1997. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. EMBO J. 16:3693-3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koromilas, A. E., A. Lazaris-Karatzas, and N. Sonenberg. 1992. mRNAs containing extensive secondary structure in their 5′ non-coding region translate efficiently in cells overexpressing initiation factor eIF-4E. EMBO J. 11:4153-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwiatkowski, D. J. 2003. Tuberous sclerosis: from tubers to mTOR. Ann. Hum. Genet. 67:87-96. [DOI] [PubMed] [Google Scholar]
- 22.Manning, B. D. 2004. Balancing Akt with S6K: implications for both metabolic diseases and tumorigenesis. J. Cell Biol. 167:399-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McManus, E. J., and D. R. Alessi. 2002. TSC1-TSC2: a complex tale of PKB-mediated S6K regulation. Nat. Cell Biol. 4:E214-E216. [DOI] [PubMed] [Google Scholar]
- 24.Pende, M., S. H. Um, V. Mieulet, M. Sticker, V. L. Goss, J. Mestan, M. Mueller, S. Fumagalli, S. C. Kozma, and G. Thomas. 2004. S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol. Cell. Biol. 24:3112-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng, X. D., P. Z. Xu, M. L. Chen, A. Hahn-Windgassen, J. Skeen, J. Jacobs, D. Sundararajan, W. S. Chen, S. E. Crawford, K. G. Coleman, and N. Hay. 2003. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 17:1352-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajasekhar, V. K., A. Viale, N. D. Socci, M. Wiedmann, X. Hu, and E. C. Holland. 2003. Oncogenic Ras and Akt signaling contribute to glioblastoma formation by differential recruitment of existing mRNAs to polysomes. Mol. Cell 12:889-901. [DOI] [PubMed] [Google Scholar]
- 27.Romanelli, A., V. C. Dreisbach, and J. Blenis. 2002. Characterization of phosphatidylinositol 3-kinase-dependent phosphorylation of the hydrophobic motif site Thr(389) in p70 S6 kinase 1. J. Biol. Chem. 277:40281-40289. [DOI] [PubMed] [Google Scholar]
- 28.Roux, P. P., B. A. Ballif, R. Anjum, S. P. Gygi, and J. Blenis. 2004. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc. Natl. Acad. Sci. USA 101:13489-13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarbassov dos, D., D. A. Guertin, S. M. Ali, and D. M. Sabatini. 2005. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307:1098-1101. [DOI] [PubMed] [Google Scholar]
- 30.Smith, E. M., S. G. Finn, A. R. Tee, G. J. Browne, and C. G. Proud. 2005. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J. Biol. Chem. 280:18717-18727. [DOI] [PubMed] [Google Scholar]
- 31.Stolovich, M., H. Tang, E. Hornstein, G. Levy, R. Cohen, S. S. Bae, M. J. Birnbaum, and O. Meyuhas. 2002. Transduction of growth or mitogenic signals into translational activation of TOP mRNAs is fully reliant on the phosphatidylinositol 3-kinase-mediated pathway but requires neither S6K1 nor rpS6 phosphorylation. Mol. Cell. Biol. 22:8101-8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang, H., E. Hornstein, M. Stolovich, G. Levy, M. Livingstone, D. Templeton, J. Avruch, and O. Meyuhas. 2001. Amino acid-induced translation of TOP mRNAs is fully dependent on phosphatidylinositol 3-kinase-mediated signaling, is partially inhibited by rapamycin, and is independent of S6K1 and rpS6 phosphorylation. Mol. Cell. Biol. 21:8671-8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Toker, A., and A. C. Newton. 2000. Akt/Protein kinase B is regulated by autophosphorylation at the hypothetical PDK-2 site. J. Biol. Chem. 275:8271-8274. [DOI] [PubMed] [Google Scholar]
- 34.Tusher, V. G., R. Tibshirani, and G. Chu. 2001. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. USA 98:5116-5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanhaesebroeck, B., and D. R. Alessi. 2000. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 346(Pt. 3):561-576. [PMC free article] [PubMed] [Google Scholar]
- 36.Vlahos, C. J., W. F. Matter, K. Y. Hui, and R. F. Brown. 1994. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J. Biol. Chem. 269:5241-5248. [PubMed] [Google Scholar]
- 37.Wang, X., W. Li, M. Williams, N. Terada, D. R. Alessi, and C. G. Proud. 2001. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J. 20:4370-4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, X., F. E. Paulin, L. E. Campbell, E. Gomez, K. O'Brien, N. Morrice, and C. G. Proud. 2001. Eukaryotic initiation factor 2B: identification of multiple phosphorylation sites in the epsilon-subunit and their functions in vivo. EMBO J. 20:4349-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams, M. R., J. S. C. Arthur, A. Balendran, J. van der Kaay, V. Poli, P. Cohen, and D. R. Alessi. 2000. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr. Biol. 10:439-448. [DOI] [PubMed] [Google Scholar]
- 40.Yang, J., P. Cron, V. Thompson, V. M. Good, D. Hess, B. A. Hemmings, and D. Barford. 2002. Molecular mechanism for the regulation of protein kinase b/akt by hydrophobic motif phosphorylation. Mol. Cell 9:1227-1240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.