Abstract
In many fungi, transcriptional responses to alkaline pH are mediated by conserved signal transduction machinery. In the homologous system in Saccharomyces cerevisiae, the zinc-finger transcription factor Rim101 is activated under alkaline conditions to regulate transcription of target genes. The activation of Rim101 is exerted through proteolytic processing of its C-terminal inhibitory domain. Regulated processing of Rim101 requires several proteins, including the calpain-like protease Rim13/Cpl1, a putative protease scaffold Rim20, putative transmembrane proteins Rim9, and Rim21/Pal2, and Rim8/Pal3 of unknown biochemical function. To identify new regulatory components and thereby determine the order of action among the components in the pathway, we screened for suppressors of rim9Δ and rim21Δ mutations. Three identified suppressors—did4/vps2, vps24, and vps4—all belonged to “class E” vps mutants, which are commonly defective in multivesicular body sorting. These mutations suppress rim8, rim9, and rim21 but not rim13 or rim20, indicating that Rim8, Rim9, and Rim21 act upstream of Rim13 and Rim20 in the pathway. Disruption of DID4, VPS24, or VPS4, by itself, uncouples pH sensing from Rim101 processing, leading to constitutive Rim101 activation. Based on extensive epistasis analysis between pathway-activating and -inactivating mutations, a model for architecture and regulation of the Rim101 pathway is proposed.
Microorganisms must regulate gene expression to cope with changes in environmental pH. In many fungi, transcriptional responses to alkaline pH are mediated by conserved signal transduction machinery (59). Extensive studies conducted on the prototypical pathway of Aspergillus nidulans have led to many of our current insights into the molecular machinery of homologous pathways (3, 59). In the A. nidulans pathway, the zinc-finger transcription factor PacC, is activated at alkaline pH to directly regulate transcription of pH-responsive genes (17, 72). Activation of PacC is exerted through proteolytic processing and removal of its C-terminal inhibitory domain at alkaline pH (21, 50, 55). The processing of PacC requires several proteins called the Pal proteins, whose deficiency causes acidity-mimicking phenotypes (2, 17, 19, 20, 48, 53, 54, 55). A set of dominant mutants of pacC that encode C-terminally truncated proteins mimicking the processed form behave as constitutively active mutants (pacCC) and cause alkalinity-mimicking phenotypes (17, 55, 72). Because the acidity-mimicking phenotypes of any of the pal mutants are suppressed by pacCC, the Pal proteins are concluded to constitute a pathway that regulates processing of PacC in response to alkaline pH (2, 17). However, the absence of other alkalinity-mimicking mutants has hindered similar genetic analyses meant to determine the order of action among the Pal proteins in the pathway.
In Saccharomyces cerevisiae, the homologous pathway regulates proteolytic processing of Rim101, which is the ortholog of PacC (59). Rim101 was originally identified as a positive regulator for meiotic gene expression (also referred to as Rim1 [68, 69]) and was subsequently shown to be responsible for alkaline pH-responsive gene expression and thereby for adaptation to alkaline conditions (25, 40, 41, 65). Rim101 also is activated by C-terminal proteolytic processing that is stimulated at alkaline pH (44). Several proteins homologous to the Pal proteins in A. nidulans are required for this processing, including the calpain-like protease Rim13/Cpl1, a putative protease scaffold Rim20, putative transmembrane proteins Rim9 and Rim21/Pal2, and Rim8/Pal3, a protein of unknown biochemical function (25, 41, 44, 73, 79). Defective mutants in any of these Rim proteins show sensitivity to alkaline conditions, as well as LiCl-containing medium. The latter phenotype is caused by reduced expression of ENA1, encoding the cation extrusion pump, whose efficient expression is dependent on Rim101 processing (41). Recently, Rim101 was shown to function as a transcriptional repressor that negatively regulates expression of several target genes, including NRG1 and SMP1; the product of the former is in turn required for repression of ENA1 (40). Like A. nidulans PacCC, C-terminally truncated mutants of Rim101 that mimic the processed form, such as Rim101ΔC531, also behave as constitutively active forms and negate the requirement for Rim13 activity or activity of any ofthe other Rim proteins needed for Rim101 processing (25, 44; E. Futai and T. Maeda, unpublished data). Therefore, molecular machinery homologous to what operates in A. nidulans appears to regulate Rim101 processing in S. cerevisiae (hereafter referred to as the “Rim101 pathway”). The mechanism by which the pathway executes regulated processing of Rim101, however, is not known, for the primary structures of the constituent components, with the exception of the calpain-like protease Rim13, whose proteolytic activity is essential for the pathway (25), provide little insight into their biochemical functions. Further, phenotypes of mutants defective in these components are indistinguishable from one another. The order of action among the Rim proteins in the pathway has also been difficult to determine, as is the case for the A. nidulans pathway. Homologous pathways are widely conserved among other fungal species, including Yarrowia lipolytica (27, 42, 73) and Candida albicans (18, 43, 61, 62).
Recently, machinery for vacuolar protein sorting was implicated in activation of the Rim101 pathway. Vacuolar sorting is mediated by the concerted action of a set of proteins collectively called vacuolar protein sorting (VPS) gene products (31). Among proteins destined for the vacuole, a membrane-bound precursor of carboxypeptidase S (CPS), as well as endocytosed cell surface receptors and transporters destined for vacuolar degradation, are recognized at the endosome and are sorted into multivesicular bodies (MVBs), endosomal structures formed by the invagination and budding of the limiting membrane into the lumen of the compartment (34). After fusion of the MVB with the vacuole, the lumenal vesicles with their cargo proteins are exposed to vacuolar hydrolases for degradation. Sorting of these cargo proteins and formation of the MVB require a group of VPS gene products categorized as “class E,” which include all components of three protein complexes called ESCRTs (for endosomal sorting complex required for transport): ESCRT-I, -II, and -III. Null mutations in any of the “class E” VPS genes commonly result in defective MVB sorting and accumulation of a malformed endosomal structure called the “class E” compartment. As a signal for MVB sorting, many cargo proteins, such as the precursor of CPS and cell surface receptors, are ubiquitinated within cytoplasmically exposed domains. Among the “class E” Vps proteins, the Stp22/Vps23 subunit of the ESCRT-I complex (composed of Stp22, Vps28, and Srn2/Vps37) and Vps27 bind to ubiquitin and thus select ubiquitinated cargo proteins for transport (7, 29, 33, 45, 60, 63, 67). Vps27 also binds the endosomally enriched lipid species phosphatidylinositol 3- phosphate and thereby initiates MVB sorting on the endosomal membrane (15, 35). ESCRT-I is then recruited for MVB sorting through direct interaction with Vps27 and binds to ubiquitinated cargo proteins (35). ESCRT-I then recruits ESCRT-II (composed of Snf8/Vps22, Vps25, and Vps36) onto the endosomal membrane, which in turn initiates assembly and recruitment of ESCRT-III onto the endosomal membrane (6, 47, 82). ESCRT-III is composed of the soluble coiled-coil-containing proteins Did4/Vps2, Vps20, Vps24, and Snf7/Vps32, which are recruited from the cytoplasm to the endosomal membrane, where they oligomerize into the complex (1, 5, 9, 37, 74). ESCRT-III contains two functionally distinct subcomplexes (5). The Vps20-Snf7 subcomplex binds to the endosomal membrane, in part through the myristoyl group of Vps20 (5). The Did4-Vps24 subcomplex binds to the Vps20-Snf7 subcomplex and thereby serves to recruit additional cofactors to the site of protein sorting, such as Vps4, which is an AAA-type ATPase catalyzing dissociation and disassembly of all three ESCRT complexes from the endosomal membrane after sorting is complete, and Doa4, which catalyzes the deubiquitination of cargo proteins to recycle ubiquitin (1, 5, 8, 56). The ESCRT complexes are thus proposed to perform a coordinated cascade of reactions that direct selection and sorting of MVB cargo proteins destined for delivery to the lumen of the vacuole.
A possible link between the Rim101 pathway and MVB sorting was suggested by several observations. For example, in a study of alkaline adaptation in Y. lipolytica, vps28, together with rim homolog mutants, was identified as a mutant in which induction of pH-responsive reporter genes was eliminated, although Rim101 processing in vps28 cells was not examined (27). In addition, a genome-wide protein interaction analysis suggested that Rim20 interacts with Snf7, and Snf7 in turn interacts with Rim13 (30, 79). Recently, Xu et al. reported that Rim101 processing is deficient in stp22Δ, vps28Δ, srn2Δ, snf8Δ, vps25Δ, vps36Δ, snf7Δ, and vps20Δ mutants but proficient in other “class E” vps mutants (80). Also in C. albicans, snf7Δ mutants are defective in Rim101 processing and show phenotypes attributed by the defect (39).
Here we screened for suppressors of two mutations, rim9Δ and rim21Δ, that cause deficiency in putative membrane-associated components of the Rim101 pathway. The three identified suppressors were all found to belong to “class E” vps mutations. These mutations cause constitutive activation of the Rim101 pathway by themselves. Based on extensive epistasis analysis between these pathway-activating mutations and pathway-inactivating rim and vps mutations, a model for the architecture of the Rim101 pathway and its regulation is proposed.
MATERIALS AND METHODS
Molecular genetic methods.
Standard Escherichia coli and yeast manipulations were performed as described previously (4). E. coli strain XL10-Gold (Stratagene, San Diego, CA) was used for plasmid propagation.
Yeast media.
Yeast extract-peptone-dextrose (YPD), synthetic dextrose (SD), synthetic complete (SC) dropout, and sporulation media were prepared as described previously (16). SD was supplemented with appropriate auxotrophic requirements. SD and SC were buffered where noted in Results with 50 mM morpholinepropanesulfonic acid and 50 mM morpholineethanesulfonic acid and adjusted to pH 3.5 or 4 with HCl or to pH 5.5, pH 7, or pH 7.5 with NaOH. In order to clone the corresponding genes for the suppressors, SD was supplemented with 7.5 μM erythrosin B (12).
Yeast strains.
Yeast strains are listed in Table 1. All strains used were derived in the S288C background (14, 77) except for those used for screening (38). RIM101 in CH1305 and RIM9 or RIM21 in CH1462 were disrupted by replacement with a kanMX6 cassette amplified by PCR as described previously (46) to generate FI1, FI3, and FI5, respectively. FI10-2d and FI10-5a were constructed by crossing FI1 with FI3. FI11-3c and FI11-13b were constructed by crossing FI1 with FI5.
TABLE 1.
Yeast strains
| Strain | Genotype | Source or reference |
|---|---|---|
| CH1305 | MATaade2 ade3 leu2 ura3 lys2 | 38 |
| CH1462 | MATα ade2 ade3 leu2 ura3 his3 | 38 |
| FI1 | Same as CH1305 except rim101::kanMX6 | This study |
| FI3 | Same as CH1462 except rim9::kanMX6 | This study |
| FI5 | Same as CH1462 except rim21::kanMX6 | This study |
| FI10-2d | Same as CH1305 except rim101::kanMX6 rim9::kanMX6 | This study |
| FI10-5a | Same as CH1462 except rim101::kanMX6 rim9::kanMX6 | This study |
| FI11-3c | Same as CH1305 except rim101::kanMX6 rim21::kanMX6 | This study |
| FI11-13b | Same as CH1462 except rim101::kanMX6 rim21::kanMX6 | This study |
| A6 | Same as FI10-5a except did4-H1 | This study |
| A108-9a | Same as FI10-5a except vps24-H1 | This study |
| TM141 | MATaura3-52 leu2Δ1 trp1Δ63 his3Δ200 | Lab stock (77) |
| TM225 | MATα ura3-52 leu2Δ1 his3Δ200 lys2Δ202 | FY838 (77) |
| MTM100 | MATaura3-52 leu2Δ1 trp1Δ63 rim13::URA3 | 25 |
| FM81 | Same as TM141 except rim8::HIS3 | This study |
| FM91 | Same as TM141 except rim9::HIS3 | This study |
| FM201 | Same as TM141 except rim20::HIS3 | This study |
| FM301 | Same as TM141 except rim21::HIS3 | This study |
| VB101-6b | Same as TM141 except did4::kanMX6 | This study |
| VB181-3a | Same as TM141 except rim8::HIS3 did4::kanMX6 | This study |
| VB191-11c | Same as TM141 except rim9::HIS3 did4::kanMX6 | This study |
| VB121-5d | Same as TM141 except rim20::HIS3 did4::kanMX6 | This study |
| VB131-1a | Same as TM141 except rim21::HIS3 did4::kanMX6 | This study |
| VB151-5d | Same as TM141 except rim13::URA3 did4::kanMX6 | This study |
| VB271-26c | Same as TM141 except snf7::His3MX6 did4::kanMX6 | This study |
| VB321-1a | Same as TM141 except vps20::His3MX6 did4::kanMX6 | This study |
| VX101-10a | Same as TM141 except vps24::kanMX6 | This study |
| VX181-6a | Same as TM141 except rim8::HIS3 vps24::kanMX6 | This study |
| VX191-6d | Same as TM141 except rim9::HIS3 vps24::kanMX6 | This study |
| VX121-2c | Same as TM141 except rim20::HIS3 vps24::kanMX6 | This study |
| VX131-5a | Same as TM141 except rim21::HIS3 vps24::kanMX6 | This study |
| VX151-1c | Same as TM141 except rim13::URA3 vps24::kanMX6 | This study |
| VX271-13d | Same as TM141 except snf7::His3MX6 vps24::kanMX6 | This study |
| VX321-4d | Same as TM141 except vps20::His3MX6 vps24::kanMX6 | This study |
| FT11 | Same as TM225 except vps4::kanMX6 | This study |
| FT101 | Same as TM141 except vps4::kanMX6 | This study |
| FT181 | Same as TM141 except rim8::HIS3 vps4::kanMX6 | This study |
| FT191 | Same as TM141 except rim9::HIS3 vps4::kanMX6 | This study |
| FT121 | Same as TM141 except rim20::HIS3 vps4::kanMX6 | This study |
| FT131 | Same as TM141 except rim21::HIS3 vps4::kanMX6 | This study |
| FT151 | Same as TM141 except rim13::URA3 vps4::kanMX6 | This study |
| SG11 | Same as TM225 except snf7::His3MX6 | This study |
| SG101-9b | Same as TM141 except snf7::His3MX6 | This study |
| VT11 | Same as TM225 except vps20::His3MX6 | This study |
| VT101-6d | Same as TM141 except vps20::His3MX6 | This study |
| VX401 | Same as TM225 except vps24::His3MX6 | This study |
| BY4741 | MATaura3Δ0 leu2Δ0 his3Δ1 met15Δ0 | Open Biosystems (14) |
| 3416 | Same as BY4741 except stp22::kanMX4 | Open Biosystems (78) |
| 2763 | Same as BY4741 except vps28::kanMX4 | Open Biosystems (78) |
| 2826 | Same as BY4741 except snf8::kanMX4 | Open Biosystems (78) |
| 2580 | Same as BY4741 except vps25::kanMX4 | Open Biosystems (78) |
| 5325 | Same as BY4741 except vps36::kanMX4 | Open Biosystems (78) |
| VV7-2c | MATaura3 leu2 his3 met15Δ0 vps24::His3MX6 | This study |
| VV3-3c | MATaura3 leu2 his3 met15Δ0 stp22::kanMX4 | This study |
| VV3-9b | MATaura3 leu2 his3 met15Δ0 stp22::kanMX4 vps24::His3MX6 | This study |
| VV7-10d | MATaura3 leu2 his3 met15Δ0 vps28::kanMX4 | This study |
| VV7-7b | MATaura3 leu2 his3 met15Δ0 vps28::kanMX4 vps24::His3MX6 | This study |
| VV11-14a | MATaura3 leu2 his3 met15Δ0 snf8::kanMX4 | This study |
| VV11-3a | MATaura3 leu2 his3 met15Δ0 snf8::kanMX4 vps24::His3MX6 | This study |
| VV15-9c | MATaura3 leu2 his3 met15Δ0 vps25::kanMX4 | This study |
| VV15-4b | MATaura3 leu2 his3 met15Δ0 vps25::kanMX4 vps24::His3MX6 | This study |
| VV19-10a | MATaura3 leu2 his3 met15Δ0 vps36::kanMX4 | This study |
| VV19-16d | MATaura3 leu2 his3 met15Δ0 vps36::kanMX4 vps24::His3MX6 | This study |
FM81, FM91, FM201, and FM301 were constructed by replacing RIM8, RIM9, RIM20, and RIM21 of TM141 with a HIS3 cassette amplified by PCR as described previously (14). DID4 in FM81, FM91, FM201, FM301, and MTM100 was disrupted by replacement with kanMX6 amplified by PCR. The resultant double disruptant strains were backcrossed with TM225 to generate VB181-3a, VB191-11c, VB121-5d, VB131-1a, VB151-5d, and VB101-6b. VPS24 in TM141, FM81, FM91, FM201, FM301, and MTM100 was disrupted by replacement with kanMX6 amplified by PCR. The resultant double disruptant strains were backcrossed with TM225 to generate VX101-10a, VX181-6a, VX191-6d, VX121-2c, VX131-5a, and VX151-1c. VPS4 in TM225 was disrupted by replacement with kanMX6 amplified by PCR to generate FT11. FT101, FT181, FT191, FT121, FT131, and FT151 were constructed by crossing TM141, FM81, FM91, FM201, FM301, and MTM100 with FT11. SNF7, VPS20, and VPS24 in TM225 were disrupted by replacement with His3MX6 amplified by PCR to generate SG11, VT11, and VX401, respectively. SG101-9b, VB271-26c, and VX271-13d were constructed by crossing VB101-6b and VX101-10a with SG11. VT101-6d, VB321-1a, and VX321-4d were constructed by crossing VB101-6b and VX101-10a with VT11. All gene disruptions constructed with kanMX6 or His3MX6 cassettes were confirmed by Southern analyses.
The stp22Δ (strain 3416), vps28Δ (strain 2763), snf8Δ (strain 2826), vps25Δ (strain 2580), vps36Δ (strain 5325), and parental (strain BY4741) strains were obtained from Open Biosystems (Huntsville, AL) (14, 78). The authenticity of each disruptant was confirmed as follows. These mutant strains exhibited temperature-sensitive growth and LiCl-sensitive phenotypes (data not shown). Plasmids containing the corresponding wild-type alleles were cloned and found to complement both phenotypes of the respective disruptants (data not shown). These results indicate that in each of above strains, the correct gene had been disrupted.
VV3-3c, VV3-9b, VV15-9c, VV15-4b, VV7-2c, VV7-10d, VV7-7b, VV11-14a, VV11-3a, VV19-10a, and VV19-16d were constructed by crossing strains 3416, 2580, 2763, 2826, and 5325 with VX401.
Plasmids.
pRIM101-2, an RIM101 plasmid with a NotI site introduced upstream of the first codon, has been described (25). Oligonucleotides encoding three tandem copies of the hemagglutinin (3× HA) epitope were inserted into the NotI site. The resultant 3HA-RIM101 fragment (2.1 kb, BamHI-EcoRI) and an ADE3 fragment (3.7 kb, BamHI-NheI) were inserted into the BamHI-EcoRI sites and the XbaI site of the pRS415 polylinker, respectively, to construct pFI1.
pAS416 was obtained from a YCp50 yeast genomic library as a plasmid rescuing the slow-growth phenotype of a did4 mutant (A6). Formation of larger and whiter colonies on SD plates containing erythrosin B was used as rescue criteria. pAS416 was found to harbor a 3.8-kb insert containing DID4. A 2.5-kb HindIII fragment containing DID4 and a portion of vector sequence was subcloned into the HindIII site of YCp50 to generate pAS4163.
pAS31 was obtained from the YCp50 yeast genomic library as a plasmid rescuing the slow-growth of a vps24 mutant (A108-9a), as described for DID4. The plasmid was found to harbor a 5.1-kb insert containing VPS24. A 1.7-kb ClaI fragment containing VPS24 was subcloned into the ClaI site of YCp50 to generate pAS311.
A 1.1-kb HindIII fragment containing URA3 was inserted in pRS415 with modified multiple cloning sites to obtain pTB554. Immediately upstream of the initiation codon of URA3 on pTB554, a 1.0-kb PCR product upstream of YPL277C containing its promoter was inserted by homologous recombination to construct pAT003.
Screening for suppressors.
FI10-2d, FI10-5a, FI11-3c, and FI11-13b transformed with pFI1 were mutagenized with ethyl methanesulfonate as described previously (16) (viability 30 to 40%), diluted, plated on YPD plates containing 0.16 or 0.21 M LiCl, and incubated for a week. Nonsectoring “red-only” colonies were selected and retested for the phenotype by streaking onto YPD plates containing 0.21 M LiCl. Candidate clones were retested on the same plates after incubation on YPD plates for 1 day to promote plasmid loss. To exclude mutants that restored LiCl tolerance due to activating mutations in pFI1 plasmid-borne RIM101, the plasmid was cured from each mutant and, after retransformation with pFI1, mutants were retested on the same plates. Finally, by Western blotting with an anti-HA antibody, mutants harboring processed HA-Rim101 were selected.
Detection of HA epitope-tagged Rim101.
Cells transformed with pFI1 were precultured in SD, inoculated in YPD at an optical density at 600 nm (OD600) of ca. 0.4, and incubated for 4 h. Cells were collected, washed with 1 mM phenylmethylsulfonyl fluoride (this extra washing step is only for the experiments in Fig. 2A, 3A, and 4A), suspended in Laemmli sample buffer, and boiled for 5 min. Samples were then cooled on ice and vortexed with glass beads for 30 s eight times with 30-s intervals on ice. Cell lysates corresponding to OD of 0.5 were boiled for 1 min and subjected to Western blotting with the anti-HA monoclonal antibody 12CA5 and IRDye-conjugated anti-mouse antibody (Rockland, Gilbertsville, PA). To detect actin for a loading control, the anti-actin monoclonal antibody C4 (ICN, Aurora, OH) was used as a primary antibody. Signals were detected by using the infrared imaging system Odyssey (LI-COR, Lincoln, NE) according to the manufacturer's instructions. For the experiments in Fig. 2A, cells were precultured to late log phase (OD = 0.6 to 1) in SD at pH 5.5, inoculated in each buffered SD medium at an OD of 1, and harvested after 30 min. Sample preparation and Western blotting were performed as described above.
FIG. 2.
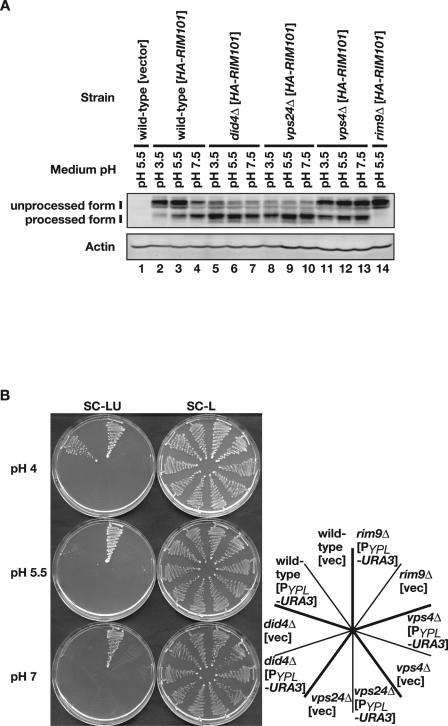
did4Δ, vps24Δ, and vps4Δ mutations activate the Rim101 pathway constitutively. (A) Constitutive and pH-independent processing of Rim101 is observed in did4Δ, vps24Δ, and vps4Δ mutants. HA-Rim101 was expressed from pFI1 in wild-type (TM141), did4Δ (VB101-6b), vps24Δ (VX101-10a), vps4Δ (FT101), and rim9Δ (FM91) strains. Cells were grown to logarithmic phase in SD buffered at pH 5.5 and then transferred to SD buffered at pH 3.5, 5.5, or 7.5 for 30 min. Cell lysates were prepared, and processed and unprocessed forms of HA-Rim101 (indicated) were detected as described in the legend to Fig. 1. Actin Western blotting was used as a loading control. (B) Constitutive and pH-independent repression of the Rim101-repressible YPL277C promoter is observed in did4Δ, vps24Δ, and vps4Δ mutants. The PYPL277C-URA3 reporter plasmid pAT003 (PYPL-URA3) or pRS415 (vec) was introduced in the strains used in panel A. The transformants were streaked on SC-LU and SC-L plates adjusted to indicated pH and then incubated at 30°C for 3 days.
FIG.3.
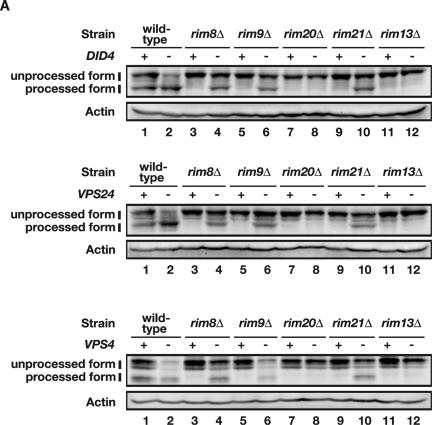
did4Δ, vps24Δ, and vps4Δ mutations suppress rim8Δ, rim9Δ, and rim21Δ but not rim20Δ or rim13Δ. (A) did4Δ, vps24Δ, and vps4Δ mutations suppress defective Rim101 processing of rim8Δ, rim9Δ, and rim21Δ mutants but not that of rim20Δ and rim13Δ mutants. HA-tagged Rim101 was expressed from pFI1 in wild-type (TM141), rim8Δ (FM81), rim9Δ (FM91), rim20Δ (FM201), rim21Δ (FM301), and rim13Δ (MTM100) strains and their did4Δ (top panel), vps24Δ (middle panel), and vps4Δ (bottom panel) derivatives: did4Δ (VB101-6b), rim8Δ did4Δ (VB181-3a), rim9Δ did4Δ (VB191-11c), rim20Δ did4Δ (VB121-5d), rim21Δ did4Δ (VB131-1a), rim13Δ did4Δ (VB151-5d), vps24Δ (VX101-10a), rim8Δ vps24Δ (VX181-6a), rim9Δ vps24Δ (VX191-6d), rim20Δ vps24Δ (VX121-2c), rim21Δ vps24Δ (VX131-5a), rim13Δ vps24Δ (VX151-1c), vps4Δ (FT101), rim8Δ vps4Δ (FT181), rim9Δ vps4Δ (FT191), rim20Δ vps4Δ (FT121), rim21Δ vps4Δ (FT131), and rim13Δ vps4Δ (FT151) strains. Processed and unprocessed forms of HA-Rim101 (indicated) were detected as described in the legend to Fig. 1. Actin Western blotting was used as a loading control. +, intact; −, deleted. (B) did4Δ, vps24Δ, and vps4Δ mutations suppress the LiCl sensitivity of rim8Δ, rim9Δ, and rim21Δ mutants but do not suppress that of the rim20Δ or rim13Δ mutant. The strains used in panel A were streaked on YPD and YPD containing 0.32 M LiCl plates and incubated at 30°C for 5 (lower panel) or 6 (upper and middle panels) days.
FIG. 4.
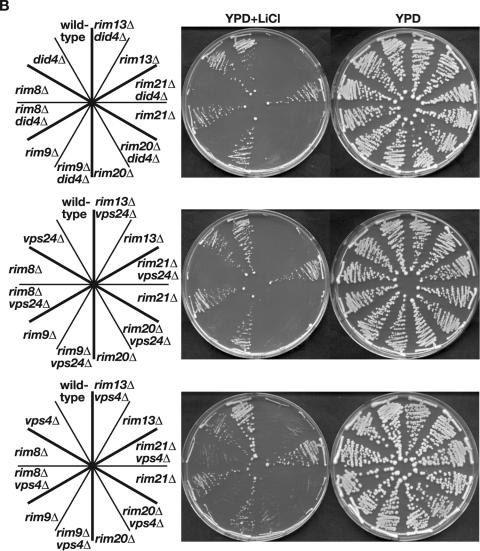
Epistasis tests between the Rim101 pathway-inactivating ESCRT mutations and did4 or vps24. (A) Defective Rim101 processing of stp22Δ and vps28Δ mutants is suppressed by vps24Δ, that of an snf8Δ mutant is suppressed moderately, and that of vps25Δ and vps36Δ mutants is not suppressed by vps24Δ. HA-tagged Rim101 was expressed from pFI1 in wild-type (BY4741), stp22Δ (VV3-3c), vps28Δ (VV7-10d), snf8Δ (VV11-14a), vps25Δ (VV15-9c), and vps36Δ (VV19-10a) strains and their vps24Δ derivatives: vps24Δ (VV7-2c), stp22Δ vps24Δ (VV3-9b), vps28Δ vps24Δ (VV7-7b), snf8Δ vps24Δ (VV11-3a), vps25Δ vps24Δ (VV15-4b), and vps36Δ vps24Δ (VV19-16d) strains. Processed and unprocessed forms of HA-Rim101 (indicated) were detected as described in the legend to Fig. 1. (B) The LiCl sensitivity of stp22Δ and vps28Δ mutants is suppressed by vps24Δ, that of an snf8Δ mutant is suppressed moderately, and that of vps25Δ and vps36Δ mutants is not suppressed by vps24Δ. The strains used in panel A were streaked on YPD and YPD containing 0.32 M LiCl plates and incubated at 30°C for 6 days. (C) Defective Rim101 processing of snf7Δ and vps20Δ mutants is not suppressed by did4Δ or vps24Δ. HA-tagged Rim101 was expressed from pFI1 in wild-type (TM141), snf7Δ (SG101-9b), and vps20Δ (VT101-6d) strains and their did4Δ and vps24Δ derivatives: did4Δ (VB101-6b), vps24Δ (VX101-10a), snf7Δ did4Δ(VB271-26c), snf7Δ vps24Δ (VX271-13d), vps20Δ did4Δ (VB321-1a), and vps20Δ vps24Δ (VX321-4d), rim8Δ (FM81), rim9Δ (FM91), rim20Δ (FM201), rim21Δ (FM301), and rim13Δ (MTM100) strains. Processed and unprocessed forms of HA-Rim101 (indicated) were detected as described in the legend to Fig. 1. (D) The LiCl sensitivity of snf7Δ and vps20Δ mutants is not suppressed by did4Δ or vps24Δ. The indicated strains used in panel C were streaked on YPD and YPD containing 0.32 M LiCl plates and incubated at 30°C for 5 days.
RESULTS
Screening for suppressors of rim9Δ or rim21Δ.
To identify new regulatory components in the Rim101 pathway and thus determine the order of action among the components in the pathway, we conducted a screen for mutations that suppressed the phenotypes of disruption alleles of RIM9 and RIM21. Rim9 and Rim21 are putative membrane proteins and thus are more likely to function upstream in the pathway (44, 73). Our initial attempts to isolate suppressors of rim9Δ and rim21Δ were, however, impeded by bypass suppressors. The screen for suppressors selected mutants whose growth in the presence of LiCl was restored, although none of the isolated mutants restored Rim101 processing (data not shown). It is likely that in these mutants LiCl tolerance was acquired through other mechanisms that also activate ENA1 expression. To isolate Rim101 pathway-specific suppressors that restored LiCl tolerance through restoration of Rim101 processing, the original screen was redesigned.
For clarity, only the screen for rim9Δ suppressors is described. Parental strains were constructed by disrupting both RIM9 and RIM101 in a pair of ade2 ade3 host strains and then transforming them with the screening plasmid pFI1, harboring HA-epitope-tagged RIM101 and ADE3, to facilitate a colony color assay (38). The parental strains had the overall genotype of rim9Δ and were therefore sensitive to LiCl. Suppressors were then sought that restored wild-type tolerance to LiCl from these parental strains. Among the mutants expected to be isolated, uninteresting bypass suppressors that restored LiCl tolerance independently of Rim101 processing would not require the pFI1 plasmid for the suppression and thus were expected to form white colonies or red colonies with many white sectors on plates containing LiCl. In contrast, the sought-after Rim101 pathway-specific suppressors would require pFI1 for the suppression and therefore were expected to form nonsectoring red colonies on plates containing LiCl. In addition, the intended pathway-specific suppressors were expected to permit formation of white or sectoring colonies on plates that did not contain LiCl because pFI1 would not then be required. This feature was used to exclude mutants that retained pFI1 for reasons other than a dependence on Rim101 for LiCl tolerance. Suppressors that restored LiCl tolerance due to activating mutations in pFI1-encoded Rim101, such as truncation of the C terminus, were then excluded by retesting the LiCl tolerance of candidate mutants after once curing the screening plasmids from them and then reintroducing genuine pFI1 by transformation. Finally, restoration of Rim101 processing was examined by Western analysis. Similar screening was also performed for suppressors of rim21Δ.
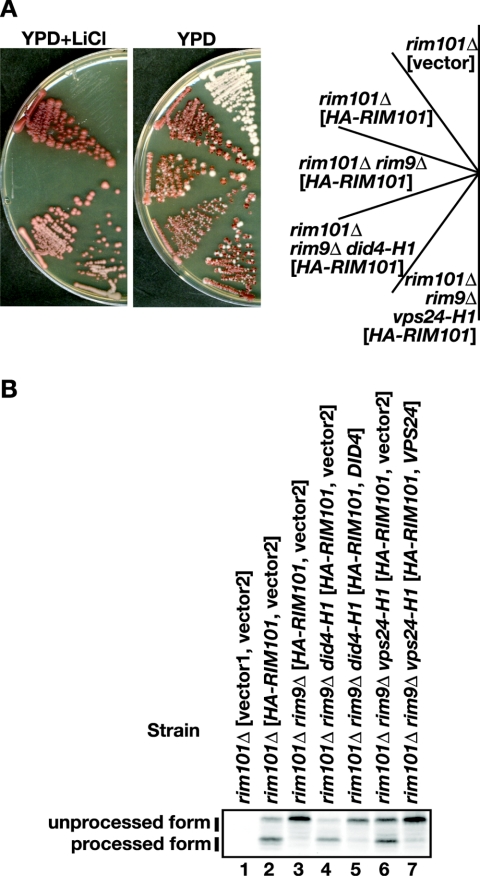
Parental strains were mutagenized with ethyl methanesulfonate treatment to a survival rate of 30 to 40%. Nineteen mutants were obtained that harbored rim9Δ suppressors restoring both LiCl tolerance and Rim101 processing among a total of 5.6 × 106 clones (Fig. 1A and B). All of the mutations were found to be recessive and were classified into two complementation groups: A and B consisting of 12 and 7 representatives, respectively. Mutants in both complementation groups commonly exhibited slow growth on SD plates and formed colonies that stained darker red than parental strains on SD plates containing the red vital dye, erythrosin B, which accumulates in dead cells (data not shown). Plasmids that restored growth on SD plates, and that also restored defective Rim101 processing were isolated from a wild-type genomic library constructed in YCp50. Subcloning of the isolated plasmids identified DID4/VPS2 (1) and VPS24 (9) as the genes corresponding to group A and B mutants, respectively (Fig. 1B). Phenotypes of the double mutants constructed using rim9Δ, and did4Δ or vps24Δ confirmed the conclusions (see below).
FIG. 1.
Screening for rim9Δ suppressors. (A) LiCl sensitivity caused by rim9Δ is suppressed by did4 and vps24. The rim101Δ strain (FI1) transformed with pFI1, a low-copy-number plasmid carrying HA-RIM101 and ADE3, formed red-only, nonsectoring colonies, whereas the same strain carrying pRS415 (vector) failed to grow on an LiCl-containing plate. The red-only, nonsectoring colony morphology indicated that LiCl tolerance was plasmid dependent. The parental strain for the screening, rim101Δ rim9Δ (FI10-5a) transformed with pFI1, failed to grow on LiCl-containing medium. Isolated LiCl-tolerant suppressor strains, rim101Δ rim9Δ did4-H1 (A6) and rim101Δ rim9Δ vps24-H1 (A108-9a), both transformed with pFI1, formed red-only, nonsectoring colonies on a plate containing LiCl because their LiCl tolerance was plasmid dependent. They formed white or sectoring colonies on a plate that did not contain LiCl because pFI1 was not then required. Cells were streaked on YPD and on YPD containing 0.21 M LiCl plates and incubated at 30°C for 11 days. (B) Defective Rim101 processing caused by rim9Δ is suppressed by did4 and vps24. The strains used in panel A were transformed with pRS415 (vector1) or pFI1, in combination with the empty YCp50 vector (vector2), pAS4163, a low-copy-number plasmid carrying DID4, or pAS311, a low-copy-number plasmid carrying VPS24, as indicated. Transformants were precultured in SD to logarithmic phase and then transferred to YPD. After a 3-h incubation, cells were collected, and cell lysates were prepared. Processed and unprocessed forms of HA-Rim101 (indicated) were detected by Western blotting with an anti-HA antibody.
In the same manner, 10 mutants were obtained that harbored rim21Δ suppressors among a total of 1.5 × 107 clones. One mutation was found to be dominant, and the other nine were found to be recessive (data not shown). Analysis of the dominant mutation will be reported elsewhere. The recessive mutations obtained were classified into three complementation groups: C, D, and E, consisting of five, three, and one representative, respectively. As the mutants harboring the rim9Δ suppressors, these mutants also exhibited slow growth on SD plates and formed colonies that stained darker red on SD plates containing erythrosin B. Transformation of representative mutants of these groups with plasmids harboring either of DID4 or VPS24 revealed that the phenotypes of group C and E mutants were rescued by VPS24 and DID4, respectively (data not shown). Cloning of the gene that rescued group D mutants identified VPS4 (8). To summarize, these results identified VPS24, VPS4, and DID4 as the genes corresponding to group C, D, and E mutants, respectively. Phenotypes of double mutants constructed using rim21Δ and vps24Δ, vps4Δ, or did4Δ confirmed our conclusions (see below).
did4Δ, vps24Δ, and vps4Δ mutations constitutively activate the Rim101 pathway.
To analyze the functions of Did4, Vps24, and Vps4 in the Rim101 pathway, DID4, VPS24, and VPS4 disruptants were constructed in the S288C isogenic background, and processing of HA-Rim101 was monitored by Western blotting. When wild-type cells were transferred to an acidic condition (pH 3.5), HA-Rim101 was mostly unprocessed (Fig. 2A, lane 2). On the other hand, when cells were transferred to an alkaline condition (pH 7.5), most HA-Rim101 was processed (Fig. 2A, lane 4). The degree of HA-Rim101 processing is between those of two conditions at pH 5.5 (Fig. 2A, lane 3). The accumulation of processed Rim101 under alkaline conditions and unprocessed form under acidic conditions are in good agreement with previous observations (44).
In did4Δ mutants transferred to any condition of pH 3.5, 5.5, or 7.5, most HA-Rim101 was processed (Fig. 2A, lanes 5 to 7) as in wild-type cells at pH 7.5 (Fig. 2A, lane 4). This was also the case in vps24Δ mutants (Fig. 2A, lanes 8 to 10). These results indicate that in did4Δ and vps24Δ mutants, HA-Rim101 processing occurs constitutively in a medium pH-independent manner and that both did4Δ and vps24Δ mutations constitutively activate the Rim101 pathway. Also in vps4Δ mutants, HA-Rim101 processing was constitutive irrespective of medium pH (Fig. 2A, lanes 11 to 13), although the degree of processing was less pronounced than in did4Δ and vps24Δ mutants. These results indicate that in vps4Δ mutants, HA-Rim101 processing occurs constitutively at a low level in a medium pH-independent manner and that a vps4Δ mutation moderately activates the Rim101 pathway.
Constitutive activation of the Rim101 pathway in did4Δ, vps24Δ, and vps4Δ mutants was confirmed by using a Rim101-responsive transcriptional reporter. Lamb and Mitchell identified several target genes for direct repression by Rim101, among which, YPL277C was most significantly upregulated by deficiency of the Rim101 pathway (40). Therefore, a reporter plasmid was constructed by connecting the promoter segment of YPL277C to immediately upstream of the initiation codon of URA3. When the reporter plasmid was introduced into wild-type cells, cells did grow on media lacking uracil at pH 4, where processing of Rim101 was kept impeded, but not at pH 7, where processing of Rim101 was stimulated (Fig. 2B). Cell grew poorly at pH 5.5 (Fig. 2B). Furthermore, when introduced into rim9Δ cells, in which processing of Rim101 did not occur irrespective of pH conditions, cells did grow on media lacking uracil at any of the three pH levels (Fig. 2B). These observations indicate that the reporter is effective in monitoring activity of Rim101. When the reporter plasmid was introduced into did4Δ, vps24Δ, and vps4Δ mutants, cells did not grow on media lacking uracil at any of the pH conditions examined (Fig. 2B). This result again shows that Rim101 is kept active irrespective of pH conditions in these three mutants.
Epistasis relationship between rim mutations and did4, vps24, or vps4.
We then examined whether did4Δ, vps24Δ, or vps4Δ were able to suppress rim8Δ, rim9Δ, rim20Δ, rim21Δ, or rim13Δ. rim8Δ, rim9Δ, rim20Δ, rim21Δ, and rim13Δ mutants were defective in HA-Rim101 processing (Fig. 3A, lanes 3, 5, 7, 9, and 11) and sensitive to LiCl (Fig. 3B) as described previously. The double mutants rim8Δ did4Δ, rim9Δ did4Δ, and rim21Δ did4Δ were capable of HA-Rim101 processing (Fig. 3A, upper panel, lanes 4, 6, and 10) and tolerant of LiCl (Fig. 3B). These observations indicate that rim8Δ, rim9Δ, and rim21Δ were suppressed by did4Δ. In contrast, the double mutants rim20Δ did4Δ and rim13Δ did4Δ were defective in HA-Rim101 processing (Fig. 3A, upper panel, lanes 8 and 12) and sensitive to LiCl (Fig. 3B, upper panel), indicating that rim20Δ and rim13Δ were not suppressed by did4Δ. This was also the case for the double mutants with vps24Δ (Fig. 3A, middle panel, lanes 4, 6, 8, 10, and 12, and Fig. 3B, middle panel) and vps4Δ (Fig. 3A, lower panel, lanes 4, 6, 8, 10 and 12, and Fig. 3B, lower panel). These observations indicate that rim8Δ, rim9Δ, and rim21Δ were suppressed but that rim20Δ and rim13Δ were not suppressed by vps24Δ or vps4Δ, although vps4 mutations were not isolated in our screen for suppressors of rim9Δ. The observations that rim20 and rim13 were epistatic to did4, vps24, and vps4, whereas did4, vps24, and vps4 were epistatic to rim8, rim9, and rim21 provide the genetic evidence that Rim8, Rim9, and Rim21 function upstream of Rim20 and Rim13 in the Rim101 pathway.
Relative to the double mutants rim8Δ did4Δ, rim9Δ did4Δ, rim21Δ did4Δ, rim8Δ vps24Δ, rim9Δ vps24Δ, and rim21Δ vps24Δ, HA-Rim101 processing was more pronounced in did4Δ and vps24Δ mutants (Fig. 3A, upper and middle panels, lanes 2, 4, 6, and 10). These results indicate that even in did4Δ and vps24Δ mutants, Rim8, Rim9, and Rim21 still function to facilitate Rim101 processing (see Discussion).
Epistasis relationship between the Rim101 pathway-inactivating ESCRT mutations and did4 or vps24.
Recently, it is reported that ESCRT-I components Stp22, Vps28, and Srn2, ESCRT-II components Snf8, Vps25, and Vps36, and ESCRT-III components Snf7 and Vps20 are required for Rim101 processing, just as are Rim8, Rim9, Rim13, Rim20, and Rim21 (80). Our own independent analysis had reached conclusions largely consistent with these results, with the sole exception of Srn2, which was dispensable for HA-Rim101 processing and LiCl tolerance in our analysis (see Discussion). We then examined whether did4Δ or vps24Δ was able to suppress phenotypes of mutants defective in these ESCRT components with respect to HA-Rim101 processing and LiCl tolerance.
stp22Δ, vps28Δ, snf8Δ, vps25Δ, vps36Δ, snf7Δ, and vps20Δ mutants were commonly defective in HA-Rim101 processing (Fig. 4A, lanes 3, 5, 7, 9, and 11, and Fig. 4C, lanes 4 and 7) and sensitive to LiCl (Fig. 4B and 4D) as described above. In contrast, stp22Δ vps24Δ and vps28Δ vps24Δ double mutants were capable of HA-Rim101 processing (Fig. 4A, lanes 4 and 6) and tolerant of LiCl (Fig. 4B). Similarly, an snf8Δ vps24Δ double mutant was moderately proficient in HA-Rim101 processing (Fig. 4A, lane 8) and somewhat tolerant of LiCl (Fig. 4B). vps25Δ vps24Δ, vps36Δ vps24Δ, snf7Δ vps24Δ, and vps20Δ vps24Δ double mutants were, however, defective in HA-Rim101 processing (Fig. 4A, lanes 10 and 12, and Fig. 4D, lanes 6 and 9) and sensitive to LiCl (Fig. 4B and 4D). snf7Δ did4Δ and vps20Δ did4Δ double mutants were also defective in HA-Rim101 processing (Fig. 4C, lanes 5 and 8) and sensitive to LiCl (Fig. 4D).
These results can be summarized as follows. Both defective Rim101 processing and LiCl sensitivity caused by ESCRT-I mutations stp22Δ and vps28Δ were suppressed by vps24Δ, those caused by ESCRT-II mutation snf8Δ were moderately suppressed, and those caused by ESCRT-II mutations vps25Δ and vps36Δ and ESCRT-III mutations snf7Δ and vps20Δ were not suppressed by vps24Δ.
DISCUSSION
New mutants that exhibit constitutive activation of the Rim101 pathway.
As of this writing, the only pathway-activating mutants that have been reported in the Rim101 pathway harbor dominant alleles of RIM101 encoding proteins with C-terminal truncations that mimic proteolytic processing (44). In the current study, we identified did4, vps24, and vps4 as pathway-activating mutants of the Rim101 pathway that are alkaline-mimicking. In our experimental system, most of the HA-Rim101 pool in wild-type cells remained unprocessed under acidic conditions (pH 3.5). The extent of processing increased as medium pH rose, and under alkaline conditions (pH 7.5) most of the HA-Rim101 pool was processed. In did4 or vps24 mutants, constitutive proteolytic processing of Rim101 was observed that occurs irrespective of medium pH. The extent of the processing was comparable to that induced in wild-type cells under alkaline conditions. In addition, did4 and vps24 mutations were found to suppress defective Rim101 processing in rim8, rim9, and rim21 mutants. Similarly, in vps4 mutants, Rim101 processing was observed even under conditions of acidic pH, although the extent of processing was less than in did4 or vps24 mutants. Consistent with this, rim8, rim9, and rim21 were also modestly suppressed by vps4.
Order of action among the Rim proteins and the ESCRT components.
Identification of did4, vps24, and vps4 as Rim101 pathway-activating mutants enabled us to perform a new set of epistasis tests that showed that Rim8, Rim9, and Rim21 function upstream of both Rim20 and Rim13 in the pathway. This conclusion is consistent with the report that Rim20 directly interacts with Rim101 (79) and also with a long-standing conjecture that calpain-like Rim13 is the protease directly catalyzing Rim101 processing. The three components placed upstream of Rim13 and Rim20, namely, putative membrane proteins Rim9 and Rim21, as well as Rim8, may constitute the pH-sensing machinery of the pathway (see below).
In contrast to the two pathway-activating mutants, did4 and vps24, most other mutants defective in ESCRT components, i.e., stp22, vps28, snf8, vps25, vps36, snf7, and vps20, show impaired Rim101 processing, as well as LiCl sensitivity, with the only exception being srn2 (see below). In this respect, these mutants share the phenotypes with rim8, rim9, rim20, rim21, and rim13 mutants. Therefore, epistasis tests similar to those conducted for the rim mutations were performed for these ESCRT mutations. Both defective Rim101 processing and LiCl sensitivity of stp22 and vps28 were suppressed by vps24, those of snf8 were moderately suppressed, and those of vps25, vps36, snf7, and vps20 were not suppressed at all by vps24. These observations suggest that components of ESCRT-II, especially Vps25 and Vps36, and components of ESCRT-III, Snf7 and Vps20, play more important roles than those of ESCRT-I with respect to Rim101 processing. This is in line with previous genetic evidence indicating that ESCRT-II acts downstream of, and plays a more fundamental role than, ESCRT-I in MVB sorting (6).
Xu et al. reported that Rim101 processing is also deficient in an srn2Δ mutant (80). In contrast, an srn2Δ mutant was capable of Rim101 processing and tolerant of LiCl in our analysis, which was the only exception among mutants defective in ESCRT components (data not shown). Bowers et al. also reported that srn2 mutants are tolerant of LiCl (13), but Eguez et al. reported that these mutants were sensitive (22). The reason for the discrepancy is currently unclear but may reflect somewhat milder phenotypes of srn2 mutants relative to other mutants in ESCRT components, as observed in a higher restrictive temperature for growth, for example (data not shown).
Possible explanation for previously reported phenotypic differences among mutants defective in different ESCRT components.
The disparity in Rim101 processing explains the difference in LiCl sensitivity among mutants defective in different ESCRT components. Full expression of ENA1, whose product is responsible for Li+ efflux, requires processing of Rim101, and the fact hence is consistent with the observation that did4 and vps24 mutants are tolerant of LiCl and mutants defective in other ESCRT components sensitive (28, 40, 41). The disparity in Rim101 processing may also explain several previously unaddressed qualitative phenotypic differences among different ESCRT mutants. CPS, a selective cargo of MVB sorting, is reported to display different processing patterns in different ESCRT mutants (5). In snf7Δ and vps20Δ mutants, CPS was processed by the hydrolytic enzymes accumulated in the “class E” compartment, although in a slightly different pattern than occurs in wild-type cells. In contrast, CPS processing was impeded in did4Δ, vps24Δ, and vps4Δ mutants. The deficiency in CPS processing in did4Δ, vps24Δ, and vps4Δ mutants has been interpreted as a result of CPS being protected from proteolysis by sequestration or concentration in a compartment with a specific role for the Snf7-Vps20 subcomplex (5). Here we propose a different explanation. Lamb and Mitchell identified PRB1, which encodes a major vacuolar protease that catalyzes CPS processing, as a target gene for repression by Rim101 (40). Therefore, in did4Δ, vps24Δ, and vps4Δ mutants, proteolytic activity of Prb1p is likely low due to transcriptional repression by Rim101, and thus the low Prb1 activity will in turn result in deficient CPS processing. The previously reported differences in the sensitivity to Ca2+, Mn2+, and calcofluor white among mutants in different ESCRT components may also be attributed to the disparity in Rim101 processing (22, 66).
Mechanism for constitutive proteolytic processing of Rim101 in did4, vps24, and vps4 mutants.
Although all of the “class E” vps mutants share the same defective MVB sorting phenotype, only did4, vps24, and vps4 mutants exhibit the constitutive proteolytic processing of Rim101. In contrast, the other mutants in ESCRT components, except for srn2, exhibit defective Rim101 processing. Furthermore, all non-ESCRT “class E” vps mutants except for vps4 are normal in Rim101 processing. This difference in Rim101 processing among the “class E” vps mutants indicates that defective MVB sorting itself does not cause constitutive Rim101 processing. Then, what is the mechanism that underlies constitutive Rim101 processing?
According to the seminal studies on the ESCRT complexes by Emr and coworkers, did4, vps24, and vps4 mutants commonly accumulate the Vps20-Snf7 subcomplex of ESCRT-III on the endosomal membrane (5, 8, 9). This accumulation is not observed in other mutants in ESCRT components (5). In contrast, Emr and coworkers have shown that many other mutants in ESCRT components are defective in formation and endosomal membrane loading of ESCRT-III. Our results reveal a consistent correlation between the reported membrane loading of Vps20-Snf7 and Rim101 processing. First, not only defects in Vps20 or Snf7 itself but also defects in ESCRT-I or ESCRT-II have been shown to prevent loading of Vps20-Snf7 onto the endosomal membrane (6). Concomitantly, mutants in ESCRT-I or ESCRT-II components are defective in Rim101 processing, with the sole exception of srn2 (see above). Second, in a vps4 background, ESCRT-II is required but ESCRT-I is not required for loading of Vps20-Snf7 on the endosomal membrane (6). This again is in good agreement with the results of our epistasis tests that show that defective Rim101 processing of the ESCRT-I mutants stp22 and vps28 is suppressed by vps24 but that of ESCRT-II mutants vps25 and vps36 is not. An exceptional component of ESCRT-II in this respect is Snf8, in that defective Rim101 processing in a snf8 mutant is partially suppressed by vps24. Because possible accumulation of Vps20-Snf7 on the endosomal membrane in snf8 did4, snf8 vps24, or snf8 vps4 double mutants has not been examined, this point requires further study.
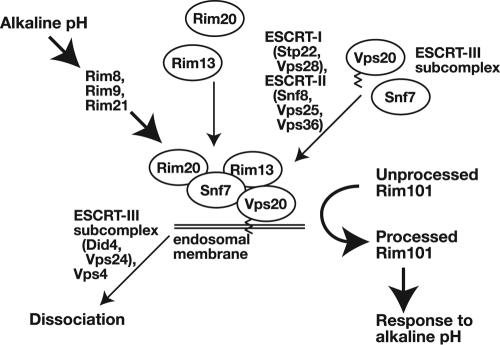
In light of these observations, we propose that either formation or the endosomal membrane loading of the Vps20-Snf7 subcomplex activates proteolytic processing of Rim101. On the other hand, it has already been suggested that a complex of Rim20-Snf7-Rim13 is responsible for catalyzing proteolytic processing of Rim101 (79). Extending this model, we then propose the following mechanism for constitutive proteolytic processing of Rim101 in did4, vps24, and vps4 mutants (Fig. 5). In these mutants, the Vps20-Snf7 subcomplex of ESCRT-III accumulates on the endosomal membrane. To this subcomplex, Rim20 and Rim13 are recruited to form a higher-order complex consisting of Rim20-(Vps20-Snf7)-Rim13 through an interaction between Rim20 and Snf7 and between Snf7 and Rim13. The formation of this complex, we propose, promotes proteolytic processing of Rim101 by recruiting Rim101 through interaction with Rim20 and thereby presenting it to activated Rim13. To test this model, localization of components of the Rim101 pathway and the ESCRT complexes need to be determined at different pH values or in various mutants of ESCRT components.
FIG. 5.
Model for activation of Rim101 proteolytic processing by components of the Rim101 pathway; ESCRT-I, -II, and -III components; and Vps4 upon exposure to alkaline pH. See Discussion for a detailed explanation of this model.
vps4 mutants exhibit constitutive processing of Rim101, although the extent of processing seems moderate relative to what was observed in did4 and vps24 mutants. This may be explained as follows. In vps4 mutants, the Did4-Vps24 subcomplex is loaded onto the Vps20-Snf7 subcomplex on the endosomal membrane (5, 9) and potentially competes with Rim20 and/or Rim13 for binding to Vps20-Snf7. In contrast, in did4 and vps24 mutants Did4-Vps24 does not exist, and thus Vps20-Snf7 is fully available for binding to Rim20 and/or Rim13.
Mechanism for alkaline-responsive proteolytic processing of Rim101.
By extending the above discussion on constitutive processing of Rim101 in did4Δ, vps24Δ, and vps4Δ mutants to the alkaline pH-responsive processing of Rim101 in wild-type cells, we propose that a similar mechanism operates in both processes. In this model, a complex of Rim20-(Vps20-Snf7)-Rim13 forms under conditions of alkaline pH in a Rim8-, Rim9-, and Rim21-dependent manner (Fig. 5). The concrete mechanism by which Rim8, Rim9, and Rim21 promote formation of the Rim20-(Vps20-Snf7)-Rim13 complex remains to be determined, although it is clear that formation of the complex also requires both ESCRT-I and ESCRT-II. Even in did4 or vps24 mutants, Rim8, Rim9, and Rim21 still play some role in facilitating Rim101 processing, because defective Rim101 processing in rim8, rim9, and rim21 mutants is not fully restored, even when suppressed by did4 or vps24: in did4 cells for example, the extent of Rim101 processing is more pronounced than in rim8 did4 cells. One speculative possibility is that, by mimicking cargo proteins for MVB sorting, either Rim9 or Rim21 recruits ESCRT-I onto the endosomal membrane, thereby promoting formation of the Rim20-(Vps20-Snf7)-Rim13 complex under alkaline conditions. If the recruitment of ESCRT-I depends on ubiquitination of Rim9 or Rim21, as is the case for known cargo proteins, the ubiquitination should occur in an alkaline pH-dependent manner. This point remains to be determined. Stimulation-dependent ubiquitination and endosomal sorting are common modes of desensitization of membrane receptors/sensors (34), and this model proposes a variant of this common mechanism that makes use of recruitment of ESCRT complexes not for downregulation, but for signaling itself.
Physiological significance of the link between the Rim101 pathway and MVB sorting.
We have shown a close relationship between the Rim101 pathway and MVB sorting. What is the physiological significance of this link? MVB sorting requires a pH gradient across the endosomal membrane. In fact, efficient sorting of vacuolar proteins is impaired under alkaline conditions (36). In addition, mutational or pharmacological inactivation of the vacuolar H+-ATPase, which disrupt acidification of the vacuolar, and perhaps the endosomal, compartment also causes defective vacuolar protein sorting (10, 36, 52, 64, 75, 81). The defects in MVB sorting under these conditions may arise from impaired formation of MVB. Consistent with this, Matsuo et al. reported that formation of MVB-like liposomes in an in vitro system required a pH gradient across the liposome membrane that reproduced the in vivo lumenal acidic pH of the endosome (49). If impairment in MVB formation under alkaline conditions blocks the process of MVB sorting after the step of ESCRT-III loading, as in did4, vps24, or vps4 mutants, formation of the active Rim20-(Vps20-Snf7)-Rim13 complex would be promoted under these conditions. This implies that failure to maintain a pH gradient across the endosomal membrane under alkaline conditions can be coupled with activation of the Rim101 pathway. This will ensure, in theory at least, the appropriate response of the Rim101 pathway to raises in environmental pH.
Finally, it should be noted that all of the components of the aforementioned protease complex have one or more mammalian homologs. Rim13 is the only calpain homolog in yeast, whereas humans have 14 genes for calpains, including PalBH/calpain 7, which is most similar to Rim13 (23, 24, 26, 70). The human homologs of Rim20, Snf7, and Vps20 are Alix/AIP1, CHMP4/hSnf7, and hVps20, respectively (32, 51, 58, 76). The existence of these homologs suggests that a similar mechanism might operate in mammalian cells. The possibility of calpain functioning at the membranes is also implied by the present study. Indeed, recent studies have shown that conventional calpains localize and function at the endoplasmic reticulum and the lysosome (11, 57, 71). Elucidation of the mechanism that regulates activation of the Rim101 pathway should shed further light on the biology of calpains in general.
Acknowledgments
We thank Fred Winston, Janice Kranz, Konnie Holm, Mark Longtine, Yoichi Noda, and Koji Yoda for yeast strains and plasmids. We also thank Eugene Futai, Koichi Suzuki, and all of the members of the Maeda laboratory for help, advice, and discussion.
This study was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas (KAKENHI 14086203) from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT) and a grant (no. 0349) from the Salt Science Research Foundation (both to T.M.).
REFERENCES
- 1.Amerik, A. Y., J. Nowak, S. Swaminathan, and M. Hochstrasser. 2000. The Doa4 deubiquitinating enzyme is functionally linked to the vacuolar protein-sorting and endocytic pathways. Mol. Biol. Cell 11:3365-3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arst, H. N., E. Bignell, and J. Tilburn. 1994. Two new genes involved in signalling ambient pH in Aspergillus nidulans. Mol. Gen. Genet. 245:787-790. [DOI] [PubMed] [Google Scholar]
- 3.Arst, H. N., and M. A. Peñalva. 2003. pH regulation in Aspergillus and parallels with higher eukaryotic regulatory systems. Trends Genet. 19: 224-231. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1988. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 5.Babst, M., D. J. Katzmann, E. J. Estepa-Sabal, T. Meerloo, and S. D. Emr. 2002. ESCRT-III: an endosome-associated heterooligomeric protein complex required for MVB sorting. Dev. Cell 3:271-282. [DOI] [PubMed] [Google Scholar]
- 6.Babst, M., D. J. Katzmann, W. B. Snyder, B. Wendland, and S. D. Emr. 2002. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 3:283-289. [DOI] [PubMed] [Google Scholar]
- 7.Babst, M., G. Odorizzi, E. J. Estepa, and S. D. Emr. 2000. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1:248-258. [DOI] [PubMed] [Google Scholar]
- 8.Babst, M., T. K. Sato, L. M. Banta, and S. D. Emr. 1997. Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 16:1820-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babst, M., B. Wendland, E. J. Estepa, and S. D. Emr. 1998. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 17:2982-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banta, L. M., J. S. Robinson, D. J. Klionsky, and S. D. Emr. 1988. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J. Cell Biol. 107:1369-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bianchi, L., B. Gerstbrein, C. Frøkj(ligae)r-Jensen, D. C. Royal, G. Mukherjee, M. A. Royal, J. Xue, W. R. Schafer, and M. Driscoll. 2004. The neurotoxic MEC-4(d) DEG/ENaC sodium channel conducts calcium: implications for necrosis initiation. Nat. Neurosci. 7:1337-1344. [DOI] [PubMed] [Google Scholar]
- 12.Bonneu, M., M. Crouzet, M. Urdaci, and M. Aigle. 1991. Direct detection of yeast mutants with reduced viability on plates by erythrosine B staining. Anal. Biochem. 193:225-230. [DOI] [PubMed] [Google Scholar]
- 13.Bowers, K., J. Lottridge, S. B. Helliwell, L. M. Goldthwaite, J. P. Luzio, and T. H. Stevens. 2004. Protein-protein interactions of ESCRT complexes in the yeast Saccharomyces cerevisiae. Traffic 5:194-210. [DOI] [PubMed] [Google Scholar]
- 14.Brachmann, C. B., A. Davies, G. J. Cost, E. Caputo, J. Li, P. Hieter, and J. D. Boeke. 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14:115-132. [DOI] [PubMed] [Google Scholar]
- 15.Burd, C. G., and S. D. Emr. 1998. Phosphatidylinositol(3)-phosphate signaling mediated by specific binding to RING FYVE domains. Mol. Cell 2: 157-162. [DOI] [PubMed] [Google Scholar]
- 16.Burke, D., D. Dawson, and T. Stearns. 2000. Methods in yeast genetics: a Cold Spring Harbor Laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 17.Caddick, M. X., A. G. Brownlee, and H. N. Arst. 1986. Regulation of gene expression by pH of the growth medium in Aspergillus nidulans. Mol. Gen. Genet. 203:346-353. [DOI] [PubMed] [Google Scholar]
- 18.Davis, D., R. B. Wilson, and A. P. Mitchell. 2000. RIM101-dependent and-independent pathways govern pH responses in Candida albicans. Mol. Cell. Biol. 20:971-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Denison, S. H., S. Negrete-Urtasun, J. M. Mingot, J. Tilburn, W. A. Mayer, A. Goel, E. A. Espeso, M. A. Peñalva, and H. N. Arst. 1998. Putative membrane components of signal transduction pathways for ambient pH regulation in Aspergillus and meiosis in Saccharomyces are homologous. Mol. Microbiol. 30:259-264. [DOI] [PubMed] [Google Scholar]
- 20.Denison, S. H., M. Orejas, and H. N. Arst. 1995. Signaling of ambient pH in Aspergillus involves a cysteine protease. J. Biol. Chem. 270:28519-28522. [DOI] [PubMed] [Google Scholar]
- 21.Díez, E., J. Alvaro, E. A. Espeso, L. Rainbow, T. Suárez, J. Tilburn, H. N. Arst, and M. A. Peñalva. 2002. Activation of the Aspergillus PacC zinc finger transcription factor requires two proteolytic steps. EMBO J. 21:1350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eguez, L., Y. S. Chung, A. Kuchibhatla, M. Paidhungat, and S. Garrett. 2004. Yeast Mn2+ transporter, Smf1p, is regulated by ubiquitin-dependent vacuolar protein sorting. Genetics 167:107-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franz, T., M. Vingron, T. Boehm, and T. N. Dear. 1999. Capn7: a highly divergent vertebrate calpain with a novel C-terminal domain. Mamm. Genome 10:318-321. [DOI] [PubMed] [Google Scholar]
- 24.Futai, E., T. Kubo, H. Sorimachi, K. Suzuki, and T. Maeda. 2001. Molecular cloning of PalBH, a mammalian homologue of the Aspergillus atypical calpain PalB. Biochim. Biophys. Acta 1517:316-319. [DOI] [PubMed] [Google Scholar]
- 25.Futai, E., T. Maeda, H. Sorimachi, K. Kitamoto, S. Ishiura, and K. Suzuki. 1999. The protease activity of a calpain-like cysteine protease in Saccharomyces cerevisiae is required for alkaline adaptation and sporulation. Mol. Gen. Genet. 260:559-568. [DOI] [PubMed] [Google Scholar]
- 26.Goll, D. E., V. F. Thompson, H. Li, W. Wei, and J. Cong. 2003. The calpain system. Physiol. Rev. 83:731-801. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez-Lopez, C. I., R. Szabo, S. Blanchin-Roland, and C. Gaillardin. 2002. Genetic control of extracellular protease synthesis in the yeast Yarrowia lipolytica. Genetics 160:417-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haro, R., B. Garciadeblas, and A. Rodríguez-Navarro. 1991. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 291:189-191. [DOI] [PubMed] [Google Scholar]
- 29.Hong, S. J., Y. S. Yi, S. S. Koh, O. K. Park, and H. S. Kang. 1998. Isolation of an extragenic suppressor of the rna1-1 mutation in Saccharomyces cerevisiae. Mol. Gen. Genet. 259:404-413. [DOI] [PubMed] [Google Scholar]
- 30.Ito, T., T. Chiba, R. Ozawa, M. Yoshida, M. Hattori, and Y. Sakaki. 2001. A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA 98:4569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones, E. W., G. C. Webb, and M. A. Hiller. 1997. Biogenesis and function of the yeast vacuole, p. 363-470. In J. R. Pringle, J. R. Broach, and E. W. Jones (ed.), The molecular and cellular biology of the yeast Saccharomyces, volume 3: cell cycle and cell biology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Katoh, K., H. Shibata, H. Suzuki, A. Nara, K. Ishidoh, E. Kominami, T. Yoshimori, and M. Maki. 2003. The ALG-2-interacting protein Alix associates with CHMP4b, a human homologue of yeast Snf7 that is involved in multivesicular body sorting. J. Biol. Chem. 278:39104-39113. [DOI] [PubMed] [Google Scholar]
- 33.Katzmann, D. J., M. Babst, and S. D. Emr. 2001. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106:145-155. [DOI] [PubMed] [Google Scholar]
- 34.Katzmann, D. J., G. Odorizzi, and S. D. Emr. 2002. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 3:893-905. [DOI] [PubMed] [Google Scholar]
- 35.Katzmann, D. J., C. J. Stefan, M. Babst, and S. D. Emr. 2003. Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J. Cell Biol. 162: 413-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klionsky, D. J., H. Nelson, and N. Nelson. 1992. Compartment acidification is required for efficient sorting of proteins to the vacuole in Saccharomyces cerevisiae. J. Biol. Chem. 267:3416-3422. [PubMed] [Google Scholar]
- 37.Kranz, A., A. Kinner, and R. Kölling. 2001. A family of small coiled-coil-forming proteins functioning at the late endosome in yeast. Mol. Biol. Cell 12:711-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kranz, J. E., and C. Holm. 1990. Cloning by function: an alternative approach for identifying yeast homologs of genes from other organisms. Proc. Natl. Acad. Sci. USA 87:6629-6633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kullas, A. L., M. Li, and D. A. Davis. 2004. Snf7p, a component of the ESCRT-III protein complex, is an upstream member of the RIM101 pathway in Candida albicans. Eukaryot. Cell 3:1609-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamb, T. M., and A. P. Mitchell. 2003. The transcription factor Rim101p governs ion tolerance and cell differentiation by direct repression of the regulatory genes NRG1 and SMP1 in Saccharomyces cerevisiae. Mol. Cell. Biol. 23:677-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamb, T. M., W. Xu, A. Diamond, and A. P. Mitchell. 2001. Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J. Biol. Chem. 276:1850-1856. [DOI] [PubMed] [Google Scholar]
- 42.Lambert, M., S. Blanchin-Roland, F. Le Louedec, A. Lepingle, and C. Gaillardin. 1997. Genetic analysis of regulatory mutants affecting synthesis of extracellular proteinases in the yeast Yarrowia lipolytica: identification of a RIM101/pacC homolog. Mol. Cell. Biol. 17:3966-3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li, M., S. J. Martin, V. M. Bruno, A. P. Mitchell, and D. A. Davis. 2004. Candida albicans Rim13p, a protease required for Rim101p processing at acidic and alkaline pHs. Eukaryot. Cell 3:741-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li, W., and A. P. Mitchell. 1997. Proteolytic activation of Rim1p, a positive regulator of yeast sporulation and invasive growth. Genetics 145:63-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, Y., T. Kane, C. Tipper, P. Spatrick, and D. D. Jenness. 1999. Yeast mutants affecting possible quality control of plasma membrane proteins. Mol. Cell. Biol. 19:3588-3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Longtine, M. S., A. McKenzie, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 47.Luo, W., and A. Chang. 2000. An endosome-to-plasma membrane pathway involved in trafficking of a mutant plasma membrane ATPase in yeast. Mol. Biol. Cell 11:579-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maccheroni, W., G. S. May, N. M. Martinez-Rossi, and A. Rossi. 1997. The sequence of palF, an environmental pH response gene in Aspergillus nidulans. Gene 194:163-167. [DOI] [PubMed] [Google Scholar]
- 49.Matsuo, H., J. Chevallier, N. Mayran, I. Le Blanc, C. Ferguson, J. Fauré, N. S. Blanc, S. Matile, J. Dubochet, R. Sadoul, R. G. Parton, F. Vilbois, and J. Gruenberg. 2004. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 303:531-534. [DOI] [PubMed] [Google Scholar]
- 50.Mingot, J. M., J. Tilburn, E. Diez, E. Bignell, M. Orejas, D. A. Widdick, S. Sarkar, C. V. Brown, M. X. Caddick, E. A. Espeso, H. N. Arst, and M. A. Peñalva. 1999. Specificity determinants of proteolytic processing of Aspergillus PacC transcription factor are remote from the processing site, and processing occurs in yeast if pH signaling is bypassed. Mol. Cell. Biol. 19: 1390-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Missotten, M., A. Nichols, K. Rieger, and R. Sadoul. 1999. Alix, a novel mouse protein undergoing calcium-dependent interaction with the apoptosis-linked-gene 2 (ALG-2) protein. Cell Death Differ. 6:124-129. [DOI] [PubMed] [Google Scholar]
- 52.Morano, K. A., and D. J. Klionsky. 1994. Differential effects of compartment deacidification on the targeting of membrane and soluble proteins to the vacuole in yeast. J. Cell Sci. 107:2813-2824. [DOI] [PubMed] [Google Scholar]
- 53.Negrete-Urtasun, S., S. H. Denison, and H. N. Arst. 1997. Characterization of the pH signal transduction pathway gene palA of Aspergillus nidulans and identification of possible homologs. J. Bacteriol. 179:1832-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Negrete-Urtasun, S., W. Reiter, E. Diez, S. H. Denison, J. Tilburn, E. A. Espeso, M. A. Peñalva, and H. N. Arst. 1999. Ambient pH signal transduction in Aspergillus: completion of gene characterization. Mol. Microbiol. 33:994-1003. [DOI] [PubMed] [Google Scholar]
- 55.Orejas, M., E. A. Espeso, J. Tilburn, S. Sarkar, H. N. Arst, and M. A. Peñalva. 1995. Activation of the Aspergillus PacC transcription factor in response to alkaline ambient pH requires proteolysis of the carboxy-terminal moiety. Genes Dev. 9:1622-1632. [DOI] [PubMed] [Google Scholar]
- 56.Papa, F. R., and M. Hochstrasser. 1993. The yeast DOA4 gene encodes a deubiquitinating enzyme related to a product of the human tre-2 oncogene. Nature 366:313-319. [DOI] [PubMed] [Google Scholar]
- 57.Pattni, K., T. H. Millard, and G. Banting. 2003. Calpain cleavage of the B isoform of Ins(1,4,5)P3 3-kinase separates the catalytic domain from the membrane anchoring domain. Biochem. J. 375:643-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peck, J. W., E. T. Bowden, and P. D. Burbelo. 2004. Structure and function of human Vps20 and Snf7 proteins. Biochem. J. 377:693-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peñalva, M. A., and H. N. Arst. 2002. Regulation of gene expression by ambient pH in filamentous fungi and yeasts. Microbiol. Mol. Biol. Rev. 66: 426-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Piper, R. C., A. A. Cooper, H. Yang, and T. H. Stevens. 1995. VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J. Cell Biol. 131:603-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Porta, A., A. M. Ramon, and W. A. Fonzi. 1999. PRR1, a homolog of Aspergillus nidulans palF, controls pH-dependent gene expression and filamentation in Candida albicans. J. Bacteriol. 181:7516-7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramon, A. M., A. Porta, and W. A. Fonzi. 1999. Effect of environmental pH on morphological development of Candida albicans is mediated via the PacC-related transcription factor encoded by PRR2. J. Bacteriol. 181: 7524-7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rieder, S. E., L. M. Banta, K. Köhrer, J. M. McCaffery, and S. D. Emr. 1996. Multilamellar endosome-like compartment accumulates in the yeast vps28 vacuolar protein sorting mutant. Mol. Biol. Cell 7:985-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rothman, J. H., C. T. Yamashiro, C. K. Raymond, P. M. Kane, and T. H. Stevens. 1989. Acidification of the lysosome-like vacuole and the vacuolar H+-ATPase are deficient in two yeast mutants that fail to sort vacuolar proteins. J. Cell Biol. 109:93-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Serrano, R., A. Ruiz, D. Bernal, J. R. Chambers, and J. Ariño. 2002. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol. Microbiol. 46:1319-1333. [DOI] [PubMed] [Google Scholar]
- 66.Shiflett, S. L., D. M. Ward, D. Huynh, M. B. Vaughn, J. C. Simmons, and J. Kaplan. 2004. Characterization of Vta1p, a class E Vps protein in Saccharomyces cerevisiae. J. Biol. Chem. 279:10982-10990. [DOI] [PubMed] [Google Scholar]
- 67.Shih, S. C., D. J. Katzmann, J. D. Schnell, M. Sutanto, S. D. Emr, and L. Hicke. 2002. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat. Cell Biol. 4:389-393. [DOI] [PubMed] [Google Scholar]
- 68.Su, S. S., and A. P. Mitchell. 1993. Identification of functionally related genes that stimulate early meiotic gene expression in yeast. Genetics 133:67-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Su, S. S., and A. P. Mitchell. 1993. Molecular characterization of the yeast meiotic regulatory gene, RIM1. Nucleic Acids Res. 21:3789-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suzuki, K., S. Hata, Y. Kawabata, and H. Sorimachi. 2004. Structure, activation, and biology of calpain. Diabetes 53(Suppl. 1):S12-S18. [DOI] [PubMed] [Google Scholar]
- 71.Syntichaki, P., K. Xu, M. Driscoll, and N. Tavernarakis. 2002. Specific aspartyl and calpain proteases are required for neurodegeneration in Caenorhabditis elegans. Nature 419:939-944. [DOI] [PubMed] [Google Scholar]
- 72.Tilburn, J., S. Sarkar, D. A. Widdick, E. A. Espeso, M. Orejas, J. Mungroo, M. A. Peñalva, and H. N. Arst. 1995. The Aspergillus PacC zinc finger transcription factor mediates regulation of both acid- and alkaline-expressed genes by ambient pH. EMBO J. 14:779-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tréton, B., S. Blanchin-Roland, M. Lambert, A. Lépingle, and C. Gaillardin. 2000. Ambient pH signalling in ascomycetous yeasts involves homologues of the Aspergillus nidulans genes palF and paIH. Mol. Gen. Genet. 263:505-513. [DOI] [PubMed] [Google Scholar]
- 74.Tu, J., L. G. Vallier, and M. Carlson. 1993. Molecular and genetic analysis of the SNF7 gene in Saccharomyces cerevisiae. Genetics 135:17-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Umemoto, N., T. Yoshihisa, R. Hirata, and Y. Anraku. 1990. Roles of the VMA3 gene product, subunit c of the vacuolar membrane H+-ATPase on vacuolar acidification and protein transport. A study with VMA3-disrupted mutants of Saccharomyces cerevisiae. J. Biol. Chem. 265:18447-18453. [PubMed] [Google Scholar]
- 76.Vito, P., L. Pellegrini, C. Guiet, and L. D'Adamio. 1999. Cloning of AIP1, a novel protein that associates with the apoptosis-linked gene ALG-2 in a Ca2+-dependent reaction. J. Biol. Chem. 274:1533-1540. [DOI] [PubMed] [Google Scholar]
- 77.Winston, F., C. Dollard, and S. L. Ricupero-Hovasse. 1995. Construction of a set of convenient Saccharomyces cerevisiae strains that are isogenic to S288C. Yeast 11:53-55. [DOI] [PubMed] [Google Scholar]
- 78.Winzeler, E. A., D. D. Shoemaker, A. Astromoff, H. Liang, K. Anderson, B. Andre, R. Bangham, R. Benito, J. D. Boeke, H. Bussey, A. M. Chu, C. Connelly, K. Davis, F. Dietrich, S. W. Dow, M. El Bakkoury, F. Foury, S. H. Friend, E. Gentalen, G. Giaever, J. H. Hegemann, T. Jones, M. Laub, H. Liao, and R. W. Davis. 1999. Functional characterization of the Saccharomyces cerevisiae genome by gene deletion and parallel analysis. Science 285:901-906. [DOI] [PubMed] [Google Scholar]
- 79.Xu, W., and A. P. Mitchell. 2001. Yeast PalA/AIP1/Alix homolog Rim20p associates with a PEST-like region and is required for its proteolytic cleavage. J. Bacteriol. 183:6917-6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu, W., F. J. Smith, R. Subaran, and A. P. Mitchell. 2004. Multivesicular body-ESCRT components function in pH response regulation in Saccharomyces cerevisiae and Candida albicans. Mol. Biol. Cell 15:5528-5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yamashiro, C. T., P. M. Kane, D. F. Wolczyk, R. A. Preston, and T. H. Stevens. 1990. Role of vacuolar acidification in protein sorting and zymogen activation: a genetic analysis of the yeast vacuolar proton-translocating ATPase. Mol. Cell. Biol. 10:3737-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yeghiayan, P., J. Tu, L. G. Vallier, and M. Carlson. 1995. Molecular analysis of the SNF8 gene of Saccharomyces cerevisiae. Yeast 11:219-224. [DOI] [PubMed] [Google Scholar]