Abstract
During most of the 20th century, the epidemiology of tick-borne rickettsioses could be summarized as the occurrence of a single pathogenic rickettsia on each continent. An element of this paradigm suggested that the many other characterized and noncharacterized rickettsiae isolated from ticks were not pathogenic to humans. In this context, it was considered that relatively few tick-borne rickettsiae caused human disease. This concept was modified extensively from 1984 through 2005 by the identification of at least 11 additional rickettsial species or subspecies that cause tick-borne rickettsioses around the world. Of these agents, seven were initially isolated from ticks, often years or decades before a definitive association with human disease was established. We present here the tick-borne rickettsioses described through 2005 and focus on the epidemiological circumstances that have played a role in the emergence of the newly recognized diseases.
INTRODUCTION
Tick-borne rickettsioses are caused by obligate intracellular bacteria belonging to the spotted fever group (SFG) of the genus Rickettsia within the family Rickettsiaceae in the order Rickettsiales (276). These zoonoses are among the oldest known vector-borne diseases. In 1899, Edward E. Maxey reported the first clinical description of Rocky Mountain spotted fever (RMSF), the prototypical tick-borne rickettsiosis (198). In 1906, Howard T. Ricketts reported the role of the wood tick in the transmission of the causative agent, subsequently named Rickettsia rickettsii (283, 284, 365). In 1919, S. Burt Wolbach provided definitive experimental evidence that R. rickettsii, referred to as “Dermacentroxenus rickettsii” at that time, was maintained by ticks and also described the fundamental histopathologic lesions of RMSF (365). For approximately the next 90 years, R. rickettsii would be the only tick-borne rickettsia conclusively associated with disease in humans in the Western Hemisphere. During the 20th century, many other formally described or incompletely characterized SFG rickettsiae were detected in North American ticks, including Rickettsia parkeri in 1939, Rickettsia montanensis (formerly R. montana) in 1963, and Rickettsia rhipicephali in 1978. However, these rickettsiae were generally considered nonpathogenic (267, 276).
Distinctions between the occurrences of a single pathogenic tick-borne rickettsia and the various other nonpathogenic rickettsiae that resided in ticks were also made by investigators from other continents. In 1910, the first case of Mediterranean spotted fever (MSF) was reported in Tunis (72). The typical inoculation eschar was described in 1925 in Marseille (223). In the 1930s, the roles of the brown dog tick, Rhipicephalus sanguineus, and the causative agent Rickettsia conorii were described (43). For several decades, R. conorii was considered to be the only agent of tick-borne SFG rickettsioses in Europe and Africa. In a similar manner, Rickettsia sibirica (in the former USSR and China) and Rickettsia australis (in Australia) were generally believed to be the sole tick-borne rickettsial agents associated with these respective locations (276).
Until relatively recently, the diagnosis of tick-borne SFG rickettsioses was confirmed almost exclusively by serologic methods (174, 276). The Weil-Felix test, the oldest but least specific serological assay for rickettsioses, is still used in many developing countries. This test is based on the detection of antibodies to various Proteus antigens that cross-react with each group of rickettsiae, including the SFG. This assay lacks sensitivity and specificity and can suggest only possible spotted fever group rickettsiosis in a patient. Even with the microimmunofluorescence (MIF) assay, the current reference method in rickettsial serology, there are wide antigenic cross-reactions among SFG rickettsiae (276). In this context, when only one antigen is used (i.e., the agent known to be pathogenic for humans in the considered location), a positive serologic reaction does not necessarily imply that the patient's illness was caused by the rickettsial species used as the antigen in the assay. Inferences made from the results of relatively nonspecific serologic assays have likely hampered the correct identification of several novel SFG rickettsioses.
The recognition of multiple distinct tick-borne SFG rickettsioses during the last 20 years has been greatly facilitated by broad use of cell culture systems and the development of molecular methods for the identification of rickettsiae from human samples and ticks (267). As a consequence, during 1984 through 2005, 11 additional rickettsial species or subspecies were identified as emerging agents of tick-borne rickettsioses throughout the world (267, 276). In 1984, an emerging SFG rickettsiosis was identified in Japan (183). Its agent was isolated from a patient in 1989 and subsequently named Rickettsia japonica (342, 343). Thereafter emerging pathogens throughout the world were described, including “Rickettsia conorii subsp. caspia” (proposed name) in Astrakhan, Africa, and Kosovo; Rickettsia africae in sub-Saharan Africa and the West Indies; Rickettsia honei in Flinders Island (Australia), Tasmania, Thailand, and perhaps the United States; Rickettsia slovaca in Europe; “Rickettsia sibirica subsp. mongolitimonae” (proposed name) in China, Europe, and Africa; “Rickettsia heilongjanghensis” (proposed name) in China and the Russian Far East; Rickettsia aeschlimannii in Africa and Europe; “Rickettsia marmionii” (proposed name) in Australia (N. Unsworth, J. Stenos, and J. Graves, Abstr. 4th Int. Conf. Rickettsiae Rickettsial Dis., abstr. O-50, 2005), and R. parkeri in the United States (267). The last rickettsia is probably the best illustration, as R. parkeri was considered a nonpathogenic rickettsia for more than 60 years. Furthermore, the pathogenicity of Rickettsia massiliae has been recently demonstrated, 13 years after its isolation from ticks (349). Other recently described rickettsiae, including Rickettsia helvetica strains in Europe and Asia, have been presented as possible pathogens (267).
The last major review on rickettsioses was published in 1997 (276). Since that time, rickettsiology has undergone a significant evolution. Some SFG rickettsiae detected or isolated from ticks only and presented as potential pathogens in 1997 are now formally described and recognized as emerging pathogens. Many previously unrecognized rickettsiae of unknown pathogenicity have been recently detected in or isolated from ticks. The use of PCR and sequencing methods for the identification of SFG rickettsiae in ticks has led to new questions regarding the geographical distribution of tick-borne rickettsiae and the tick-rickettsia association. We present here an overview of the various tick-borne rickettsioses described to date and focus on some epidemiological circumstances that have contributed to the emergence of these newly recognized diseases. We also discuss some of the questions remaining to be resolved in the future.
RECENT DEVELOPMENTS AND CONTINUING GAPS IN RICKETTSIOLOGY
Microbiology and Taxonomy: What Defines a Rickettsia sp.?
In recent years, the rickettsial field has undergone a significant evolution, particularly due to technological advances in molecular genetics. Wolbach, using a modified Giemsa stain, was the first to note the intracellular nature of R. rickettsii (365). In the 1930s and 1940s, Castaneda and Machiavello used a modified Giemsa stain to describe the tinctorial properties of rickettsiae. Rickettsiae first appear in reference books of bacteriology during the late 1930s (e.g., reference 40a). These bacteria were described as a group based on filterability, poor staining with aniline dyes, gram negativity, and staining with Giemsa or Castaneda stains. Hans Zinsser correctly insisted that some rickettsia-like forms (e.g., the agent of trench fever) were not obligatorily intracellular and could be cultivated on artificial media and therefore did not belong in the genus Rickettsia (375). However, bacteria of the order Rickettsiales have long been described simply as short, gram-negative rods that retained basic fuchsin when stained by the method of Gimenez, which was described in the mid-1950s (122).
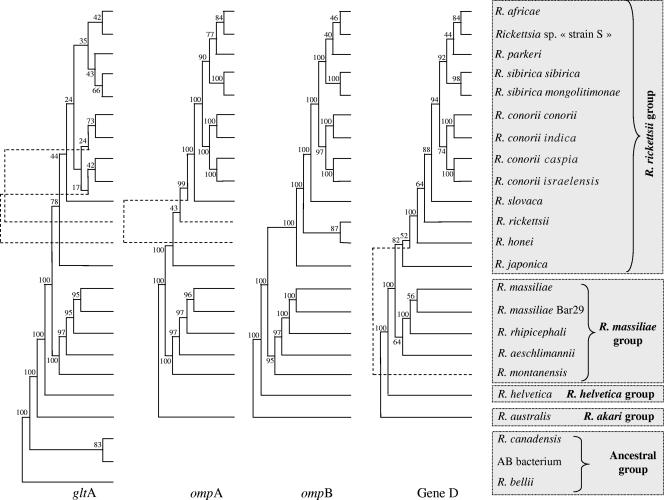
During the last decade, the taxonomy of rickettsiae has undergone extensive reorganization (134, 276). The family Bartonellaceae (including Bartonella quintana, the agent of trench fever) as well as Coxiella burnetii, the agent of Q fever, were removed from the order Rickettsiales, which includes now two families, the Anaplasmataceae and Rickettsiaceae. The classification of this order continues to be modified as new data become available. Currently, all tick-associated rickettsiae (with the exception of Rickettsia bellii and Rickettsia canadensis) belong to the spotted fever group of the genus Rickettsia within the Rickettsiaceae (Fig. 1).
FIG. 1.
Phylogenetic organization of tick-transmitted rickettsiae based on the comparison of gltA, ompA, ompB, and gene D sequences by using the parsimony method.
Traditional identification methods used in bacteriology cannot be routinely applied to rickettsiae because of the strictly intracellular nature of these organisms. Of the major rickettsial protein antigens, three high-molecular-mass surface proteins (OmpA, OmpB, and PS120) contain species-specific epitopes which provide the basis for rickettsial serotyping using comparative MIF techniques. The MIF serotyping was long considered the reference method for the identification of rickettsiae (276). Indeed, since the pioneering work of Philip et al. in 1978, two rickettsial strains were considered to have different serotypes if they exhibited a specificity difference of ≥3 (252). However, with the development of robust molecular approaches, the use of MIF serotyping as a reference method should be reconsidered. Even when serotyping by immunofluorescence or monoclonal antibodies is available, the information provided by genotypic approaches (discussed below) is characteristically more objective and definitive.
The comparison of 16S rRNA sequences is not useful for the taxonomy of rickettsiae because greater than 97% similarity exists between any two taxa. Several other genes can be used including gltA, ompA, ompB, and gene D. By use of molecular tools, one of the difficulties in rickettsiology has been the determination of a cutoff in the percent divergence among gene sequences that define a species, subspecies, or strain within the Rickettsia genus. Recent genetic guidelines for the classification of rickettsial isolates at the genus, group, and species levels, using the sequences of five rickettsial genes, including a 16S rRNA (rrs) gene, gltA, ompA, ompB, and gene D, have been proposed (104). This work was done using universally recognized species. According to these guidelines, to be classified as a new Rickettsia species, an isolate should not have more than one of the following degrees of nucleotide similarity, with the most homologous validated species: ≥99.8 and ≥99.9% for the rrs and gltA genes, respectively, and, when amplifiable, ≥98.8, ≥99.2, and ≥99.3% for the ompA and ompB genes and gene D, respectively (104). However, these guidelines may later be updated by the introduction of additional genetic or phenotypic characteristics and of new Rickettsia species.
The utility of multiple-gene sequencing for taxonomy has been discussed for the reevaluation of species definitions in bacteriology. Further, polyphasic taxonomy, which integrates phenotypic and phylogenetic data, seems to be particularly useful for rickettsial taxonomy, as demonstrated for other bacteria (326, 346). However, experts in the field of rickettsiology frequently do not agree on defining a species. One example concerns the closely related rickettsiae of the so-called R. conorii complex, including R. conorii strain Malish (the agent of MSF), Israeli spotted fever rickettsia (ISFR), R. conorii strain Indian (Indian tick typhus rickettsia [ITTR]), and Astrakhan spotted fever rickettsia (AFR).
In 1978, Philip et al., using mouse MIF serotyping, concluded that R. conorii isolates Malish, Moroccan, and Kenya belonged to the same serotype as ITTR (252). Using complement fixation, Bozeman et al. were also unable to distinguish ITTR from R. conorii isolates (F. M. Bozeman, J. W. Humphries, J. M. Campbell, and P. L. O'Hara, Symp. Spotted Fever Group Rickettsiae, p. 7-11, 1960). In contrast, Goldwasser et al. (R. A. Goldwasser, M. A. Klingberg, W. Klingberg, Y. Steiman, and T. A. Swartz, 12th Int. Congr. Intern. Med., p. 270-275, 1974), using mouse polyclonal antibodies, and Walker et al. (355), using monoclonal antibodies, observed that ITTR differed substantially from other R. conorii isolates. Regarding ISFR and AFR, we reported that PCR-restriction fragment length polymorphism (RFLP) allowed differentiation of these rickettsiae (292), and we demonstrated that AFR was different from ISFR and R. conorii on the basis of sodium dodecyl sulfate-polyacrylamide gel electrophoresis and pulsed-field gel electrophoresis profiles (95). In 1995, Walker et al., using serotyping, Western blotting (WB), monoclonal antibody reactivity, and PCR amplification of the tandem repeats within ompA, concluded that ISFR belonged to the R. conorii species (352). However, in 1998, Dasch and colleagues differentiated R. conorii from ISFR by using PCR-RFLP and then proposed the names “R. sharonii” and “R. caspii” for ISFR and AFR, respectively (77, 149). However, phylogenetically, these rickettsiae constitute a homogeneous cluster supported by significant bootstrap values and are distinct from other Rickettsia species. In 2003, using the combination of genotypic criteria described above, we demonstrated that ITTR, AFR, and ISFR were not genetically different enough to be considered new species but belonged to the R. conorii species (104). Moreover, these rickettsiae exhibit differentiable serotypes and cause diseases with distinct clinical features in defined geographic locations.
To clarify the situation, we recently considered the report of the ad hoc committee on reconciliation of approaches to bacterial systematics which proposed that bacterial isolates within a species could be considered distinct subspecies if they were genetically close but diverged in phenotype (359). Therefore, we estimated the degrees of genotypic variation among 31 isolates of R. conorii, 1 isolate of ITTR, 2 isolates and 3 tick amplicons of AFR, and 2 isolates of ISFR by using multilocus sequence typing (MLST). Also, 16S rRNA and gltA genes, as well as three membrane-exposed protein-encoding genes, ompA, ompB, and sca4 (formerly gene D), were incorporated in MLST. To further characterize the specificities of distinct MLST types, we incorporated a prototype isolate from each of these into a multispacer typing (MST) assay, which we have previously demonstrated to be more discriminant than MLST at the strain level for R. conorii (see below). Furthermore, mouse serotypes were obtained for each of these MLST types. It is important to emphasize that this work was not a pure sequence-based classification. Among the 39 isolates or tick amplicons studied, four MLST genotypes were identified: (i) the Malish type, (ii) the ITTR type, (iii) the AFR type, and (iv) the ISFR type. Among these four MLST genotypes, the pairwise similarity in nucleotide sequence varied from 99.8 to 100%, 99.4 to 100%, 98.2 to 99.8%, 98.4 to 99.8%, and 99.2 to 99.9% for 16S rRNA genes, gltA, ompA, ompB, and sca4, respectively. Representatives of the four MLST types were also classified within four types by using MST genotyping as well as mouse serotyping. By using these results, we proposed to modify the nomenclature of the R. conorii species through the creation of the following subspecies: “R. conorii subsp. conorii subsp. nov.” (type strain Malish, ATCC VR-613), “R. conorii subspecies indica subsp. nov.” (type strain ATCC VR-597) (formerly Indian tick typhus rickettsia), “R. conorii subspecies caspia subsp. nov.” (type strain A-167) (formerly Astrakhan fever rickettsia), and “R. conorii subspecies israelensis subsp. nov.” (type strain ISTT CDC1) (formerly Israeli spotted fever rickettsia) (374). The description of R. conorii has been emended to accommodate the four subspecies (for detailed descriptions of the four subspecies, see reference 374 ). The same approach has been recently proposed for R. sibirica, for which two subspecies have been proposed, including “R. sibirica subsp. sibirica” and “R. sibirica subsp. mongolitimonae” (P. E. Fournier, Y. Zhu, and D. Raoult, Abstr. 4th Int. Conf. Rickettsiae Rickettsial Dis., abstr. P-180, 2005). Nonetheless, rickettsial taxonomy remains an evolving and controversial field; in this context, there is no universal consensus on the current classification, and some rickettsiologists believe that there are too many described species of Rickettsia.
The Genome Era
Until 2001, the genome size of rickettsiae, estimated by using pulsed-field gel electrophoresis, ranged from 1.1 to 1.6 Mb (222). In 2001, the first genome of a tick-transmitted rickettsia (R. conorii strain Seven) was fully sequenced and revealed several unique characteristics among bacterial genomes (220, 221), including long-palindromic-repeat fragments irregularly distributed throughout the genome. Further comparison of the R. conorii genome with that of R. prowazekii (the agent of epidemic typhus and included in the typhus group of the genus Rickettsia) provided additional data on the evolution of rickettsial genomes, the latter appearing to be a subset of the former (221). Recently, the genomes of R. sibirica, R. rickettsii, R. akari, R. felis, and R. typhi have been reported (187, 203). Those of R. bellii, R. massiliae, R. africae, and R. slovaca are currently being sequenced. These data will provide insights into the mechanism of rickettsial pathogenicity (282) and will provide new molecular diagnostic targets and new tools for phylogenetic and taxonomic studies.
Until recently, there was no formal genotypic method to describe rickettsiae at the strain level. In 2004, Fournier et al. tested the hypothesis that the most suitable sequences for genotyping bacterial strains are those that are found to be most variable when the genomes of two closely related bacteria are aligned (114). Using the nearly perfectly colinear genomes of R. conorii (a spotted fever group rickettsia) and R. prowazekii (a typhus group rickettsia), they found that the most variable sequences at the species level were variable intergenic spacers, which are significantly more than conserved genes, split genes, remnant genes, and conserved spacers (P values of <10−2 in all cases). These spacers were also the most variable at the strain level. Using a combination of sequences from three highly variable spacers in a multispacer tool, they identified 27 genotypes among 39 strains of R. conorii subsp. conorii strain Malish (Seven). Further, this technique, which was named multispacer typing (MST), appeared to be a valuable tool for tracing rickettsial isolates from a single source with a difference in culture history of at least 60 passages. It was also found to be more discriminatory for strain genotyping than multiple-gene sequencing (P < 10−2) (114). The advantages of MST include high discrimination, reproducibility, simplicity of interpretation, and ease of incorporation of the data obtained into accessible databases. MST could be used for tracking isolates from a wide variety of sources, including isolates from a single strain with different passage histories, and even be applied to clinical specimens.
Tick-Rickettsia Relationships
Ticks belonging to the family Ixodidae, also called “hard” ticks, can act as vectors, reservoirs, or amplifiers of SFG rickettsiae. These bacteria do not normally infect humans during their natural cycles between their arthropod and vertebrate hosts. Ecological characteristics of the tick vectors influence the epidemiology and clinical aspects of tick-borne diseases (245). As an example, European Dermacentor species ticks that bite humans are most active during early spring, autumn, and occasionally winter and are well known to bite on the scalp. Because R. slovaca is transmitted by Dermacentor ticks, the inoculation eschar of R. slovaca infection is characteristically located on the scalp during these seasons (275). Similarly, because the principal vectors of RMSF in the United States (i.e., Dermacentor variabilis and D. andersoni) and MSF in southern Europe (i.e., Rhipicephalus sanguineus) are most active during the late spring and summer, most cases of RMSF and MSF occur during these months. Further, Rhipicephalus sanguineus lives in peridomestic environments shared with dogs (e.g., kennels, yards, and houses) but has a relatively low affinity for humans. Infection rates of Rhipicephalus sanguineus with SFG rickettsiae are generally under 10%. Because of these circumstances, cases of MSF are sporadic and typically encountered in urban areas. In contrast, Amblyomma hebraeum (the southern African bont tick), the principal vector of R. africae in southern Africa, is an aggressive, human-biting tick and demonstrates high rates of infection with this rickettsia (145, 242). Because of these particular characteristics, cases of African tick bite fever (ATBF) often occur in clusters and are frequently described among groups of persons who venture into rural or undeveloped areas on safari or adventure races (66). More details on biology and behaviors of ticks and their consequences in tick-borne bacterial diseases have been reviewed recently (245).
Questions regarding the specificity of associations among rickettsiae and a particular tick species are unresolved, in part because specific characterizations of species and subspecies in both phyla may lack sensitivity. Consequently, it is difficult to determine how long a tick species has been associated with a rickettsial species and if coevolution has occurred. Some rickettsiae, such as R. rickettsii, may be associated with several different tick vectors from several different genera. This contrasts with other rickettsiae, such as R. conorii, which appear to be associated with only one tick vector (276). Between these extremes, there are certain rickettsiae which are associated with several species within the same genus, such as R. africae and R. slovaca with various Amblyomma spp. and Dermacentor spp., respectively (245). Finally, there is some evidence to indicate that some typhus group rickettsiae, particularly Rickettsia prowazekii, may in certain circumstances be associated with ticks (A. Medina-Sanchez, D. H. Bouyer, C. Mafra, J. Zavala-Castro, T. Whitworth, V. L. Popov, I. Fernandez-Salas, and D. H. Walker, Abstr. 4th Int. Conf. Rickettsiae Rickettsial Dis., abstr. O-53, 2005) (54).
In the 1980s, Burgdorfer et al. showed that ticks infected with the SFG rickettsia Rickettsia peacockii were refractory to infection with and maintenance of R. rickettsii (51). Recent studies of interspecies competition between different rickettsiae in the same tick, using cohorts of R. montanensis-infected and R. rhipicephali-infected D. variabilis organisms, have demonstrated similar inhibitory effects between rickettsiae: rickettsia-infected ticks exposed to the other rickettsial species by capillary feeding were incapable of maintaining both rickettsial species transovarially. It was suggested that rickettsial infection of tick ovaries may alter the molecular expression of the oocytes and cause interference or blocking of the second infection (182). The process of rickettsial “interference,” i.e., one species of SFG rickettsia successfully outcompeting another for the microenvironment inside the tick, may have profound implications regarding the distribution and frequency of various pathogenic rickettsiae and the specific diseases they cause (F. M. Bozeman, J. W. Humphries, J. M. Campbell, and P. L. O'Hara, Symp. Spotted Fever Group Rickettsiae).
The life cycles of most tick-borne rickettsiae are also incompletely known. In natural vertebrate hosts, infections may result in a rickettsemia that allows noninfected ticks to become infected and for the natural cycle to be perpetuated. For example, R. rickettsii has been isolated from various small mammals, and some, including meadow voles, golden-mantled ground squirrels, and chipmunks, develop rickettsemias of sufficient magnitude and duration to infect laboratory-reared ticks. However, other wild and domesticated animals susceptible to infection with R. rickettsii, including dogs, cotton rats, and wood rats, produce rickettsemias too low or too transiently to routinely infect ticks (50, 219).
Ticks may also acquire rickettsiae through transovarial passage (transfer of bacteria from adult female ticks to the subsequent generation of ticks via the eggs). Because ixodid ticks feed only once at each life stage (245), rickettsiae acquired during blood meal acquisition from a rickettsemic host or through tranovarial route can be transmitted to another host only when the tick has molted to its next developmental stage and takes its next blood meal. This so-called transstadial passage (transfer of bacteria from stage to stage) is a necessary component for the vectorial competence of the ticks. When rickettsiae are transmitted efficiently both transstadially and transovarially in a tick species, this tick will serve as a reservoir of the bacteria and the distribution of the rickettsiosis will be identical to that of its tick host (245). R. slovaca multiplies in almost all organs and fluids of its tick host, particularly in the salivary glands and ovaries, which enables transmission of rickettsiae during feeding and transovarially, respectively (279). Two other methods for acquiring rickettsiae have been reported. Sexual transmission of R. rickettsii from infected males to noninfected female ticks has been described, but this process is unlikely to significantly propagate the infection in tick lineages, as venereally infected females do not appear to transmit rickettsiae transovarially (307). A second suggested method of acquisition of rickettsiae by ticks is the process of cofeeding, which occurs as several ticks feed in proximity on the host. In this circumstance, direct spread of bacteria from an infected tick to an uninfected tick may occur during feeding at closely situated bite sites, as demonstrated with R. rickettsii and D. andersoni (250).
Although tick-rickettsia relationships were a focus of interest by many pioneering rickettsiologists, most early studies concentrated on the role of ticks as vectors. Considerably less attention was directed to the relationships of rickettsiae with various tick cells, tissues, and organs and with specific physiologic processes of acarines. Transovarial transmission of rickettsiae in their recognized vectors has been demonstrated for several SFG species, including R. rickettsii (307), R. slovaca (279), R. sibirica (297), R. africae (154), R. helvetica (46), and R. parkeri (124). In some instances, transovarial transmission of a particular Rickettsia species may occur in a particular tick but cannot be sustained for more than one generation (182).
The percentage of infected eggs obtained from females of the same tick species infected with the same rickettsial strain may vary for as yet unknown reasons (47, 55). For some rickettsia-tick relationships, such as R. montanensis in D. variabilis (182), R. slovaca in Dermacentor marginatus (279), and R. massiliae in Rhipicephalus sanguineus group ticks (196), maintenance of rickettsiae via transovarial transmission may reach 100% and have no effect on the reproductive fitness and viability of the tick host. In contrast, transovarial transmission of R. rickettsii in D. andersoni diminishes survival and reproductive capacity of tick filial progenies. Recent experiments have shown that R. rickettsii is lethal for the majority of experimentally and transovarially infected D. andersoni ticks. In one study, most nymphs infected as larvae by feeding on rickettsemic guinea pigs died during the molt into adults, and most of adult female ticks infected as nymphs died prior to feeding. Rickettsiae were vertically transmitted to 39.0% of offspring, and significantly fewer larvae developed from infected ticks (214). The lethal effect of R. rickettsii on its acarine host, coupled with the competitive interactions among different rickettsiae that inhabit the tick microenvironment, may influence the low prevalence of ticks infected with R. rickettsii in nature and affect its enzootic maintenance (214).
Interestingly, basic questions about the tick-rickettsia relationship remain for MSF, one of the oldest recognized tick-borne rickettsioses. In this context, we are unaware of any well-documented demonstration of transovarial transmission of R. conorii in Rhipicephalus sanguineus. In 1932, Blanc and Caminopetros demonstrated that larvae, nymphs, and adults could act as vectors of MSF. Furthermore, over winter, unfed males and females were shown to be able to transmit the agent (38). It was also shown that when eggs or larvae obtained from infected Rhipicephalus sanguineus females were crushed and inoculated into humans, MSF was obtained. These data suggest that transovarial transmission of the MSF agent occurs in ticks (38). However, neither the transovarial transmission rate (the proportion of infected females giving rise to at least one positive egg or larva) nor the filial infection rate (proportion of infected eggs or larvae obtained from an infected female), which would be useful for comparison with infection rates in nature, is known to our knowledge. It is not known if transovarial transmission of R. conorii is maintained from generation to generation of Rhipicephalus sanguineus. In a similar manner, the effect of R. conorii on its tick host and potential interactions with other rickettsiae associated with the same tick species, such as R. massiliae, are unknown (25).
Female D. andersoni ticks infected with R. rickettsii and incubated at 4°C show a lower mortality rate than infected ticks at 21°C (214). This discrepancy may be linked with the long-recognized but poorly explained phenomenon known as reactivation (325). In nature, stress conditions encountered by rickettsiae within the tick include starvation and temperature shifts. As an example, as ticks enter diapause, weeks to months may pass until they obtain their next blood meal. In the laboratory, R. rickettsii in D. andersoni ticks loses its virulence for guinea pigs when the ticks are subjected to physiological stress, such as low environmental temperature or starvation. However, subsequent exposure of these same ticks to 37°C for 24 to 48 h or the ascertainment of a blood meal may restore the original virulence of the bacteria. During tick blood-feeding, rickettsiae undergo various physiological changes and proliferate intensively as they reactivate from a dormant avirulent state to a pathogenic form (47, 325, 363, 364).
The precise molecular mechanisms responsible for the adaptation of rickettsiae to different host conditions and for reactivation of virulence are unknown. However, the stress adaptation in some gram-negative bacteria, also called the stringent response, has been shown to be mediated by the nucleotide guanosine-3,5(bis)pyrophosphate(ppGpp), which is modulated by spoT genes. Interestingly, annotation of R. conorii genome reveals five spoT paralogs, and environmental stress conditions are accompanied by a variable spoT1 transcription in R. conorii (296). This phenomenon could play a role in adaptation of rickettsiae to ticks and during the process of reactivation. It has also been hypothesized that changes in outer surface proteins occur during alternating infection in ticks and in mammals (296). Further studies of the molecular dynamics of between rickettsiae and ticks, similar to work on molecular interactions at the tick ovary-rickettsia interface recently published by Mulenga et al. (210), will be needed to better understand these processes.
Pathogenicity of Tick-Borne Rickettsiae
Throughout the 20th century, many spotted fever group rickettsiae were isolated from or detected in ticks. Many of these rickettsiae were initially characterized as symbionts, endosymbionts, or nonpathogenic bacteria. The rationale for these characterizations was generally supported by limited pathogenicity testing in animals and the fact that these rickettsiae had been isolated in places where a single, previously recognized tick-borne rickettsial pathogen already existed. Interestingly, the view that multiple and distinct pathogenic rickettsiae may circulate in one or several species of ticks in a given geographic area is a relatively contemporary concept.
The first component for a rickettsia to be a potential pathogen of humans is the likelihood of the bacterium to be transmitted through the tick bite, which generally implies that the rickettsia can localize to the salivary glands of the tick. Some SFG rickettsiae, including R. peacockii, produce heavy infections in the ovaries but do not invade the salivary glands of their tick hosts, precluding subsequent transmission to potential vertebrate hosts during blood meal acquisition (215). A potential pathogen must also be associated with a tick with some proclivity to bite a human host. In this context, tick-host specificity is a key component of the epidemiology of tick-borne diseases. Certain tick species rarely, if ever, bite humans, and even if these ticks are associated with highly pathogenic rickettsiae, disease in humans will rarely, if ever, be associated with these species. However, it may also be possible that tick-borne rickettsiae that are excreted in tick feces might initiate infection via abraded skin. For example, viable R. slovaca have been isolated from the feces of D. marginatus collected in nature (279). The abundance of a particular tick vector, the prevalence of the infection within ticks, and the prevalence of natural hosts of the ticks that come in contact with humans are other elements that affect the frequency of tick-borne rickettsioses that have been discussed elsewhere (245).
When transmitted to a susceptible human host, pathogenic tick-borne SFG rickettsiae localize and multiply in endothelial cells of small- to medium-sized blood vessels, causing a vasculitis which is responsible for the clinical and laboratory abnormalities that occur in tick-borne rickettsioses (276). Molecular characteristics and the expression of particular rickettsial gene products likely contribute to differences in pathogenicity among various species of spotted fever group rickettsiae. The expression of OmpA by R. rickettsii allows adhesion of and entry into host endothelial cells by this pathogen (178). Despite its close phylogenetic placement to R. rickettsii, R. peacockii (another SFG rickettsia found in D. andersoni) possesses an ompA gene that contains three premature stop codons and is unable to express the OmpA protein; this rickettsia is considered a nonpathogen (18). Also, it has been suggested that the OmpB plays a role in the adherence to and invasion of host cells by R. japonica (344).
After phagocytosis and internalization, the phagocytic vacuole is rapidly lysed and rickettsiae escape the phagocytic digestion to multiply freely in the host cell cytoplasm and nucleus, the latter a characteristic specific for bacteria in the spotted fever group of the genus Rickettsia (276). Rickettsiae can move from cell to cell by actin mobilization (357). Escape from vacuole is suspected to be mediated by an enzyme, possibly phospholipase A2 (351). However, the presence of a gene encoding a phospholipase D has been recently shown, and this gene may be a key factor for virulence (281). More recently, a R. conorii surface protein, RickA, was identified in vitro as an activator of the Arp2/3 complex, which is essential in actin polymerization (126).
Many aspects of rickettsial pathogenesis remain unknown. Animal models have been used to predict the pathogenicity to humans of other symbionts found in arthropods; however, this technique is unreliable with the rickettsiae. For example, the T-type strain of R. rickettsii causes only a mild illness in guinea pigs but is highly pathogenic in humans. In the past, pathogenicity of various rickettsiae in guinea pigs was considered an indication of the pathogenicity of the agent in humans; however, the pathogenic role of a tick-borne rickettsia can only be determined conclusively by isolating or detecting the organisms from patients with signs of disease. In this context, nonpathogenic rickettsiae are better characterized as rickettsiae of unknown pathogenicity until clear evidence exists to show that the particular bacterium does not cause disease in humans.
In this review, the rickettsiae designated as human pathogens have been isolated in cell culture or detected by molecular methods from blood or tissues from patients with illnesses clinically compatible with spotted fever rickettsioses. When cases are documented solely by serologic methods, the pathogenicity of the rickettsia used as an antigen can only be presumed, particularly when a limited number of rickettsial antigens are used in the evaluation. Other rickettsiae can be considered potential pathogens, particularly if they have been detected in the salivary glands of tick species readily biting people.
TICK-BORNE RICKETTSIAE IDENTIFIED AS HUMAN PATHOGENS
Pathogens Described Prior to 1984
Rickettsia rickettsii (Rocky Mountain spotted fever)
Rocky Mountain spotted fever was first described as a specific clinical entity by Maxey in 1899 (284). The role of Dermacentor ticks in the transmission of the disease was documented in reports by King (156) and Ricketts (283) in 1906. Ricketts also isolated the causative organism in guinea pigs and demonstrated that it circulated between ticks and mammals in nature and that infected ticks could transmit the bacterium transovarially to their progeny (284, 285). Ricketts, as well as another famous rickettsiologist, von Prowazek, died of typhus, and the agents of typhus and RMSF were subsequently named Rickettsia prowazekii and R. rickettsii, respectively, in their honor.
RMSF remains the most severe of all tick-borne rickettsioses. Prior to the discovery of effective antibiotics and appropriate supportive therapy, persons with RMSF frequently succumbed to the infection: from 1873 to 1920, 283 (66%) of 431 reported cases resulted in death (68). RMSF also claimed the lives of many early investigators, including entomologists and laboratorians, who worked with R. rickettsii (261). This disease continues to cause significant mortality in the United States. Five to 39 deaths were reported annually to public health authorities during 1983-1998; however, the magnitude of underreporting may be profound, and it is estimated that approximately 400 additional RMSF deaths were not reported during this same interval (229). Despite its name, RMSF has been reported throughout most of the continental United States, except for Maine and Vermont (194).
Although most cases are associated with rural or semirural areas, autochthonous cases have been described in large urban centers, including New York City, where rickettsia-infected D. variabilis were found in parks and vacant lots (302). The disease is most prevalent in the southeastern and midwestern United States, with the largest number of reported cases originating from North Carolina, Oklahoma, Tennessee, Arkansas, South Carolina, Maryland, and Virginia (194, 337). Because of the seasonal activity associated with the tick vectors of R. rickettsii, RMSF demonstrates a similar pattern, with a peak in cases observed during mid-spring through late summer in the United States. From 1997 through 2002, 3,649 cases of RMSF were reported to the Centers for Disease Control and Prevention, and approximately 90% of confirmed cases occurred from April through September. The average annual incidence of RMSF for this period was 2.2 cases per million persons (67).
Multiple and diverse factors contribute to the incidence rates of complex zoonoses, including RMSF and other tick-borne SFG rickettsioses, and annual case counts are generally subject to wide regional and temporal variabilities. The annual number of cases of RMSF in the United States, as determined by passive surveillance, has fluctuated markedly since the beginning of systematic collection of these data in 1920. These numbers may have been affected by one or more of the following: changes in surveillance affected by improved recognition and disease reporting, cyclic changes in the transmission caused by competition or interference with other tick-borne rickettsiae, diminished tick populations caused by widespread use of pesticides (particularly dichlorodiphenyltrichloroethane), and increased human contact with tick-infested habitats through recreational activities (68). For example, the average annual incidence of RMSF in the United States fluctuated from a low of 1.4 cases per million persons in 1998 to a high of 3.8 cases per million in 2002, representing the lowest and highest incidence rates, respectively, recorded since 1993 (67).
The primary vector of RMSF for most of the United States is the American dog tick D. variabilis (Fig. 2). This tick inhabits the Great Plains region, the Atlantic Coast, California, and southwestern Oregon. It has also been described in southeastern Saskatchewan Province in Canada and as far south as northern Mexico. Adult and nymphal activity generally begins in March or April and extends through August or September. The ticks at immature stages feed almost exclusively on small rodents. D. andersoni (the Rocky Mountain wood tick) is an important vector in the Rocky Mountain states and Canada. The distribution of this tick occurs in the mountainous regions of the western United States and the southern parts of British Columbia. Adults feed primarily on large animals such as horses, cattle, sheep, coyotes, deer, and bear. Immature stages feed largely on small mammals. The Rocky Mountain wood tick is most abundant in areas where small rodent share habitats with large wild and domestic animals. This situation was prevalent in the Bitterroot valley of Western Montana during early investigations of RMSF, when a large population of Columbian ground squirrels (Citellus columbianus columbianus) lived in close association with humans and domestic animals (44).
FIG. 2.
Dermacentor variabilis, the primary vector of Rocky Mountain spotted fever in most of the United States. From left to right, male, female, nymph, and larva. Bar scale, 1 cm.
Other species of ticks found in the United States have been shown to be naturally infected with R. rickettsii or have been demonstrated to be potential vectors of the pathogen in the laboratory. These include Haemaphysalis leporispalustris (the rabbit tick), Ixodes dentatus, Dermacentor occidentalis, Dermacentor parumapertus, Amblyomma americanum (the lone star tick), Rhipicephalus sanguineus, and the soft tick Ornithodoros parkeri (78, 200, 231, 234, 235). Some of these tick species seldom bite humans (e.g., H. leporispalustris and D. parumapertus), and for others, contemporary evidence incriminating the tick as an important vector of RMSF is lacking (e.g., O. parkeri or A. americanum) (69). It is likely that several tick species are involved in maintaining and disseminating R. rickettsii in nature (313). Although ticks serve as a natural reservoir for R. rickettsii, the deleterious effect of this pathogen for all stages of its acarine host may explain the low prevalence of infected ticks in nature and may affect its enzootic maintenance (214). Small mammals, such as chipmunks, voles, ground squirrels, and rabbits are common blood meal sources for immature ticks of many species naturally infected with R. rickettsii. Some of these animals are highly susceptible to rickettsial infection and may serve as R. rickettsii-amplifying hosts (40, 53).
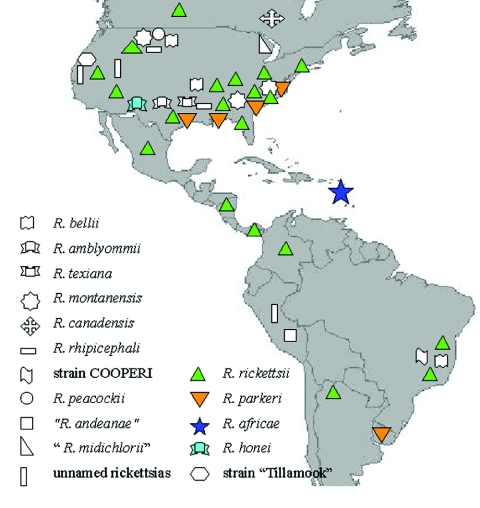
In Central and South America, natural infections with R. rickettsii have been identified in Amblyomma cajennense (the Cayenne tick) specimens collected in Mexico (59), Panama (84), and Brazil (86) and in Amblyomma aureolatum specimens in Brazil (303). Considerable evidence accumulated by investigators in Mexico during the early to mid-1940s convincingly demonstrated a role of Rhipicephalus sanguineus in the transmission cycle of a severe spotted fever rickettsiosis (presumably RMSF) to humans in several northern and central states of that country, including Coahuila, Durango, San Luis Potosí, Sinaloa, Sonora, and Veracruz (58, 59, 189). Surprisingly, despite historical data on natural infections in and vector competency of Rhipicephalus sanguineus (235), and a generally ubiquitous and peridomestic distribution of this tick, similar studies to conclusively incriminate the brown dog tick as an important vector of RMSF in other regions of the Western Hemisphere were absent until 2002-2004, when 15 cases of RMSF were identified in two rural communities in eastern Arizona. In both locales, only Rhipicephalus sanguineus ticks were found in the areas frequented by case patients, typically in peridomestic settings associated with abundant pet and stray dogs. Rhipicephalus sanguineus ticks were also found occasionally attached to individuals in the community, most often children, and infesting the local dog population. R. rickettsii was identified by using culture and PCR in ticks collected at case households (82). It is likely that similar ecologic scenarios exist in other areas of the Western Hemisphere and that investigators will subsequently identify other peridomestic cycles of RMSF that involve Rhipicephalus sanguineus.
The mean incubation period of RMSF following tick bite is 7 days (range, 2 to 14 days). Only approximately 60% of patients recall a tick bite (194, 317), as these bites are generally painless and the tick may attach in places of the body difficult to observe, including the scalp, axillae, and inguinal areas (245). In contrast with most other tick-borne SFG rickettsiae, R. rickettsii does not generally elicit an eschar at the tick bite site. The onset of the disease includes high fever and a headache that may be associated with malaise, myalgias, nausea, vomiting, anorexia, generalized or focal abdominal pain, and diarrhea. When such nonspecific symptoms dominate the clinical presentation, misdiagnosis and treatment delay can occur. The rash of RMSF is usually not apparent until the third day of fever or later and begins as small, irregular, pink macules that typically appear first on wrists, ankles, and forearms. The rash may later evolve to papules or petechiae. The characteristic spotted rash of RMSF is generally observed in persons on or after the fifth day of illness and heralds progression of the infection to more severe disease (313, 317). In approximately 10% of patients, the rash may be absent, which may delay diagnosis and therapy (317).
RMSF may result in various neurological manifestations, including deafness, convulsions, and hemiplegia. Other manifestations of severe disease include pulmonary and renal failure, myocarditis, and necrosis and gangrene of the fingers, toes, earlobes, and external genitalia. The case fatality rate of untreated RMSF is 10 to 25%, depending on patient's age, and approximately half of the deaths occur on or before the eighth day of illness (194). Recently, risk factors for death of 6,388 RMSF-confirmed (81%) and probable RMSF cases reported during 1981-1998, including 213 deaths (average annual case fatality rate, 3.3%), were studied. Older patient age, onset-to-treatment interval of ≥5 days, lack of tetracycline treatment, and chloramphenicol-only treatment remained significantly associated with fatal outcome (137). Although chloramphenicol and tetracyclines were considered effective antibiotic therapies for RMSF, the results of this study indicate that tetracylines are superior to chloramphenicol for the treatment of this disease. Doxycycline is currently considered the drug of choice for nearly all patients with RMSF, including young children (137, 194, 265). Unfortunately some physicians are not aware of this: among 84 primary care physicians in Mississippi who participated in a 2002 survey examining the knowledge, attitudes, and practices regarding diagnosis and treatment of RMSF, only 21% of family practice physicians and only 25% of emergency medicine physicians correctly identified doxycycline as the antibiotic of choice for treating children with RMSF (224).
RMSF is likely underdiagnosed and underreported in the United States, particularly in states where physicians are less aware of the disease (350). Surprisingly few research teams in the United States currently work with SFG rickettsiae, even though many questions posed by Ricketts and others in 1909 are still unanswered (350). Early investigators commented on differences between case fatality rates of RMSF identified among patients residing in certain areas in the United States (i.e., 5% in Idaho versus 65 to 80% in the Bitterroot Valley of Montana) (284, 365). In a similar manner, several contemporary studies have commented on the frequency of antibodies reactive with R. rickettsii among persons with no history of an illness of the severity generally associated with RMSF. Using these data, some have inferred that mild or subclinical infections with R. rickettsii may occur. For example, serum specimens of 32 (9.1%) of 352 children showed immunoglobulin G (IgG) titers of ≥64 to R. rickettsii antigen when tested by indirect fluorescent-antibody assay (IFA); however, only 8 of these children had experienced a febrile illness accompanied by rash or headache in the previous year, and none had ever been hospitalized or treated for RMSF (335). Another recent study identified IgG titers of ≥64 to R. rickettsii in the sera of 239 (12%) 1999 children 1 to 17 years of age, collected from various medical facilities in six southeastern and south-central states in the United States. These investigators suggested that at least some of the seroreactivity identified in this study could be directed against other spotted fever group rickettsiae not previously considered pathogenic and that most infections with SFG rickettsia may be relatively mild (192).
More compelling are recent prospective evaluations of individuals who seroconvert to R. rickettsii following tick bites and for whom mild or no illness is reported. In two recent studies involving military personnel exposed to ticks during training exercises in rural areas, only 20 to 44% of persons with recent evidence of spotted fever rickettsial infection by IFA or enzyme immunoassay tests developed symptoms compatible with rickettsiosis (e.g., fever, rash, myalgia, or headache), and none of the 67 individuals from these studies who seroconverted to R. rickettsii developed an illness severe enough to require hospitalization (199, 370). These findings, particularly viewed in context with the historically recognized severity of RMSF and the known cross-reactivity of spotted fever group rickettsial antigens, strongly suggest that many serologically confirmed cases of RMSF in the United States represent infections with spotted fever group rickettsiae other than R. rickettsii.
RMSF has also been identified in several provinces of Canada (201), several states in Mexico (56, 57), and in Panama (83), Costa Rica (115), Colombia (247), Brazil (86), and Argentina (286). Outside of the United States, RMSF has been most extensively described in Brazil, where R. rickettsii has been associated of with various synonymous diseases termed “São Paulo exanthematic typhus,” “Minas Gerais exanthematic typhus,” and “Brazilian spotted fever” since the early 1930s (86, 118, 232). A primary vector of R. rickettsii in Brazil is A. cajennense, a tick that feeds on various medium to large wild and domesticated animals, including tapirs, capybaras, horses, and dogs (168). Despite the extensive investigation of this disease by South American scientists during the years shortly following its discovery, relatively little attention was directed to the study of spotted fever in Brazil, and few cases were identified until the mid-1980s. During the last 20 years, a resurgence in identified cases, accompanied by increasing interest in rickettsioses by Brazilian investigators and others, have intensified the study of these diseases in this region (79, 80, 117, 207, 318). Cases of spotted fever have been documented by serology or immunostaining of tissues in several states, particularly in the southeast region of the country, including Minas Gerais, São Paulo, Rio de Janeiro, and Espirito Santo (79, 118), where most cases occur between July and December. Recently, Angerami et al. reported 23 patients with a confirmed diagnosis of Brazilian spotted fever either by isolation of R. rickettsii from blood or skin (13 patients) or a fourfold rise in MIF titers (8 patients). They were admitted with fever at the Hospitalas das Clinicas da Unicamp, São Paulo, Brazil. Relevant clinical features included myalgias (80%), headache (66%), icterus (52%), exanthema (47%), consciousness impairment (43%), vomiting (42%), abdominal pain (38%), respiratory distress (37.5%), acute renal insufficiency (35.3%), and hypotension and shock (33%). Hemorrhagic manifestations, including petechiae and suffusions, were frequent (69.5%). The case fatality rate was 30% (R. N. Angerami, M. R. Resende, S. B. Stuchi Raquel, G. Katz, E. Nascimento, and L. J. Silva, Abstr. 4th Int. Conf. Rickettsiae Rickettsial Dis., abstr. P-160, 2005).
The first confirmed cases of spotted fever rickettsiosis in Argentina were described in 1999. Between November 1993 and March 1994 in Jujuy Province in northwestern Argentina, six children with fever, rash, and a history of recent tick bite were evaluated for rickettsial infections. Immunohistochemical staining of tissues obtained at an autopsy of one fatal case confirmed a spotted fever group rickettsiosis, and the serum of another patient convalescing from the illness showed high antibody titers to R. rickettsii when tested by MIF. A. cajennense ticks were collected from dogs and pets in the area (286). The recent identification of spotted fever rickettsiosis in Peru (37) indicates that R. rickettsii or other related rickettsiae are also endemic in this country (36). The incidence and distribution of RMSF and other tick-borne rickettsioses in Latin America are undoubtedly underestimated and await the collaborative efforts of physicians, rickettsiologists, entomologists, and epidemiologists to characterize the magnitude and public health impact of these infections in these regions (350).
“Rickettsia conorii subsp. conorii” (Mediterranean spotted fever)
In 1910, the first case of MSF was reported in Tunis (72). The disease was thereafter also known as “boutonneuse fever” because of a papular rather than macular rash. The typical inoculation eschar at the tick bite site, the hallmark of many SFG rickettsioses, was described in 1925 in Marseille by Boinet and Pieri (223, 276). In the 1930s, the role of Rhipicephalus sanguineus and the causative agent subsequently named R. conorii were described (43). As discussed previously, these isolates may be collectively identified as Rickettsia conorii subsp. conorii subsp. nov. (104, 374). Three strains of R. conorii subsp. conorii include (i) Seven or Malish (the most common strain identified in our laboratory from France, Portugal, and northern Africa), (ii) Kenyan, and (iii) Moroccan, which is apparently a unique isolate (D. Raoult, unpublished data).
MSF is endemic in the Mediterranean area, including northern Africa and southern Europe. Cases continue to be identified in new locations within this region, as some cases were recently described in Turkey (165). In Italy, approximately 1,000 cases are reported each year (8). MSF is a reportable disease in Portugal (14), where the annual incidence rate of 9.8 cases per 100,000 persons is the highest of the rates of all Mediterranean countries (85). As with all rickettsioses, this rate likely underestimates the true incidence, and some authors suggest that there are seven times more cases than officially reported (85). Some cases have also been sporadically reported in northern and central Europe, including Belgium (172), Switzerland (248), and northern France (312), where Rhipicephalus sanguineus can be imported with dogs and survive in peridomestic environments providing acceptable microclimatic conditions, including kennels and houses (245). MSF is also encountered infrequently in sub-Saharan Africa and around the Black Sea (276). Although an MSF-like disease was described in Vladivostok in the eastern part of Russia in 1966 (332), no direct evidence of R. conorii infection has been reported there since that time.
In Europe, cases are encountered in late spring and summer, when the tick vectors are most active. In France, most cases are diagnosed during July and August, because of increased outdoor activity associated with the peak of activity of immature ticks that are far smaller than adults and difficult to observe even when attached to the body (276). Similarly, most samples submitted for diagnostic evaluation in Portugal at the Center for Vectors and Infectious Disease Research, National Institute of Health, are received between July and September (14). In Croatia, >80% of cases occur between July and September, with a peak in August (263).
An increase in the numbers of MSF cases observed in France, Italy, Spain, and Portugal during the 1970s paralleled similar increases in RMSF observed in the United States during this same decade (188). This increase in incidence was correlated with higher temperatures and lower rainfall in Spain and with a decrease in the number of days of frost during the preceding year in France (121). Although Rhipicephalus sanguineus adapts well to urban environments, it is relatively host specific and rarely feeds on people unless its preferred host (the domestic dog) is not available. For this reason, the incidence of MSF is relatively low in southern France (approximately 50 cases per year per 100,000 persons), despite the fact that 5% to 12% of Rhipicephalus sanguineus ticks in the region are infected with spotted fever group rickettsiae. To our knowledge, a recent report describing 22 Rhipicephalus sanguineus (1 adult and 21 nymphs) attached to an alcoholic homeless man living with his dog near Marseille (135) was the first documentation of more than one Rhipicephalus sanguineus feeding on a human host (121). Because this infestation was associated with the highest summer temperatures noted in France during the past 50 years in France, it is possible that host-seeking and feeding behaviors of this tick were altered by unusual climactic circumstances (245). In this context, it is also likely that other homeless persons who live and sleep in proximity to Rhipicephalus sanguineus-infested dogs are at increased risk for MSF (248, 277). Most recently, another unusual case of MSF including three inoculation eschars has also been observed in southern France (D. Raoult, unpublished data).
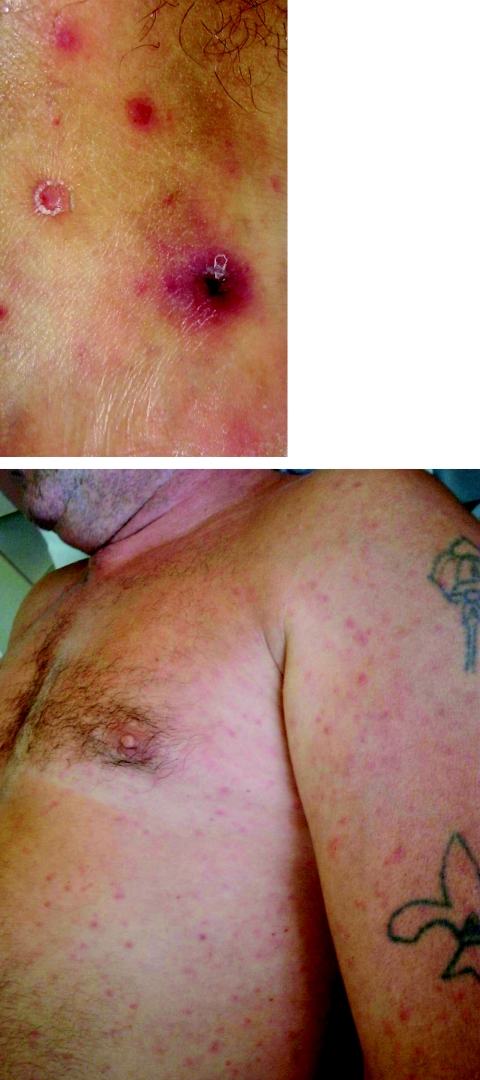
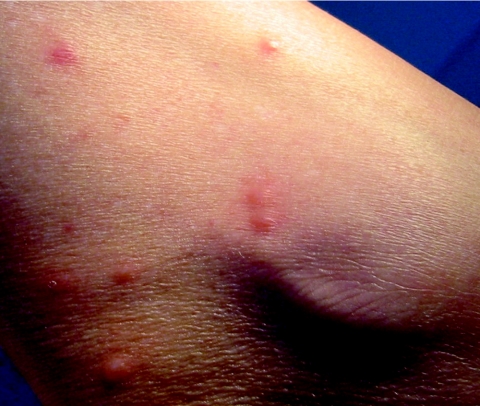
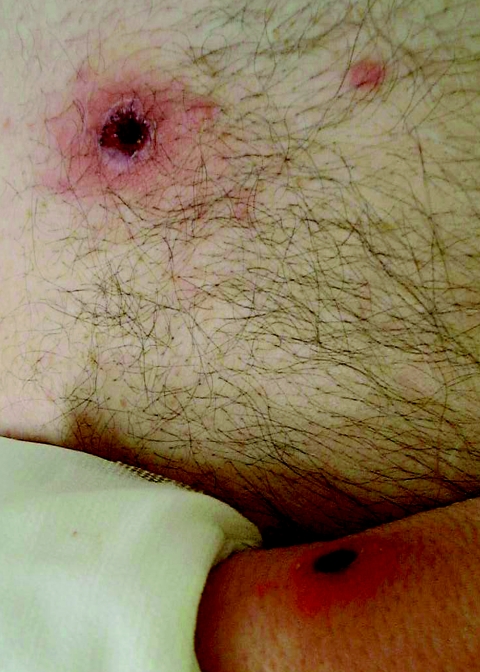
After an asymptomatic incubation of 6 days, the onset of MSF is abrupt and typical cases present with high fever (>39°C), flu-like symptoms, a black eschar (tache noire) at the tick bite site (7, 276) (Fig. 3). In a few cases, the inoculation occurred through conjunctivae and patients presented with conjunctivitis. One to 7 days (median, 4 days) following the onset of fever, a generalized maculopapular rash that often involves the palms and soles but spares the face develops (Fig. 3). Usually, patients will recover within 10 days without any sequelae. However, severe forms, including major neurological manifestations and multiorgan involvement may occur in 5 to 6% of the cases (1, 274). In France, MSF involves mostly males under 10 years of age or older than 50 years. The mortality rate is usually estimated around 2.5% among diagnosed cases (1.50% in the last decade in Portugal, including 2.58% in 1997) (1, 14). Classic risk factors for severe forms include advanced age, immunocompromised situations, chronic alcoholism, glucose-6-phosphate-dehydrogenase deficiency, prior prescription of an inappropriate antibiotic, and delay of treatment (276). In 1997 in Beja, a southern Portuguese district, the case fatality rate in hospitalized patients with MSF was 32.3%, the highest ever obtained there since 1994. Interestingly, when risk factors for fatal outcome were studied in 105 patients hospitalized between 1994 and 1998, the risk of dying was significantly associated with diabetes, vomiting, dehydration, and uremia (85). Some differences in the severity of MSF in different areas, even in the same country, such as Catalonia in northern Spain, have been noted. There, the disease seems to be milder than elsewhere in the country (102). However, cases in this area could be caused by rickettsiae different than R. conorii. For example, a new spotted fever group rickettsial strain (R. massiliae Bar 29) of unknown pathogenicity for humans was isolated there in 1996 from Rhipicephalus sanguineus ticks. We know now that this rickettsia is pathogenic for humans (349).
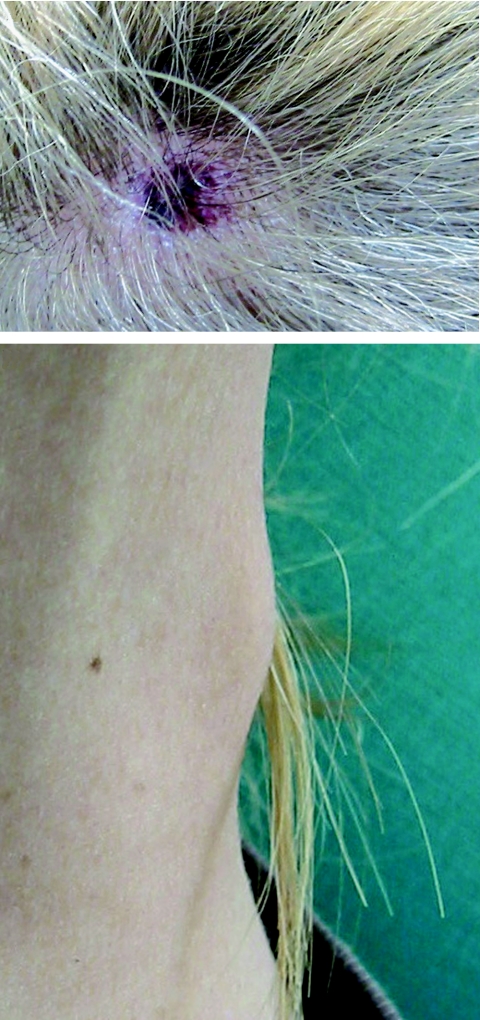
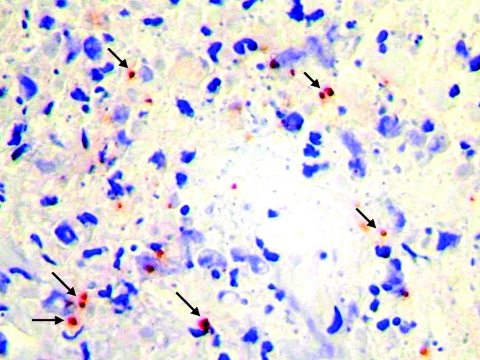
FIG. 3.
Inoculation eschar (top panel) and maculopapular rash (bottom panel) on a patient with Mediterranean spotted fever.
We proposed in the last several years a diagnostic score to help clinicians for the diagnosis of MSF (276). It was recently presented as an helpful tool even using clinical and epidemiological criteria only, when 62 consecutive charts of patients with suspected MSF were retrospectively reviewed in Tunisia (176).
“Rickettsia conorii subsp. israelensis” (Israeli spotted fever)
The first cases of rickettsial spotted fever in Israel were reported in the late 1940s (345), and the number of cases increased following the development of new settlements in the rural areas of this country (276). Clinically, the disease appeared milder and with a shorter duration than classical MSF, and the typical inoculation eschar was usually lacking. These preliminary clinical data led some investigators to suspect than the cause of this rickettsiosis was different from the agent of MSF. In 1971, the agent of Israeli spotted fever was isolated from a patient (R. A. Goldwasser, M. A. Klingberg, W. Klingberg, Y. Steiman, and T. A. Swartz, Front. Intern. Med., 12th Int. Congr. Intern. Med., p. 270-275, 1974). Two other antigenically identical agents were isolated from Rhipicephalus sanguineus ticks collected on the dogs of two patients with serologically documented Israeli spotted fever. These three isolates were characterized as rickettsiae closely related to but slightly different from R. conorii isolates obtained from patients with MSF (R. A. Goldwasser, M. A. Klingberg, W. Klingberg, Y. Steiman, and T. A. Swartz, Front. Intern. Med., 12th Int. Congr. Intern. Med., p. 270-275, 1974). This observation has been confirmed by recent molecular studies (111, 294, 295, 310), and it has been recently proposed that the agent of Israeli spotted fever constitutes a subspecies of R. conorii identified as Rickettsia conorii subsp. israelensis subsp. nov. (374).
Israeli spotted fever appears as a typical spotted fever, but the eschar at the inoculation site is absent in >90% of cases and resembles a small pinkish papule rather than a real eschar (130). Splenomegaly and hepatomegaly are seen in 30 to 35% of patients. The disease may be acquired even without direct contact with animals, through exposure to ticks in places frequented by dogs, as demonstrated in three grouped cases in children (319). Several fatal cases and severe forms have been described, especially in children and in people with glucose-6-phosphate dehydrogenase deficiency, and the prevalence of the disease seems to be increasing (130, 278, 369). Although asymptomatic infections have been described by seroconversion, the test used was not specific enough to ensure that the Israeli isolate was definitely the agent provoking the serologic response (306).
In 1999, R. conorii subsp. israelensis was isolated from three patients living in semirural areas along the River Tejo in Portugal (12). Of interest was the fact that none of the patients had traveled away from Portugal during the previous year, and none reported an eschar. All patients had severe disease, and two patients died with septic shock and multiorgan failure. More recently, Sousa et al. reported the clinical data of 44 patients infected with R. conorii subsp. israelensis in Portugal between 1994 and 2004. Cases were confirmed by isolation of the rickettsia from blood or by PCR on skin biopsy specimens. The absence of an eschar was noted for 54% of the patients. All but two patients presented with a rash. A total of 10 patients died. These clinical characteristics were not statistically different from those of 44 patients infected with R. conorii subsp. conorii at the same period (R. Sousa et al., Abstr. 4th Int. Conf. Rickettsiae Rickettsial Dis., abstr. O-22).
The occurrence of R. conorii subsp. israelensis in Portugal indicates that the geographic distribution of Israeli spotted fever is wider than previously appreciated. This was confirmed more recently when R. conorii subsp. israelensis was detected in Sicilian Rhipicephalus sanguineus ticks (120).
“Rickettsia sibirica subsp. sibirica” (Siberian tick typhus or North Asian tick typhus)
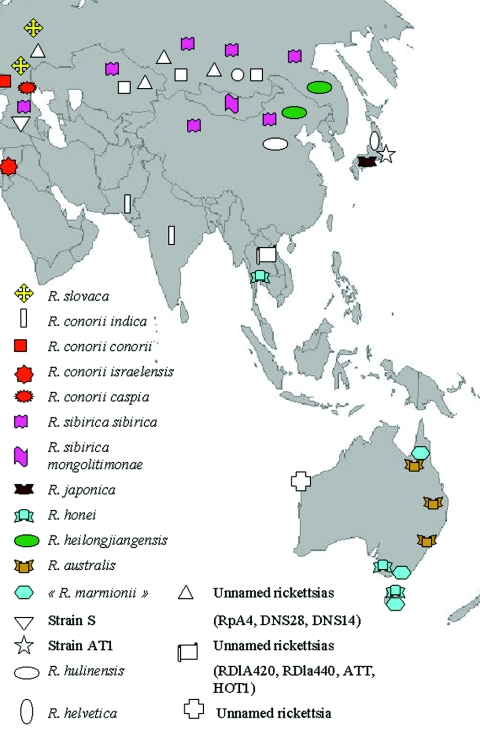
R. sibirica is the agent of Siberian tick typhus, a spotted fever group rickettsiosis that was first described in Primorye in the spring-summer season of 1934 to 1935 by Shmatikov (280). Human isolates obtained in 1946 were used as reference strains for molecular studies many years later (17). Siberian tick typhus is well documented in the former USSR, but relatively few descriptions are available in the English medical literature (280). Active foci of the disease are widely spread in Asiatic Russia, with more than 80% of the cases being observed in Altai (Western Siberia) and Krasnoyarsk regions. The disease is frequently reported during spring and summer months. Since 1979, a constant increase of the number of cases has been observed. Between 1979 and 1997, 23,891 cases were recorded (297).
R. sibirica has been found in several species of ticks, and some of them have been presented as the principal vectors Siberian tick typhus (297). These include Dermacentor nuttalli in the mountainous steppe of western and eastern Siberia, D. marginatus in the steppe and meadow regions of western Siberia and northern Kazakhstan, Dermacentor silvarum in forest shrubs, and Haemaphysalis concinna in swampy tussocks of some southern and far eastern territories of Siberia (17, 297). Isolates obtained from these species of ticks, respectively, in 1949, 1959, 1983, and 1986 are available at the Gamaleya Research Institute of Epidemiology and Microbiology in Moscow (17). These ticks may act as vectors but also reservoirs of R. sibirica which is maintained in ticks through transstadial and transovarial transmission, as demonstrated at least for D. nuttalli. More recently, a rickettsial strain that had been isolated from Ixodes persulcatus and maintained at the Omsk Research Institute of Natural Foci Infections was identified as R. sibirica (S. Shpynov, P. E. Fournier, N. Rudakov, I. Samoilenko, T. Reshetnikova, V. Yastrebov, M. Schaiman, I. Tarasevich, and D. Raoult, Abst. 4th Int. Conf. Rickettsiae Rickettsial Dis., abstr. P-179).
The incubation period is usually 4 to 7 days following a tick bite. Clinical features include a high fever associated with an inoculation eschar that is often accompanied by regional lymphadenopathy. Severe headache, myalgia, and digestive disturbances are concomitant symptoms and can last for 6 to 10 days without treatment. The rash, which may be purpuric, usually occurs 2 to 4 days after the onset of symptoms. Although central neurological involvement may occur, this disease is usually mild and is seldom associated with severe complications (297).
Infection due to R. sibirica is also prevalent in northern China, where it is known as North Asian tick typhus (98, 371). There an isolate was obtained from D. nuttalli in 1974, and from patients in 1984 when five patients with characteristic symptoms of spotted fever were seen in Xinjiang, China. D. nuttalli specimens were attached to four patients, and the last patient recalled a tick bite (99). About 20 strains of SFG rickettsiae in China have been identified as R. sibirica from patients, various species of ticks, rodents (Microtus fortis), and hedgehogs (99, 372). Recently, a distinct strain of R. sibirica, currently considered a subspecies (see “Rickettsia sibirica subsp. mongolotimonae” below), emerged as a pathogen for humans in Europe and Africa.
Rickettsial strains antigenically identical to R. sibirica have also been isolated from several species of ticks in Pakistan (289). However, because only serological methods have been used to characterize these strains, to our knowledge, there is no definitive evidence of the prevalence of R. sibirica in Pakistan.
Rickettsia australis (Queensland tick typhus)
Queensland tick typhus has been clinically recognized since 1946. The first cases were observed among Australian troops training in the bush of northern Queensland State in eastern Australia. Rickettsiae were isolated from 2 of 12 infected soldiers (4). Using serological methods, this agent was found to be a new spotted fever group rickettsia (256) and was named R. australis in 1950 (249). Thereafter, Queensland tick typhus has been recognized along the entire eastern coast of Australia east of the Great Dividing Range (127, 314, 316). In regions south of Queensland, cases were recorded in the 1990s in the eastern coastal region of New South Wales, including its capital, Sydney (93). Spotted fevers with slight clinical and epidemiological differences were subsequently reported in Victoria and on Flinders Island (328). Although these cases were primarily assumed to be due to R. australis (316), it is now known that a different pathogenic rickettsia, R. honei, occurs at least in Flinders Island, where it was shown to be responsible for the cases (see below). To date, R. australis has been definitely isolated only from patients in Queensland (4, 257).
R. australis has been identified in Ixodes holocyclus, a common, human-biting tick in Queensland (60). This tick also feeds on a broad range of vertebrate hosts. It is distributed primarily in coastal regions but is also prevalent in the rain forests of Queensland (287). R. australis has also been isolated from Ixodes tasmani, a species that exists along the coast as well as in the interior regions of south and western Australia (287). This tick rarely bites humans but may play a role in the enzootic maintenance of R. australis in small animals (60, 127). An uncharacterized SFG rickettsia was recently identified in the hemolymph of an Ixodes cornuatus tick removed from a human in Victoria (129). This tick is prevalent in south costal New South Wales, eastern Victoria, and Tasmania (288). A number of vertebrates, including bush rats, bandicoots, and domestic dogs, are common hosts for all these ticks. In one study, antibodies reactive with R. australis were detected in 54 of 307 bandicoots and rodents trapped in northern Queensland; however, the precise role of vertebrates as reservoirs of R. australis is not known (74).
Although Queensland tick typhus is a notifiable disease in Australia, it is seldom reported. A review on 62 cases of spotted fever recorded in Australia between 1946 and 1989 (16) indicated that 37 of these cases that originated from Queensland and New South Wales could be considered infections due to R. australis. A total of 78% of the cases occurred between June and November, and cases from both urban and suburban areas were reported. Approximately 76% of patients recall an antecedent tick bite. The disease is characterized by a sudden onset characterized by fever, headache, and myalgia, followed within 10 days by maculopapular or vesicular rash. An inoculation eschar is identified in approximately 65% of cases, and lymphadenopathy is identified in 71% of cases. The disease ranges from mild to severe, but only two patients with fatal disease have been described (127, 315).
Emerging Pathogens (1984 to 2004)
Rickettsia japonica (Japanese or Oriental spotted fever)
Between May and July 1984, the Japanese physician Fumihiko Mahara identified three patients with high fever and rash. All lived in the same rural area and had collected shoots from bamboo plantations on the same mountain. For two patients, an eschar was observed. Scrub typhus, caused by the mite-borne pathogen Orientia tsutsugamushi, was initially suspected because of the clinical similarity to the illnesses and because it is a well-known zoonotic disease in Japan (358). However, the results of the Weil-Felix test showed positive OX2 serum agglutinins, indicating a possible spotted fever group rickettsiosis, whereas OXK serum agglutinins (used for the diagnosis of scrub typhus) were negative (183). Patient sera were then shown to have antibodies reactive with spotted fever group rickettsial antigens when tested by immunofluorescence (186, 339). The disease was called Japanese spotted fever. The causative agent was first isolated from patients in Shikoku in 1985 (340, 341) and was subsequently characterized as a new rickettsia of the spotted fever group and named Rickettsia japonica (342, 343).
Since 1984, approximately 30 to 40 cases have been reported annually, mainly along the coast of southwestern and central Japan (184, 185). The disease occurs from April to October. High-risk areas for acquiring the infection include bamboo plantations, crop fields, and coastal hills and forests. Japanese spotted fever has an abrupt onset with headache, high fever (39 to 40°C), and chills. A macular rash appears after two or three days, all over the body, including the palms and soles. It becomes petechial after 3 or four days and disappears in two weeks. An inoculation eschar was observed in 91% of 34 patients diagnosed at Mahara Hospital in 1984-1997, and 38% of the patients recalled a tick bite (184, 185). Severe cases, including those of patients with encephalitis, disseminated intravascular coagulopathy, multiorgan failure, and acute respiratory distress syndrome, have been reported (9, 158, 159, 161). In a series of 28 patients hospitalized during 1993-2002, 6 (21%) were classified as severe, including 1 fatality (160, 161).
R. japonica has been detected in or isolated from six species of ticks in Japan. Of these, Haemaphysalis flava, Haemaphysalis longicornis, Dermacentor taiwanensis, and Ixodes ovatus commonly feed on humans and are considered as the most likely vectors of the disease (106, 148, 185).
“Rickettsia conorii subsp. caspia” (Astrakhan fever)
Since the 1970s, in Astrakhan, a region of Russia located by the Caspian Sea, cases of a febrile exanthema have been observed in patients of rural areas. Prospective surveillance during 1983 through 1988 identified 321 cases of Astrakhan fever. Most patients were adults (94%), specifically males (61%), and the cases occurred during summer months (85%, including 43% in August). The disease was similar to MSF, including fever associated with a maculopapular rash in 94% of the cases. However, the presence of a tache noire was reported in only 23% of the patients. Conjunctivitis was seen in 32% of the cases. No fatal cases were reported in this series (333). Most of the patients had dogs and reported having contact with Rhipicephalus sanguineus dog ticks. The Gamaleya Institute for Epidemiology and Microbiology in Moscow tested sera from patients with Astrakhan fever using the complement fixation test and observed the presence of antibodies reactive with R. conorii in 72% of patients (334). These results were also confirmed by MIF when tested at the Unité des Rickettsies in Marseille (96).
A rickettsial isolate was obtained from a patient with Astrakhan fever in 1991 (95). In 1992, it was shown that restriction endonuclease patterns of DNA fragments of rickettsia amplified from the blood of a patient were identical to those of rickettsial DNA amplified Rhipicephalus sanguineus ticks collected in Astrakhan and related to those of R. conorii strains from Israel (88). In 1994, Rhipicephalus pumilio ticks were also shown to harbor rickettsiae with identical genomic patterns. This species usually feeds on domesticated and wild mammals, including rabbits and large rodents, but may occasionally bite people (97).
During the summer of 2001, French United Nations troops in Kosovo collected ticks on asymptomatic soldiers and dogs in the Morina region. By molecular methods, Rickettsia conorii subsp. caspia was detected in four Rhipicephalus sanguineus organisms, including three collected on dogs and one taken from an asymptomatic soldier. The man with the positive tick remained asymptomatic (105).
A rickettsial isolate was obtained recently from a patient from Chad, Africa. The patient presented with fever, dyspnea, a maculopapular rash, an inoculation eschar on the leg, and conjunctivitis of the right eye. Five days before the onset of the symptoms, she had traveled to Lake Chad, where she had walked into the bush but recalled no tick bite. The 16S rRNA gene, gltA, and ompA sequences of the isolate, obtained by inoculating the eschar biopsy specimen in cell culture, were found to be 99.7%, 99.6%, and 99.5% identical to those of Rickettsia conorii subsp. caspia, respectively. Based on these molecular characteristics, the Chad isolate is considered a variant strain of Rickettsia conorii subsp. caspia (113). Thus, Astrakhan fever might be a cause of spotted fever in Kosovo and Chad, and the area of distribution of this rickettsia could be wider than initially suspected in Astrakhan.
It has been proposed recently that the Astrakhan fever rickettsia actually constitutes several subspecies of R. conorii. R. conorii subsp. caspia subsp. nov. has been proposed as the name of the agent of Astrakhan spotted fever (374).
Rickettsia africae (African tick bite fever)
The etiologic agent of African tick bite fever was discovered twice. In 1911, an influenza-like disease named tick bite fever was described in Mozambique and South Africa (204, 305). Thereafter, there was debate as to whether these cases were cases of MSF, described 1 year earlier in Tunisia (see “R. conorii subsp. conorii” above). In the 1930s in South Africa, Pijper described tick bite fever as a rural disease occurring in people having contact with cattle ticks, whereas MSF was typically acquired in urban areas with dog ticks (254, 255). Tick bite fever was a far milder disease than MSF and was not associated with skin rash. Thus, Pijper considered tick bite fever to be a distinct disease. He isolated a rickettsia from a patient and demonstrated that it was different from R. conorii in cross-protection studies (254, 255). Unfortunately, this isolate was lost and subsequent workers were unable to confirm these findings (119). Erroneously, MSF and tick bite fever were considered synonyms, and R. conorii remained the only recognized agent of tick bite fever in Africa until the 1990s.
In 1990, Kelly et al. isolated rickettsial strains from Amblyomma hebraeum ticks in Zimbabwe and demonstrated these strains to be distinct from R. conorii by MIF typing (155). In 1992, a rickettsia was isolated by shell vial cell culture from a patient suffering from tick bite fever in Zimbabwe (150). The rickettsial isolate was found to be distinct from other SFG rickettsia but indistinguishable from the rickettsial isolates previously obtained from A. hebraeum and from a strain isolated from an Amblyomma variegatum specimen collected in Ethiopia 20 years earlier (54, 152). Kelly et al. proposed the name African tick bite fever for the disease and the name Rickettsia africae for the newly recognized rickettsia causing the disease. These names were officially adopted when the rickettsia was definitively characterized (151). This finding confirmed Pijper's earlier work and validated the presence of a second tick-transmitted rickettsiosis in Africa approximately 60 years after the initial description.
In southern Africa, A. hebraeum, a tick of large ruminants and wildlife species is a recognized vector and reservoir of R. africae. Transstadial and transovarial transmission of R. africae in this tick have been demonstrated (154). Frequent human cases may be attributed to efficient transovarial transmission of the agent when larvae, which are small and difficult to notice when attached to the skin, are involved as vectors. R. africae has also been detected in A. variegatum throughout west, central, and eastern sub-Saharan Africa (181, 242) and in A. lepidum from the Sudan (242). Three uncultivated rickettsiae, named Rav1, Rav3, and Rav9, detected by PCR from A. variegatum ticks in Niger and Mali, appear to be closely related, probably variants of R. africae, based on molecular analyses of the ompA and gltA genes (242).
Infection rates are remarkably high, and as many as 100% of Amblyomma spp. may be infected with R. africae. Because A. hebraeum ticks readily bite humans, cases of African tick bite fever often occur in clusters and patients often present with multiple inoculation eschars (273). Further, high prevalences (30 to 80%) of antibodies to spotted fever group rickettsiae have been shown in persons throughout the continent, including children parallel to the distribution of Amblyomma ticks (145). During the Zimbabwean war of independence in the late 1970s, army medical authorities reported that several thousand cases of tick typhus occurred in European and African soldiers, particularly those of urban origin who were deployed to rural areas and presumptively nonimmune to the infection. Despite high seroprevalence to R. africae among native Africans, nearly all acute cases of ATBF described in the literature have occurred in European or American travelers (145, 205, 273). Recently however, Ndip et al. reported cases of ATBF documented by serology (26 patients) and molecular techniques (7 patients) among indigenous patients in Cameroon (212, 213).
Game hunting, traveling to southern Africa, and traveling during November through April have recently been identified as independent risk factors of ATBF in travelers (146). Serological evidence of recent spotted fever group rickettsial infection was detected in 11% of patients returned to Germany from southern Africa (144). Further, specific antibodies to R. africae were detected in 9% of first-time Norwegian travelers to rural subequatorial Africa (147). Finally, in a prospective cohort study of 940 Norwegian travelers (mostly short-term) to rural subequatorial Africa, the incidence rates of African tick bite fever ranged from 4 to 5% (146).
Between 1983 and 2003, 171 published cases have been microbiologically confirmed as ATBF. Another 78 cases could, based on positive (but not species-specific) tests in combination with typical clinical and epidemiological features, retrospectively be classified as probable ATBF (145, 273). The mean age of these 249 cases was 40 years; most patients (72%) were male, and most (64%) originated from Europe. Although cases have been reported from western, central and eastern Africa, more than 80% of the patients acquired ATBF in South Africa. Risk areas include wildlife attractions in natural parks, where the tick vectors are highly prevalent and attack readily any person who enters their biotope. Up to 74% of travel-associated cases of African tick bite fever occur in clusters (62, 110). The incubation period ranges from 5 to 7 days, up to 10 (145). In the largest published series, known tick bites or tick contact were reported in 44% of the patients. Fever occurred in 88% and clinical signs were generally mild. High prevalence of multiple inoculation eschars was seen in patients (55%) and characterizes ATBF from other spotted fever rickettsioses. Eschars were predominantly on the lower limbs (62%) presumably because Amblyomma spp. attack from the ground to the legs and generally bite as soon as possible (Fig. 4). Enlargement of lymph nodes draining area of the eschar (s) is common (43%). A rash is seen in 49% of the patient and may be vesicular (50%). To date no deaths or severe manifestations have been reported in patients with ATBF. However, differences in clinical presentation appear between retrospectively collected cases (including many which were hospitalized and whose samples were referred to reference labs) (273) and 38 consecutive Norwegian cases (including only 2 hospitalized cases) (146). When examining consecutive cases, many patients had mild disease, whereas cases submitted to reference labs and later presented in a retrospective fashion had more pronounced symptoms.
FIG. 4.
Multiple tick bites on a patient with African tick bite fever caused by R. africae.
Recently, ATBF has been identified from locations away from the African mainland. In 1998, the first case of naturally acquired R. africae infection in the Western Hemisphere was described for a tick-bitten patient returning from Guadeloupe Island in the French West Indies (243). R. africae was subsequently isolated from A. variegatum ticks collected on cattle, sheep and goats on Guadeloupe (246). A second human case was documented in 2001 (273). R. africae may have been introduced into the West Indies from Africa during the 18th or 19th centuries with A. variegatum ticks on cattle shipped from Senegal to Guadeloupe (19). Over the past 50 years, A. variegatum have propagated and invaded more than 15 islands in the Caribbean, by livestock movements and migration of birds. More recently, R. africae was detected from A. variegatum collected on others islands in the Caribbean including Martinique (237), St. Kitts and Nevis (153) and Antigua (S. A. Thornton, P. E. Olson, M. Medina, J. Robinson, M. Eremeeva, J. W. Sumner, T. Parakh, M. L. Lim, and G. A. Dasch, Abstr. 41st Annu. Meet. Infect. Dis. Soc. Am., p. 40, 2003). Without effective control measure, great potential exists for A. variegatum and R. africae to become established on the American mainland. R. africae has also been recently identified in A. variegatum ticks collected on Reunion Island, a French Island in the Indian Ocean, where ticks where introduced with animals by humans in the 17th century (238).
Rickettsia honei (Flinders Island spotted fever)
Flinders Island spotted fever was described in 1991 by R.S. Stewart, the only medical doctor among approximately 1,000 inhabitants on Flinders Island, a small island off the south-east coast of Australia near Tasmania. He described 26 cases observed over 12 years of a seasonal, febrile rash illness. The rash was erythematous in the majority of patients and purpuric in two patients with severe cases associated with thrombocytopenia (328). Other findings included an inoculation eschar and enlarged local nodes in 25% and 55% of cases, respectively. Cases occurred in spring and summer, predominantly during December and January. Incidence was approximately 150 per 100,000 persons. The patients' sera were initially evaluated by the Weil-Felix test and subsequently by microimmunofluorescence, which confirmed that the disease was caused by a SFG rickettsia. At the time of Stewart's observations, these were considered to be cases of Queensland tick typhus, although clinical and epidemiological differences were noted when compared to cases originating from Queensland. The usual features in Flinders Island spotted fever were a sudden onset of fever, headache, arthromyalgias with joint swelling and slight cough. However, the rash which appeared few days later, was maculopapular and there was no vesiculation, unlike Queensland tick typhus caused by R. australis. Although most of the reported cases of R. australis occurred during June through November, and were reported from both urban and suburban areas, cases in Flinders Island spotted fever occurred predominantly during December through January (328).
In 1992, isolates were obtained from 2 patients with Flinders Island spotted fever (15). The rickettsia was characterized by molecular methods and proposed as a new species, R. honei (16, 327). One patient from whom a rickettsial isolate was obtained from blood had been bitten by a tick 9 days prior becoming ill. The tick was identified as Aponomma hydrosauri (129) a tick that usually bites reptiles. During the same summer season of 1990, eight people collected the ticks that had bitten them. Six were I. tasmanii (the main tick that bites humans on Flinders Island) and 2 were A. hydrosauri. Further, 29/46 (63%) of A. hydrosauri removed from 12 Australian blue-tongued lizard on Flinders Island were shown to harbor R. honei by molecular techniques (128, 362). DNA of R. honei has been detected by PCR in eggs obtained from engorged female ticks of this species, suggesting transovarial transmission of R. honei in the acarine host (128).
In addition to Flinders Island, A. hydrosauri is widespread in south Australia including Victoria area on the Mainland and Tasmania. It has also been noted in New South Wales (288). In 1995, a similar rickettsiosis emerged in Tasmania, where patients were found by PCR to be infected with R. honei (70). A. hydrosauri ticks removed from a Tasmanian were also positive by PCR, and recent work suggested again that transovarial transmission may occur in ticks (362). However, the definitive implication of A. hydrosauri as a vector and possibly reservoir of R. honei, requires further study.
Interestingly, in 1962 a rickettsia was isolated in Thailand from pooled larval Ixodes sp. and Rhipicephalus sp. ticks collected on a Rattus rattus specimen, designated Thai tick typhus rickettsia TT-118 (289); however, this rickettsia has been shown recently to be a strain of R. honei (327). More recently, DNA of R. honei has been recently detected in Thai Ixodes granulatus ticks collected from R. rattus in Thailand in 1974 (162).
Finally, a rickettsia closely related to R. honei was detected by molecular tools in 2 Amblyomma cajennense adult ticks collected on cattle in south Texas (35). Sequence analysis of segments of the 17-kDa gene, gltA gene, and the gene encoding OmpA amplified from these ticks showed the highest degree of similarity to the Thai tick typhus rickettsia (homologies of 99.5, 99.5, and 100%, respectively). To date, no case of spotted fever group rickettsioses has been linked to R. honei infection in Thailand or the United States.
“Rickettsia sibirica subsp. mongolitimonae.”
In 1991, a rickettsia was isolated from Hyalomma asiaticum ticks collected in Inner Mongolia in China. It was antigenically and genotypically unique among SFG rickettsiae and was named isolate HA-91 (371). In 1996, a genetically indistinguishable isolate was obtained from the blood and the skin of a 63-year-old patient in southern France. The patient was hospitalized in March (an atypical month for MSF) with a mild disease characterized by only a discrete rash and an inoculation eschar involving the groin. This patient was a resident of Marseille and had no travel history; however, the patient had collected compost from a garden where migratory birds were resting (271). The name “Rickettsia mongolotimonae” was first proposed for the rickettsia to refer to the disparate sources of the isolates (i.e., Mongolia and La Timone Hospital in Marseille). Using gene sequence-based criteria to define Rickettsia species (104), “R. mongolotimonae” was identified as a member of the R. sibirica species complex. However, in phylogenetic studies, all strains of “R. mongolotimonae” group together in clusters separated from other strains of R. sibirica. In addition, “R. mongolotimonae” exhibits serotypic and ecological differences from other members of this complex. For these reasons, and in accordance with Latin nomenclature, this agent is now called “R. sibirica subsp. mongolitimonae,” and the agent of North Asian tick typhus called R. sibirica sensu stricto (107) (P. E. Fournier, et al., Abstr. 4th Int. Conf. Rickettsiae Rickettsial Dis., abstr. P-180, 2005).
A second human case of infection with R. sibirica subsp. mongolitimonae was diagnosed in 1998 in an HIV-positive patient who had gardened in a rural area of Marseille. The patient presented with fever, headache, an eschar, lymphangitis and painful satellite lymphadenopathy (112). From January 2000 to June 2004, R. sibirica subsp. mongolitimonae infection has been diagnosed in 7 additional patients. In 3, the bacterium was cultivated from the inoculation eschar. The other 4 patients were diagnosed using PCR from the eschar (2 patients) or blood (2 patients), plus specific Western blot before (2 patients) and after (2 patients) cross-adsorption (CA).
Based on evaluation of these nine cases, R. sibirica subsp. mongolitimonae infection differs from other tick-borne rickettsioses in the Mediterranean area. Specific characteristics include an incidence from March to early July, the occasional findings, alone or in combination, of multiple eschars (Fig. 5), and draining lymph nodes, and a lymphangitis that extends from the inoculation eschar to the draining node. The unique clinical features of this new rickettsiosis have led to the moniker lymphangitis-associated rickettsiosis (107).
FIG. 5.
Multiples eschars on a patient infected with R. sibirica subsp. mongolitimonae.
R. sibirica subsp. mongolitimonae was reported in sub-Saharan Africa in 2001, when it was detected in Hyalomma truncatum (242). The first proven human infection with R. sibirica subsp. mongolitimonae in Africa was reported in a construction worker, working in South Africa's Northern Province. The patient presented with an eschar on his toe, lymphangitis, severe headache, and fever. An eschar biopsy specimen was used for PCR amplification of the rickettsial OmpA gene which showed >99% similarity with the corresponding OmpA gene fragment of R. sibirica subsp. mongolitimonae (258). More recently, a second African case was documented in a patient returning to France after a trip in Algeria. She presented with fever and 2 inoculation eschars. She had been in contact with camels which are highly parasitized by ticks (107). Thus, lymphangitis-associated rickettsiosis should be considered in the differential diagnosis of tick-borne rickettsioses in Europe, Africa and Asia.
Specific vectors of R. sibirica subsp. mongolitimonae have yet to be described, particularly in southern France. It has been hypothesized that French patients may have been bitten by ticks from migratory birds. The detection of R. sibirica subsp. mongolitimonae in Hyalomma spp. in Mongolia and Niger suggests a possible association of this rickettsia with ticks of this genus that are also prevalent in southern France (209). More arguments for this hypothesis have been provided recently when two cases of R. sibirica subsp. mongolitimonae have been documented in Crete, Greece. Indeed, in one patient, this rickettsia was simultaneously detected on a H. anatolicum excavatum tick parasitized on him (A. Psaroulaki, A. Germanakis, E. Scoulica, B. Papadopoulos, A. Gikas, and Y. Tselentis, Abstr. 4th Int. Conf. Rickettsiae Rickettsial Dis., abstr. P-207, 2005).
Rickettsia slovaca
R. slovaca was first isolated in 1968 from Dermacentor marginatus ticks in Czechoslovakia (279). Subsequently, it has been detected or isolated from ticks in all European countries where D. marginatus and D. reticulatus have been evaluated for rickettsiae, including France, Switzerland, Slovakia, Ukraine, Yugoslavia, Armenia, and Portugal. Infection prevalence in ticks varies from 1 to 17% (311). These ticks are generally common throughout Europe and central Asia, except for D. marginatus, which has not been identified in northern Europe. They are active during early spring, autumn, and winter in southern Europe. Adult ticks inhabit forests and pastures and frequently bite people entering these biotopes, particularly on the scalp. These ticks may act as vectors but also reservoirs of R. slovaca, which is maintained in ticks through transstadial and transovarial transmission (279).
For more than 20 years following its discovery, R. slovaca was considered a nonpathogenic rickettsia; however, in 1997, the first documented case of human infection with R. slovaca was reported, found in a woman who presented with a single eschar of the scalp and enlarged cervical lymph nodes following the bite of a Dermacentor sp. tick in France. This case was documented by seroconversion and molecular detection of R. slovaca in the eschar's biopsy specimen and by isolation of the bacterium from the tick which had been kept (270). Clinically similar but undocumented cases had been seen previously in France, Slovakia, and Hungary, where this clinical syndrome had been named “TIBOLA” (for tick-borne lymphadenopathy) (Fig. 6).
FIG. 6.
Inoculation eschar of the scalp (top panel) and enlarged cervical lymph nodes (bottom panel) of a patient with tick-borne lymphadenopathy (R. slovaca infection).
From January 1996 through April 2000, the role of R. slovaca infection in this syndrome was evaluated in 67 patients from France and Hungary presenting with tick-borne lymphadenopathy (275). A total of 17 cases of R. slovaca infection were confirmed in this cohort by molecular methods. Infections were most likely to occur in children and in patients who were bitten during the colder months of the year. Fever and rash were uncommon, and sequelae included localized alopecia at the bite site and chronic fatigue. During the study period, R. slovaca infection was shown to represent 19% of the European tick-transmitted rickettsioses documented in the Unité des Rickettsies in Marseille. Cases have also recently been reported in Bulgaria (163) and Spain (226). Finally, the isolation of R. slovaca from a patient has been recently reported, providing definitive evidence that R. slovaca is a human pathogen (65).
Recently, 22 cases of a similar disease have been reported in Spain, where the clinical syndrome was called “DEBONEL” (for Dermacentor-borne necrosis-erythema-lymphadenopathy) (225). In half of the cases, patients were bitten by ticks identified as D. marginatus. Cases occurred between October and April, with a peak in November. The incubation period was approximately 4 days (range, 1 to 8 days). All patients had an eschar at the tick bite site (86% of the eschars were on the scalp) associated with regional painful lymphadenopathy, and all but one reported a headache. Low-grade fever was present in 45% of patients. After antibiotic treatment (doxycycline, except for a child who received josamycin) all patients recovered, but the eschar resulted in alopecia lasting for several months for several patients. In this series, the infection was not definitely confirmed to be due to R. slovaca. A weak and late serological response against this rickettsia was observed in 25% of the cases analyzed. This serological profile had been previously reported within French and Hungarian patients (275). R. slovaca was, however, detected by PCR in an engorged D. marginatus female removed from the scalp of one of the patient. It is also interesting that, in addition to R. slovaca, another rickettsia of unknown pathogenicity and belonging to R. massiliae genogroup has been detected from D. marginatus ticks in Spain (190, 225), as previously in other European countries. As R. slovaca seems to be involved in most but not all cases of Dermacentor-borne necrosis-erythema-lymphadenopathy or tick-borne lymphadenopathy, one should pay attention to other rickettsiae associated with Dermacentor ticks.
Rickettsia heilongjiangensis
In 1982, a rickettsial isolate (HLJ-054) was obtained from Dermacentor silvarum ticks collected in Suifenhe in the Heilongjiang Province of China (99). Between May and July 1992, 12 patients presenting with fever, headache, rash, eschar, regional lymphadenopathy, and conjunctivitis following a tick bite in the same area were reported to have antibodies against this rickettsial strain. Between May and June 1996, rickettsial isolates were obtained from seven patients with clinical manifestations of SFG rickettsiosis in Suifenhe (99). Serological typing and PCR-RFLP analysis showed that these isolates were identical to identical to HLJ-054 (also called “Rickettsia heilongjiangii”), providing the first direct evidence of the pathogenic role of this rickettsia (368). Recent genomic analysis allowed a definitive characterization of this rickettsia (373), which is now called Rickettsia heilongjiangensis (104). The most closely related rickettsia is R. japonica. Until recently, infections caused by R. heilongjanghensis were attributed to R. sibirica (99).
More recently, 13 patients from the Russian Far East were shown to have been infected by R. heilongjiangensis (206). Rickettsial DNA (four fragments of three genes) was amplified from the patients' skin biopsy specimens and blood samples. Further, the presence of specific antibodies against R. heilongjiangensis was shown when the serum samples of 11 patients were tested with a panel of rickettsial antigens. All the patients had a history of tick bite or tick exposure or a stay in an epidemiologically suspected location. In 12 cases, a macular or maculopapular rash appeared but was faint in most cases. Also, 12 patients had eschars. Two patients were shown to have lymphangitis and regional lymphadenopathy. R. sibirica infection (Siberian tick typhus) that is endemic in Russia peaks at the end of April, and the seasonal peak of the newly described rickettsiosis was at the end of June and July. Further, the infection due to R. heilongjiangensis seemed to affect younger people (only one under 45 years) and to be relatively mild and without any reported deaths (206). The epidemiology of infections caused by the variant of R. heilongjiangensis remains to be studied. Recently, the DNA of the same rickettsia was amplified from Haemaphysalis concinna ticks from Siberia (321) and from H. concinna and Haemaphysalis japonica douglasi ticks from Russian Far East (O. Mediannikov, Y. Sildelnikov, L. Ivanov, E. Mokretsova, P. E. Fournier, I. Tarasevich, and D. Raoult, Abstr. 4th Int. Conf. Rickettsiae Rickettsial Dis., abstr. O-52, 2005). The name Far Eastern spotted fever has been proposed for this emerging infectious disease.
Rickettsia aeschlimannii
R. aeschlimannii was first characterized as a new spotted fever group rickettsia following its isolation from Hyalomma marginatum marginatum ticks in Morocco in 1997 (23). However, genotypically similar organisms had previously been detected in Hyalomma marginatum rufipes in Zimbabwe and in H. m. marginatum in Portugal (22). R. aeschlimannii was also later detected in H. m. rufipes in Niger and Mali (242). In Europe, R. aeschlimannii has been recently identified in H. m. marginatum ticks in Croatia (264), from six tick species in Spain, including H. m. marginatum (101), and from H. m. marginatum ticks in Cephalonia, the largest Ionian islands of Greece (A. Psaroulaki, D. Ragiadakou, G. Kouris, B. Papadopoulos, B. Chaniotis, and Y. Tselentis, Abstr. 4th Int. Conf. Rickettsiae Rickettsial Dis., abstr. P-208, 2005). This rickettsia was recently isolated from H. m. marginatum ticks collected on various mammals and from H. m. rufipes ticks collected on migratory birds coming from Africa, collected in Corsica (197). In this work, R. aeschlimannii was shown to be transstadially and transovarially transmitted in ticks indicating that Hyalomma ticks may be not only vectors but also reservoirs of R. aeschlimannii. As a consequence, the geographic distribution of R. aeschlimannii in Europe would be at least that of H. m. marginatum ticks throughout southern Europe (197).
In 2002, the first human infection caused by R. aeschlimannii, in a patient returning from Morocco to France, was reported. This patient presented with an inoculation eschar on his ankle, fever, and a generalized maculopapular rash. MIF and Western blot assays showed that the patient's serum reacted more intensively with R. aeschlimannii proteins than with proteins of the other tested SFG rickettsiae. The definitive diagnosis was obtained using PCR amplification of rickettsial DNA in the patients' early serum and sequencing of a fragment of the ompA gene of R. aeschlimannii (272). A second case, in a patient returning from a hunting and fishing trip in South Africa, was reported. A Rhipicephalus appendiculatus tick was attached to his thigh, and an eschar around the attachment site was noted (259). He removed the tick and self-prescribed doxycycline. No further symptoms developed. A skin biopsy specimen was, however, taken from the eschar. Molecular studies of both the biopsy specimen and the tick allowed the amplification of ompA fragments sharing >99% similarity with the ompA of R. aeschlimannii.
Interestingly, for 11 of 144 cases of spotted fever rickettsioses recently reported in Spain, patients presented with multiple eschars (7). Although all cases were diagnosed as MSF caused by R. conorii, this diagnosis is doubtful. Rhipicephalus sanguineus, the vector of R. conorii, has a low affinity to bite people, and the infection rate by SFG rickettsiae is generally less than 10%. Thus, the probability of being bitten simultaneously by several infected Rhipicephalus sanguineus ticks is likely to be low (245). Conversely, H. marginatum ticks readily bite humans, and persons may receive multiple simultaneous tick bites. For example, from 2001 to 2005, a total of 496 ticks were removed from people at the Hospital de La Rioja, Spain. A total of 170 were identified as H. m. marginatum, and 3 of them (1.8%) were shown to harbor R. aeschlimannii (J. A. Oteo, A. Portillo, S. Santibáñez, L. Pérez-Martínez, J. R. Blanco, S. Jiménez, V. Ibarra, A. Pérez-Palacios, and M. Sanz, Abstr. 4th Int. Conf. Rickettsiae Rickettsial Dis., abstr. P-92, 2005). Furthermore, infection rates of H. marginatum may be high, such as that reported in Croatia (64.7%) (264).
Therapeutic failures with rifampin administration to children with MSF have been reported in Catalonia, Spain, although R. conorii, the agent of MSF, is susceptible to rifampin (28, 290); however, R. aeschlimannii has been shown to be resistant to rifampin (290). In this context, it is possible that some cases of tick bite spotted fever acquired in southern Europe and presenting with several eschars are caused by R. aeschlimannii.
Rickettsia parkeri
In 1939, R. R. Parker isolated a rickettsia from Gulf Coast ticks (Amblyomma maculatum) collected from cows in south Texas (233). Subsequent studies characterized this bacterium as a distinct SFG rickettsia, and it was named Rickettsia parkeri (170). R. parkeri was generally characterized as a nonpathogenic species and received relatively little attention for the remainder of the 20th century. In 2004, Paddock et al. reported the first recognized case of infection with R. parkeri in a human 65 years after the initial isolation of the rickettsia from ticks (230). It occurred in a 40-year-old man living in suburban area in southeast Virginia who presented with fever, headache, diffuse myalgias and arthralgias, and multiple eschars on his lower extremities. There was no travel history, and no arthropod bite was recalled, although the patient was frequently exposed to ticks and fleas. An erythematous maculopapular rash developed on his trunk and spread to his extremities, including his palms and soles. The patient was initially diagnosed with infected arthropod bite and treated unsuccessfully with a penicillin-class antibiotic. He was subsequently diagnosed with rickettsialpox and successfully treated with doxycycline. R. parkeri was isolated in cell culture from an eschar biopsy specimen and definitively identified by use of molecular methods (230).
A remarkable clinical feature was the occurrence of multiple inoculation eschars. The occurrence of multiple eschars has been also described for a several other tick-borne rickettsioses, including African tick bite fever and the rickettsiosis caused by R. sibirica subsp. mongolotimonae (245), but is generally an unusual clinical feature of most SFG rickettsial infections. Indeed, eschars are seldom described for patients with RMSF, and it has been suggested that rare observations of eschars in patients with supposed RMSF (76, 354) could be caused by infection with R. parkeri rather than R. rickettsii (230). A 1994 report describing PCR-restriction fragment length polymorphism profiles of several SFG rickettsial stains isolated from patients presumably infected with R. rickettsii noted close similarity of one profile with that of R. parkeri (266). More recently, an analysis of several Western blot profiles of serum samples from patients presumed to be infected with R. rickettsii revealed reactivity with a 120-kDa protein of R. parkeri in a pattern compatible with the profile observed from the index patient with R. parkeri infection (268). Collectively, these findings suggest that other cases of R. parkeri rickettsiosis were previously diagnosed as RMSF (230).
The distribution and significance of R. parkeri rickettsiosis in the Americas await further investigation. In the United States, A. maculatum ticks are distributed throughout several southeastern states that border the Gulf and southern Atlantic coasts. R. parkeri has been identified in Gulf Coast ticks collected throughout its range in the United States. Some clinical, epidemiologic, and serologic evidence suggesting that human infections with R. parkeri have also occurred in Florida, Mississippi, and South Carolina exists (76, 230). R. parkeri has also been detected rarely in the lone star tick (Amblyomma americanum), and a study suggests that this rickettsia may be transovarially and transstadially transmitted in this widely distributed tick (124). Preliminary studies also suggest that A. cajennense will support the growth and survival of R. parkeri (303), and a recent investigation has demonstrated DNA of R. parkeri in Amblyomma triste (formerly A. maculatum) ticks collected from humans and animals in Uruguay (347). These findings, coupled with other reports of eschar-associated spotted fever rickettsioses in patients following bites of A. triste in Uruguay (73, 87), suggests that R. parkeri rickettsiosis also occurs in areas of South America. In Uruguay, it is likely that rickettsiosis caused by R. parkeri has been diagnosed as infection with R. conorii based on nonspecific serologic tests (87).
Rickettsia massiliae
In 1992, a rickettsial agent was isolated from Rhipicephalus sanguineus ticks collected near Marseille. It was characterized as a distinct species within the SFG group of rickettsiae and named R. massiliae (21). In Europe, this rickettsia has been detected by molecular methods in Rhipicephalus sanguineus in Greece (11) and Rhipicephalus turanicus in Portugal (13). It has been also detected in Africa in Rhipicephalus muhsamae, Rhipicephalus lunulatus, and Rhipicephalus sulcatus in the Central African Republic (92) and in Rhipicephalus muhsamae collected on cattle in Mali (242). In 1996, a variant strain of R. massiliae (Bar 29) was isolated in Rhipicephalus sanguineus tick from Catalonia, Spain (26). It was thereafter detected in Switzerland (33). We recently demonstrated transstadial and transovarial transmission of R. massiliae Bar 29 in ticks of the R. sanguineus group molecularly identified as Rhipicephalus turanicus, which may also be considered to be reservoirs (196).
R. massiliae exhibits a natural resistance to rifampin in cell cultures (290). Interestingly, therapeutic failures with rifampin for the treatment of MSF in children have been reported in Catalonia, although R. conorii is susceptible to rifampin (28, 290). R. massiliae Bar 29 has been detected in tick saliva, suggesting that the bacteria could be transmitted through the tick bite (196). In 2003, among 15 patients of MSF in Catalonia, Spain, eight sera reacted at high titers with only R. conorii and R. massiliae Bar 29 antigens, and the titers against R. massiliae Bar 29 were clearly higher than those against R. conorii, which implied the possible pathogenic role of R. massiliae Bar 29 for humans (61).
The first recognition of infection with R. massiliae in a human occurred in 2005, 20 years after the isolate was initially obtained from the patient. A spotted fever rickettsia, presumed to be R. conorii, was isolated in 1985 from the blood of a 45-year-old man hospitalized in Palermo, Italy, with fever, a necrotic eschar, a maculopapular rash involving palms and soles, and mild hepatomegaly. Treatment with a cephalosporin antibiotic failed, but he recovered completely after receiving a tetracycline antibiotic. His antibody titer to R. conorii antigens rose from 0 to 80 by MIF, and the patient was considered to have Mediterranean spotted fever caused by R. conorii. The rickettsial isolate was stored for 2 decades before being identified as R. massiliae by sequencing of portions of the ompA and gltA rickettsial genes at the Unité des Rickettsies (349).
“Rickettsia marmionii.”
During 2003-2005, six patients from the states of Queensland, Tasmania, and South Australia, Australia, were diagnosed with a SFG rickettsiosis characterized by fever (all patients), headache (83%), arthralgia (50%), cough (50%), maculopapular rash (33%), and pharyngitis (33%). An eschar was observed for only one patient. Genetic analyses of an isolate obtained from one of the patients showed close similarity to but distinction from R. honei, with 99.0%, 99.7%, and 99.6% homology to the 17-kDa antigen, gltA, and 16S rRNA genes, respectively. Investigators have proposed the name “R. marmionii” to describe this rickettsia and the term “Australian spotted fever” to describe the rickettsiosis it causes. The definitive status of “R. marmionii” as a distinct species or as a subspecies of R. honei remains to be determined. Potential vectors include Haemaphysalis novaeguineae (N. Unsworth, J. Stenos, and J. Graves, Abstr. 4th Int. Conf. Rickettsiae Rickettsial Dis., abstr. O-53, 2005).
TICK-BORNE SFG RICKETTSIAE PRESUMPTIVELY ASSOCIATED WITH HUMAN ILLNESSES
“Rickettsia conorii subsp. indica” (Indian Tick Typhus)
Indian tick typhus (ITT) is a tick-borne rickettsiosis prevalent in India. Although the disease has been clinically recognized at the beginning of the century, the etiologic agent has never been isolated in patients. However, a SFG rickettsia was isolated in 1950 from a Rhipicephalus sanguineus tick collected in India (C. B. Philip, L. E. Hughes, K. N. A. Rao, S. L. Kalra, Arq. 5th Congr. Int. Microbiol., p. 1-571, 1958). This bacterium, classified as R. conorii, was considered to be the cause of ITT. However, subsequent serologic studies including cross-adsorption and MIF serotyping demonstrated significant differences in antibody responses of patients of ITT with the ITT rickettsia and type strains of R. conorii (21, 252). Using molecular methods, it was recently demonstrated that these rickettsiae are closely related, although their diversity is reflected in antigenic variation (111, 294, 295). As discussed above, we have recently proposed that the agent of ITT constitutes a subspecies of R. conorii different from the agent of MSF designated Rickettsia conorii subsp. indica subsp. nov.
Indian tick typhus differs from MSF in that the rash is frequently purpuric and an inoculation eschar at the bite site is rarely identified (143, 211). Cases are documented infrequently and generally by using nonspecific serological methods, such as the Weil-Felix test, which provides indirect evidence of possible rickettsial infection but does not allow a definitive diagnosis (195, 211). In 2001, MIF, Western blotting, and cross-absorption assays were used to document the first serologically confirmed case of severe infection in a returning traveler caused by “Rickettsia conorii subsp. indica” (241). More recently, in Kerala, a state at the southernmost tip of India, seven cases were documented for the first time by MIF. Patients were laborers working in tea estates and presented with fever and a generalized maculopapular rash occurring, involving palms and soles in three patients (330). Recent studies also suggest that two patients with SFG rickettsioses, including one presenting with a rash and an eschar, may have been infected with “Rickettsia conorii subsp. indica” in Thailand (244).
Rickettsia canadensis
R. canadensis was first isolated from Haemaphysalis leporispalustris ticks removed from rabbits in Ontario, Canada (202). It was initially considered a member of the typhus group rickettsiae on the basis of antigenic similarities (140). However, recent molecular studies (104, 293, 295, 310) showed that it should be representative of a distinct group within the genus rickettsia, such as R. bellii. The role of R. canadensis as a human pathogen has not been definitively established. Serological evidence of human infection has been reported in four patients presenting with an RMSF-like disease in California and Texas (39). A role for R. canadensis in acute cerebral vasculitis was also suspected in a patient from southwestern Ohio, based on serological studies that included immunoblot analysis (179).
“Rickettsia amblyommii”
“R. amblyommii” (proposed name), also referred to as strains 85-1034, WB-8-2, and MOAa, was first isolated from lone star ticks (A. americanum) collected in Tennessee in 1974 and subsequently identified throughout the range of the lone star tick (C. Pretzman, D. R. Stothard, D. Ralph, and A. Fuerst, Proc. 11th Sesquiannu. Meet. Am. Soc. Rickettsiol. Rickettsial Dis., p. 24, 1994) (49, 52, 361). R. amblyommii has also been recently detected in A. cajennense and Amblyomma coelebs ticks collected from the western Amazon forest of Brazil (169). The role of R. amblyommii as an agent of human disease has been suggested by a study that examined Western blot profiles of 12 members of a military unit that developed mild illnesses and antibodies reactive with spotted fever group rickettsiae following field maneuvers in tick-infested habitats in Arkansas and Virginia. Investigators determined that five of these patients exhibited specific profiles of reactivity to major surface proteins antigens of strain 85-1034, suggesting infection with this agent [G. A. Dasch, D. J. Kelly, A. L. Richards, J. L. Sanchez, and C. C. Rives, abstract from Program and Abstracts of the Joint Annual Meeting of the American Society of Tropical Medicine and Hygiene and the American Society of Parasitologists, Am. J. Trop. Med. Hyg. 49(Suppl):220, 1993]. Some investigators have speculated that this rickettsia may be the agent described as “Rickettsia texiana” (proposed name) (see below). In some areas of the United States, 40% or more of A. americanum may be infected with this rickettsia (125). Because lone star ticks are especially abundant in the southern and midwestern United States, and because A. americanum of all three stages readily bite humans, infections with R. amblyommii could prove to be relatively frequent if this agent is definitively identified as a human pathogen. Recent investigations have also identified a spotted fever group rickettsia (strain Aranha) in Amblyomma longirostre ticks collected from Brazil that shows a very close phylogenetic relationship with R. amblyommii (167). To our knowledge, no isolate of R. amblyommii is currently available to the scientific community.
“Rickettsia texiana”
In 1975, Anigstein and Anigstein presented work completed approximately 30 years earlier that proposed a rickettsial etiology for Bullis fever, a forgotten epidemic which involved more than 1,000 soldiers participating in field training exercises at Camp Bullis, Tex., during the spring and summer months of 1942 and 1943 (5). Considerable epidemiologic and entomologic evidence collected during the outbreak implicated an infectious agent transmitted by the bite of lone star ticks (41). Because all patients reported a history of tick bites, most notably bites from A. americanum, the disease was also initially referred to as “Texas tick fever” and “lone star fever.” Clinical features included fever, chills, orbital and postoccipital headache, weakness, weight loss, and leukopenia. All patients demonstrated enlargement of at least some lymph nodes, and many had a generalized lymphadenopathy. A maculopapular rash involving the trunk was noted in 10% of cases. The illnesses varied from a mild febrile syndrome of short duration to severe disease and included one death (367).
Impression smears made from biopsied lymph nodes stained by the Machiavello technique showed small intracellular fuchsinophilic granule and rods morphologically similar to rickettsiae (180). Rickettsia-like organisms were also isolated from blood and lymph nodes of patients and from A. americanum ticks collected in the area (6). Isolates from humans and ticks, passaged in chicken embryo culture and in animals, subsequently produced clinical features compatible with Bullis fever when inoculated in human volunteers. The name Rickettsia texiana was proposed for this agent (5). Because A. americanum ticks frequently harbor spotted fever group rickettsiae (125), most notably R. amblyommii, it has been suggested that R. texiana may have been represented by a strain of this rickettsia; however, no isolate of R. texiana is known to exist today, precluding analyses by contemporary molecular tools.
Bullis fever vanished in 1947, and the cause of this outbreak remains enigmatic and controversial. At the time of the outbreak, R. R. Parker and E. A. Steinhaus of the Rocky Mountain Laboratory concluded that no association between the isolated rickettsia and Bullis fever could be firmly established based on serological responses in patients and experimentally infected animals. These investigators suggested that “there might be pathogens in the local tick population that we have failed to demonstrate, either because of absence in the particular ticks tested or that they may not be demonstrable by the techniques employed” (5). Other tick-borne agents have been suggested as the etiology of Bullis fever, including Coxiella burnetii (171) and Ehrlichia chaffeensis (123); however, the clinical presentation of Bullis fever does not match entirely with either of these diseases, and its cause remains a mystery.
Rickettsia helvetica
R. helvetica was first isolated in Ixodes ricinus ticks (the vector of Lyme borreliosis) in Switzerland in 1979 (24, 46). Because transstadial and transovarial transmission of this rickettsia has been demonstrated in I. ricinus, this tick represents both a potential vector and natural reservoir of R. helvetica. R. helvetica has been identified in I. ricinus ticks in many European countries, including France, Sweden, Slovenia, Portugal, Italy, and Bulgaria (32, 71, 216, 239, 304). Recently, it has been shown that the distribution of R. helvetica is not limited to Europe but extends to Asia. Rickettsiae identical with or closely related to R. helvetica have been isolated from I. ovatus, I. persulcatus, and Ixodes monospinosus ticks collected in Japan (106).
For approximately 20 years after its discovery, R. helvetica was considered a nonpathogenic rickettsia; however, in 1999, R. helvetica was implicated in fatal perimyocarditis in several patients in Sweden. Infection was documented by electron microscopy, PCR, and serology (217). These researchers subsequently reported a controversial association between R. helvetica and sarcoidosis in Sweden (218). However, the validity of these associations has been questioned by prominent rickettsiologists (357). As noted by D. H. Walker et al., none of the sarcoidosis cases had supporting serologic evidence of a SFG rickettsiosis and results of immunohistochemistry and transmission electron photomicrographs appeared dubious at best (357). In addition, although histochemical stains were interpreted as showing structures consistent with bacteria, the techniques used are known for staining unidentified particles in any damaged tissues (357).
In 2000, seroconversion to R. helvetica was described for a French patient with a nonspecific febrile illness (108). In 2003, serological findings in tick bite patients from Switzerland were suggestive of acute or past R. helvetica infection (20). More recently, one patient from France and three from Italy were also diagnosed using serological criteria that included MIF, Western blotting, and cross-absorption methods. All four reported tick bites and one developed an eschar (103). Recently, five cases of SFG rickettsiosis, possibly caused by R. helvetica, were reported in patients living along the central Thai-Myanmar border. Two patients reported a tick bite, one presented with an eschar, and another patient presented with rash. Infection were documented by MIF and Western blot assays (244). Three more cases, in patients from eastern Thailand with undifferentiated febrile illnesses, were serologically documented. Although no vector of R. helvetica has been identified in Thailand, it is known that I. ovatus, which has been shown to carry R. helvetica, at least in Japan, is also prevalent in Thailand (331). Further evidence of R. helvetica infections in the far East is supported by a recent report of a Japanese traveler returning from Australia with tick-induced paralysis, due to I. holocyclus, who subsequently seroconverted to R. helvetica (141).
These data suggest that R. helvetica occurs across a much larger geographical area than previously known and is associated with Ixodes species ticks. The few patients for whom serology-based diagnosis exists had relatively mild, self-limited illnesses associated with headache and myalgias and, less frequently, with a rash and/or an eschar (103). Additional evaluation and isolation of the bacterium from clinical samples are, however, needed to confirm the pathogenicity of R. helvetica.
RICKETTSIAE ISOLATED FROM OR DETECTED IN TICKS ONLY
In addition to SFG rickettsiae that are currently recognized as human pathogens, many other rickettsiae have been detected in or isolated from ticks from several continents (Fig. 7-10). Some of these agents were isolated in culture many years ago, and others have been detected only recently by using molecular tools. Other rickettsial genomic fragments detected from ticks have been deposited in GenBank but have not been published yet. Rickettsia spp. identified in the literature or by these searches (by using keywords searches in GenBank (using “tick” and “rickettsia,” and also “tick” and the tick genera list) are listed in Table 1.
FIG. 7.
Tick-borne rickettsiae in Africa. Colored symbols indicate pathogenic rickettsiae. White symbols indicate rickettsiae of possible pathogenicity and rickettsiae of unknown pathogenicity.
FIG. 10.
Tick-borne rickettsiae in Europe. Colored symbols indicate pathogenic rickettsiae. White symbols indicate rickettsiae of possible pathogenicity and rickettsiae of unknown pathogenicity.
TABLE 1.
SFG rickettsiae of unknown pathogenicity isolated or detected in ticks
| Rickettsia sp. or strain(s) | Confirmed or potential tick vectors(s) | Locations | Comments | Reference(s) or GenBank accession no. |
|---|---|---|---|---|
| Available strains | ||||
| R. peacockii | Dermacentor andersoni | United States | Transstadially and transovarially transmitted; the presence of R. peacockii within ovaries interferes with the ability of R. rickettsii to infect the ovarian tissues and to be transovarially transmitted to progeny | 18, 51, 215, 236, 324 |
| R. montanensis (formerly R. montana) | Dermacentor variabilis, Dermacentor andersoni | United States | Transovarially transmitted in D. variabilis | 2, 3, 27, 100, 182, 260 |
| R. bellii | Dermacentor, Haemaphysalis, Amblyomma, Ornithodoros. Argas | United States, Brazil | Represents a distinct ancestral group within rickettsiae and seems to be one of the most abundant and broadly distributed rickettsiae infecting ticks in the United States | 10, 116, 251, 353 |
| R. rhipicephali | Rhipicephalis sanguineus, Dermacentor occidentalis | United States, Europe, Africa | Invades both salivary glands and ovaries of the tick | 13, 48, 89, 91, 92, 132, 262 |
| R. monacensis | Ixodes ricinus | Europe | Closely related to rickettsiae previously detected but not isolated in I. ricinus, including “the Cadiz agent,” IRS3 and IRS4 | 71, 191, 309, 323 |
| “R. tamurae” | Amblyomma testudinarium | Western Japan | Previously called strain ATI | 106 |
| “R. asiatica” | Ixodes ovatus | Central Japan | Previously called “I-OI” | 106 |
| Strain S | Rhipicephalus sanguineus | Armenia | 94 | |
| Nonclassified, incompletely characterized, or uncultivated rickettsiae awaiting further characterization | ||||
| Strain Cooleyi | Ixodes scapularis | United States | 34 | |
| Strain 364-D | Dermacentor occidentalis | United States | 75, 173 | |
| Strain Parumapertus | Dermacentor parumapertus | 329 | ||
| Strain Tillamook | Ixodes pacificus | United States | 139, 253 | |
| Unnamed | Ixodes cookei | 45 | ||
| Unnamed | Amblyomma longirostre | Brazil | Could be “R. amblyommii” | 167 |
| Strain COOPERI | Amblyomma cooperi | Brazil | 138, 166 | |
| “Candidatus Rickettsia andeanae” | Amblyomma maculatum, Ixodes boliviensis | Peru | 36, 37 | |
| Strain RDla420 | Dermacentor auratus | Thailand | 240 | |
| Strain RDla440 | Dermacentor spp. | Thailand | 240 | |
| Strain ATT | Amblyomma testudinarium | Thailand | 136 | |
| Strains HOT1 and HOT2 | Haemaphysalis ornithophila | Thailand | 136 | |
| Strain RpA4 | Rhipicephalus pumilio, Dermacentor reticulatus, Dermacentor niveus | Europe | 301 | |
| Strains DnS14 and DnS28 | Dermacentor nutallii, Dermacentor silvarum, Dermacentor niveus | Siberia, Kazakhstan | Closely related to members of the R. massiliae group together with R. rhipicephali, R. aeschlimannii, and R. montanensis | 301, 322 |
| “Candidatus Rickettsia tarasevichiae” | Ixodes persulcatus | Russia | Closely related to R. canadensis | 320 |
| Strain R300 | Haemaphysalis juxtakochi | Brazil | AY472038 | |
| Rickettsia midichlorii | Ixodes scapularis | United States | AY348295 | |
| Strain AaR/SoCarolina | A. americanum | United States | Closely related to R. amblyommii | AF45340 |
| “R. morelii” | Ixodes ricinus | Spain | Y08784, Y08785 | |
| “Candidatus Rickettsia principis” | Haemaphysalis japonica douglasi | Far Eastern Russia | AY578114, AY578115 | |
| Rickettsia sp. | Amblyomma triguttatum | Barrow Island, northwest coast of western Australia | Ticks were collected off people working on the island | —a |
H. Owen, N. Unswarth, J. Stenos, P. Clark, S. Graves, and S. Fenwick, Abstr., 4th Int. Conf. Rickettsiae Rickettsial Dis., abstr. P-200, 2005.
NEW APPROACHES TO DIAGNOSIS
Generally, the clinical symptoms of tick-borne spotted fever group rickettsioses begin 4 to 10 days after the bite and typically include fever, headache, muscle pain, rash, local lymphadenopathy, and for most of these diseases, a characteristic inoculation eschar (tache noire) at the bite site. However, these signs vary depending on the rickettsial species involved (Table 2) (276, 358). Common nonspecific laboratory abnormalities in rickettsioses include mild leukopenia, anemia, and thrombocytopenia. Hyponatremia, hypoalbuminemia, and hepatic and renal abnormalities may also occur (276). The specific methods for the diagnosis of rickettsioses have been recently reviewed (174).
TABLE 2.
Characteristics of known and potential tick-borne rickettsiae, 2005
| Rickettsia sp. | Recognized or potential tick vector(s) | Yr of first identification in ticks | Disease (yr of first clinical description) | Yr of first microbiological documentation of human cases | Selected clinical and epidemiological characteristics |
|---|---|---|---|---|---|
| Confirmed pathogens | |||||
| Rickettsia rickettsii | Dermacentor andersoni, Dermacentor variabilis, Rhipicepahlus sanguineus, Amblyomma cajennense, Amblyomma aureolatum | 1906 | Rocky Mountain spotted fever (1899) | 1906a | The prototypical and most severe tick-borne spotted fever rickettsiosis. Case fatality ratio is 20 to 25% in untreated patients. Peak occurrence during spring and summer. Eschars rarely reported. Broadly distributed in the Western Hemisphere and associated with several species of tick vectors. |
| Rickettsia conorii subsp. conorii | Rhipicephalus sanguineus | 1932 | Mediterranean spotted fever (1910) | 1932a | Disease occurs in urban (66%) and rural (33%) settings. Rash occurs in 97%. Cases generally sporadic. Single eschar. Case fatality ratio, approximately 2.5%. |
| Rickettsia conorii subsp. israelensis | Rhipicephalus sanguineus | 1974 | Israeli spotted fever (1940) | 1971a | Compared to Mediterranean spotted fever, eschars are less frequent. Mild to severe illness. |
| Rickettsia sibirica subsp. sibirica | Dermacentor nuttalli, Dermacentor marginatus, Dermacentor silvarum, Haemaphysalis concinna | Unknown | Siberian tick typhus (1934) | 1946a | Disease occurs in predominantly rural settings. Cases occur during spring and summer. Increasing reports of cases. Cases generally associated with rash (100%), eschar (77%), and lymphadenopathy. |
| Dermacentor sinicus | 1974 | North Asian Tick typhus (1977) | 1984a | ||
| Rickettsia australis | Ixodes holocyclus, Ixodes tasmani | 1974 | Queensland tick typhus (1946) | 1946a | Disease occurs in predominantly rural settings. Cases occur from June to November. Vesicular rash (100%), eschar (65%), and lymphadenopathy (71%). Two fatal cases described. |
| Rickettsia japonica | Ixodes ovatus, Dermacentor taiwanensis, Haemaphysalis longicornis, Haemaphysalis flava | 1996 | Oriental or Japanese spotted fever (1984) | 1985a | Disease occurs in predominantly rural settings. Agricultural activities, bamboo cutting. April to October. Eschar (91%) and rash (100%). May be severe. One fatal case reported. |
| Rickettsia conorii subsp. caspia | Rhipicephalus sanguineus, Rhipicephalus pumilio | 1992 | Astrakhan fever (1970s) | 1991a | Disease occurs in predominantly rural settings. Associated with eschar (23%), maculopapular rash (94%), and conjunctivitis (34%). |
| Rickettsia africae | Amblyomma hebraeum, Amblyomma variegatum | 1990 | African tick bite fever (1934) | 1992a | Disease occurs in predominantly rural settings and is associated in international travellers returning from safari, hunting, camping, or adventure races. Outbreaks and clustered cases common (74%). Symptoms include fever (88%), eschars (95%) which are often multiple (54%), maculopapular (49%) or vesicular (50%) rash, and lymphadenopathy (43%). No fatal cases reported. |
| Rickettsia honei | Aponomma hydrosauri, Amblyomma cajennense, Ixodes granulatus | 1993 | Flinders Island spotted fever (1991) | 1992a | Disease occurs in predominantly rural settings. Peak in December and January. Symptoms include rash (85%), eschar (25%), and lymphadenopathy (55%). |
| Rickettsia Sibirica subsp. mongolitimonae | Hyalomma asiaticum, Hyalomma truncatum | 1991 | Lymphangitis associated rickettsiosis (1996) | 1996a | Few cases described in southern France between March and July and South Africa. Symptoms include eschar (75%), rash (63%), and lymphangitis (25%). |
| Rickettsia slovaca | Dermacentor marginatus, Dermacentor reticulatus | 1968 | Tick-borne lymphadenopathy (1997), Dermacentor-borne necrosis and lymphadenopathy (1997) | 1997b, 2003a | Fever and rash rare. Typical eschar on the scalp with cervical lymphadenopathy. Illness mild. |
| Rickettsia heilongjiangensis | Dermacentor silvarum | 1982 | Far Eastern spotted fever (1992) | 1992, 1996a | Rash, eschar, and lymphadenopathy. No fatal cases reported. |
| Rickettsia aeschlimannii | Hyalomma marginatum marginatum, Hyalomma marginatum rufipes, Rhipicephalus appendiculatus | 1997 | Unnamed (2002) | 2002b,d | Few cases described in patients from Morocco and South Africa. Symptoms include eschar and maculopapular rash. |
| Rickettsia parkeri | Amblyomma maculatum, Amblyomma americanum, Amblyomma triste | 1939 | Unnamed (2004) | 2004a | One case reported in a patient in the United States. Symptoms include fever, multiple eschars, and rash. |
| Rickettsia massiliae | Rhipicephalus sanguineus, Rhipicephalus turanicus, Rhipicephalus muhsamae Rhipicephalus lunulatus, Rhipicephalus sulcatus | 1992 | Unnamed (2005) | 2005a | The strain was obtained from a blood of a patient from Sicily in 1985, stored, and definitively identified in 2005. |
| “Rickettsia marmionii” | Haemaphysalis novaeguineae | 2003-2005 | Australian spotted fever (2005) | 2003-2005 | Between February and June, six confirmed cases, including one with escar and two with a maculopapular rash. |
| Potential pathogens | |||||
| Rickettsia conorii subsp. indica | Rhipicephalus sanguineus | 1950 | Indian tick typhus | 2001b | Compared to Mediterranean spotted fever, rash usually purpuric. Eschar rarely found. Mild to severe. |
| Rickettsia canadensis | Haemaphysalis leporispalustris | 1967 | Possible Rocky Mountain spotted fever-like disease descibed in California and Texas. Suspected cause of acute cerebral vasculitis in Ohio. | ||
| Rickettsia amblyommii | Amblyomma americanum, Amblyomma cajennense, Amblyomma coelebs | 1974 | Unnamed (1993) | 1993d | Possible cause of mild spotted fever rickettsiosis in the United States. Rickettsiae also recently identified in Brazilian ticks. |
| Rickettsia texiana | A. americanum | 1943 | Bullis fever (1942) | 1943c | Possible agent of an epidemic which occurred among army personnel at Camp Bullis, Texas during 1942-1943. |
| Rickettsia helvetica | Ixodes ricinus, Ixodes ovatus, Ixodes persulcatus, Ixodes monospinus | 1979 | Unnamed (1999) | 1999b | Although implicated in perimyocarditis and sarcoidosis, the validity of these associations has been debated or not accepted by rickettsiologists. Few cases documented by serology only in France and in Thailand. Rash and eschar seldom occur. |
Documentation by culture.
Documentation by molecular tools.
Documentation by animal or human inoculation.
Documentation by serology.
Serology
Serological tests are the most frequently used and widely available methods for diagnosis. The Weil-Felix test, the oldest assay, is based on the detection of antibodies to various Proteus antigens that cross-react with rickettsiae. Although it lacks specificity and sensitivity, it continues to be used in many developing countries and in countries with higher level of technical development (142). For example, the results of Weil-Felix tests led F. Mahara to suspect a SFG rickettsiosis in Japan (183). This was the first diagnostic step in the recognition of R. japonica as a case of disease in this country (183). MIF is currently being considered as the reference method. For RMSF, 84.6% to 100% sensitivity and 99.8 to 100% specificity have been reported, depending on the cutoff chosen (42). For MSF, MIF sensitivity ranges from 46% (when sampling day 5 to 9) to 100% (when sampling after day 29) (42). One of the major limitations of serology is the cross-reactivity that often exists among antigens of pathogens within the same genus and occasionally in different genera (245). For example, it was noted recently that cases of a particular rickettsial infection reported among paleontologists following an expedition to Mongolia could only be considered as presumptive, as the diagnosis was supported by an MIF assay using a single antigen. Indeed, other SFG rickettsiae (pathogenic and of unknown pathogenicity) are known to occur in that region (177). To illustrate, when 16 patients with culture-positive or PCR-documented R. conorii infection and 5 patients with SFG rickettsioses contracted in Mongolia or Siberia were evaluated solely by MIF, this assay was unable to discriminate between several antigens tested, including R. conorii, R. rickettsii, R. africae, R. slovaca, R. sibirica, and R. felis (a flea-borne SFG rickettsia) (291). MIF remains the reference standard for serological diagnosis of SFG rickettsioses. However, most commercially available MIF assays offer a very limited selection of antigens (e.g., R. rickettsii in the United States or R. conorii and R. rickettsii in France). Even national reference centers may routinely test for only a few other SFG rickettsiae (e.g., R. akari or R. africae). In this context, it is important to remind the practicing physician that MIF may be adequate to diagnose the class of infection (e.g., a spotted fever rickettsiosis) but is likely to be insufficient to definitively identify the etiologic agent unless other, more-sophisticated serologic assays are performed or blood or tissue samples can be evaluated by culture- or PCR-based methods (see below). It is also important to remind clinicians that collection of acute and convalescent-phase serum specimens, separated by several weeks, in necessary to confirm disease.
Cross-absorption (CA) techniques and Western blotting (WB) can be used in reference centers to help to differentiate rickettsial infections by antibody evaluation (Fig. 11) (174). For African tick bite fever, the sensitivity of IFA, CA, WB, and IFA with CA and WB have been estimated at 26%, 83%, 53%, and 56%, respectively, when 414 patients were tested, including 39 cases confirmed by PCR or culture, and 81 considered positive on the basis of IFA with CA and WB (273). Both specificity and positive predictive value were 100% for all techniques. Currently in our laboratory, when cross-reactions are noted between several rickettsial antigens, the standard procedure comprises several steps. A rickettsial antigen is considered to represent the agent of infection when titers of IgG or IgM antibody against this antigen are at least two serial dilutions higher than titers of IgG or IgM antibody against other rickettsial antigens. When differences in titers between several antigens are lower than 2 dilutions, Western blot assays and, if needed, cross-absorption studies are performed (244).
FIG. 11.
Western blot assay of an acute MIF-positive serum showing reactivity with the 135- and 115-kDa specific protein antigens of Rickettsia conorii and Rickettsia africae, respectively. Columns 1, 3, and 5, R. conorii antigens. Columns 2, 4, and 6, R. africae antigens. Columns 1 and 2, untreated sera. Columns 3 and 4, sera absorbed with R. conorii antigens. Columns 5 and 6, sera absorbed with R. africae antigens. MW, molecular weight marker. The interpretation is that when absorption is performed with R. africae, it results in the disappearance of homologous and heterologous antibodies, but when it is performed with R. conorii, only homologous antibodies disappeared. This indicates that antibodies are specific for R. africae. Molecular masses are indicated on the left.
In this context, serology should be considered as an initial but not sole method to recognize and diagnose rickettsial diseases, particularly if no rickettsiae have been previously isolated or detected in the considered area. Cautious interpretation of serologic assays is necessary to avoid misinterpretation and assignment of a specific etiologic agent, particularly when novel or “emerging” tick-borne rickettsioses are described (245). For example, a SFG rickettsioses initially described in Uruguay in 1990 was observed in three patients who presented with fever, a small initial maculopapulous lesion on the scalp at a tick bite site followed by regional lymphadenopathy. MIF serology using R. conorii as the sole antigen was positive for all patients, and these infections were presumptively identified as spotted fever caused by R. conorii (73). During 1993-1994, 23 patients with a previous history of tick bite, including some with exanthems and inoculation eschars were identified from rural areas of Canelones County, Uruguay. These patients were also found to have titers reactive with R. conorii when tested by MIF; however, R. conorii was again the only antigen used in the assay (87). Amblyomma triste, a tick commonly found on dogs with a larval cycle on wild rodents, has been involved in these cases, and recently, R. parkeri has been isolated from this tick (347). In this context, it is possible that some or all of these patients were infected with SFG rickettsiae other than R. conorii, particularly as R. conorii has never been found in the Western Hemisphere or in Amblyomma species ticks.
The need for an effective and versatile serologic assay to identify a particular rickettsia responsible for infection cannot be overemphasized. Although other classical and contemporary methods (described below) provide a conclusive etiologic diagnosis, these techniques may require a clinical specimen that is not readily available when the patient is initially evaluated for care (e.g., tissue or acute-phase whole blood). A serum specimen, often collected from the patient days to weeks after his or her recovery, is the most frequently evaluated and often the only analyte available to laboratories that perform diagnostic tests for tick-borne rickettsioses. Whatever the technique used, it is important to emphasize that acute- and convalescent-phase sera be collected in any case in which rickettsiosis is suspected.
Culture
Although rickettsial isolation in culture remains the most definitive diagnostic method, this technique is typically performed only in reference laboratories with a P3 safety level and requires staff capable of maintaining living host cells (animal mouse models or embryonated eggs) or cell cultures (Vero, L929, HEL, XTC-2, or MRC5 cells). The centrifugation shell-vial technique using HEL fibroblasts is an effective application of this method (348). Isolation of rickettsiae can be performed routinely in this type of laboratory by using buffy coat preparations of heparinized or EDTA-anticoagulated whole blood, skin biopsy specimens, or arthropods. Although this technique is versatile, approximately one-third of rickettsial isolates may be lost when passaged to new cells.
Histochemical and Immunohistochemical Methods
Rickettsiae can been detected occasionally in tissue specimens by various histochemical stains, including Giemsa or Gimenez stains (174); however, immunohistochemical methods provide superior visualization of SFG rickettsiae when applied to formalin-fixed, paraffin-embedded tissue specimens obtained at autopsy or cutaneous biopsy samples (particularly eschars) (Fig. 12) (82, 228, 230, 300). Similar caution must be used when interpreting immunohistochemical stains, as most available assays are SFG specific but not species specific.
FIG. 12.
Immunohistochemical detection of Rickettsia sibirica subsp. mongolotimonae (arrows, rickettsiae staining red) in a skin biopsy specimen of an eschar of the patient presented in Fig. 5. Note the abundant inflammatory infiltrate with necrotic features and vascular injury in the dermis (polyclonal rabbit anti-R. sibirica subsp. mongolitimonae antibody used at a dilution of 1/2,000 with hemalin counterstain; original magnification, ×400).
Molecular Tools
PCR and sequencing methods are now used as sensitive and rapid tools to detect and identify rickettsiae in blood and skin biopsy specimens throughout the world where these facilities are available. Primers amplifying sequences of several genes, including ompA, ompB, gltA, and gene D, have been used (Table 3) (42, 104, 111, 295, 310). Ticks may also be used as epidemiological tools to detect the presence of a pathogen in a specific area, providing insights to and discoveries of rickettsiae of unknown pathogenicity (245). Recently, we proposed a PCR assay with increased sensitivity, named “suicide PCR,” that had been first developed to detect ancient DNA (269). Thereafter, we have been starting to use it in the diagnosis of SFG rickettsioses to detect DNA from blood samples, as conventional PCR generally has poor sensitivity for detecting SFG rickettsiae when applied to blood specimens. Suicide PCR is a nested PCR using single-use primers targeting a gene never amplified previously in the laboratory (90, 269). This procedure avoids vertical contamination by amplicons from previous assays, one of the limitations of extensive use of PCR. There is no positive control. Because the essential role of positive controls is to validate negative results, the absence of these controls does not impair the interpretation of positive results, which are validated by appropriate negative controls. All positive PCR products are sequenced to identify the causative agent. This technique has been successful with EDTA-blood, serum, and lymph node specimens in the diagnosis of African tick bite fever due to R. africae and infection due to R. slovaca (273, 275). This technique was also applied in our laboratory to DNA from 103 skin biopsy specimens from patients with confirmed rickettsiosis, 109 skin biopsies from patients who possibly had a rickettsiosis, and from 50 skin biopsy specimens with patients with no rickettsial diseases. Specificity was 100%. Sensitivity (68%) was 2.2 times higher than culture and 1.5 times higher than regular PCR (109). This technique requires validation studies and carefully conducted controls, regardless of sequencing results, that are usually made in reference laboratories. Other teams have successfully applied nested PCR to serum and tissue specimens from patients suffering from severe MSF (175); however, standard nested PCR assays may be highly subject to contamination and false-positive results.
TABLE 3.
Selected DNA primers used for the detection of tick-borne rickettsiae in clinical patient samples or ticks
| Gene target (rickettsial species) | Primer, sequence | Method | Sample | Reference(s) |
|---|---|---|---|---|
| Citrate synthase gene (gltA all rickettsiae) | RpCS.877p (forward), GGGGACCTGCTCACGGCGG | Standard PCR | Skin | 295 |
| RpCS.1258n (reverse), ATTGCAAAAAGTACAGTGAACA | Ticks | |||
| Outer membrane protein A gene ompA (all species except R. helvetica, R. australis, R. bellii, and R. canadensis) | Rr190.70p (forward), ATGGCGAATATTTCTCCAAAA | Standard PCR | Skin | 111 |
| Rr190.602n (reverse), AGTGCAGCATTCGCTCCCCCT | Ticks | |||
| Rr190.70p (forward), ATGGCGAATATTTCTCCAAAA | Standard PCR | |||
| Rr190.70ln (reverse), GTTCCGTTAATGGCAGCATCT | ||||
| AF1F (forward), CACTCGGTGTTGCTGCA | Suicide PCRa | Serum | 109 | |
| AF1R (reverse), ATTAGTGCAGCATTCGCTC | ||||
| AF3F (forward), GGTGGTGGTAACGTAATC | ||||
| AF3R (reverse), CGTCAGTTATTGTAACGGC | ||||
| AF2F (forward), GCTGCAGGAGCATTTAGTG | ||||
| AF2R (reverse), TATCGGCAGGAGCATCAA | ||||
| AF4F (forward), GGAACAGTTGCAGAAATCAA | ||||
| AF4R (reverse), CTGCTACATTACTCCCAATA | ||||
| Outer membrane protein B gene ompB (all species except R. helvetica, R. bellii, and R. massiliae) | BG1-21 (forward), GGCAATTAATATCGCTGACGG | Standard PCR | Skin | 294 |
| BG2-20 (reverse), GCATCTGCACTAGCACTTTC | Ticks | |||
| Gene D (most rickettsiae) | D1F (forward), ATGAGTAAAGACGGTAACCT | Standard PCR | Skin | 104, 310 |
| D928R (reverse), AAGCTATTGCGTCATCTCCG | ||||
| 17-kDa protein-encoding gene (all spotted fever group rickettsiae) | R17122 (forward), CAGAGTGCTATGGAACAAACAAGG | Nested PCR | Skin | 193, 338 |
| R17500 (reverse), CTTGCCATTGCCCATCAGGTTG | Serum | |||
| TZ15 (forward), TTCTCAATTCGGTAAGGGC | Blood | |||
| TZ16 (reverse), ATATTGACCAGTGCTATTTC | Ticks |
Note that primers used for this nested PCR are used only once in the same lab, with no positive control. Other pairs of primers may be designed each time a new screening is done. They can be selected for other rickettsial genes. These primers are given as examples.
TREATMENT
Early empirical antibiotic therapy should be prescribed in any suspected tick-transmitted rickettsiosis, before confirmation of the diagnosis (Table 4). More than 50 years after the introduction of tetracyclines, doxycycline (200 mg per day) remains the treatment of choice for tick-transmitted rickettsioses (276). Although tetracyclines are contraindicated for general use in children less than 9 years of age, doxycycline remains the treatment of choice for all patients, including young children, with RMSF (137, 194, 265). This recommendation should be expanded to include other SFG tick-borne rickettsioses, several of which may be potentially life-threatening. In general, the risk of dental staining by doxycycline is negligible when a single, relatively short (e.g., 5- to 10-day) course of therapy is administered.
TABLE 4.
Antibiotic treatments for MSF and RMSF
| Rickettsiosis | Patient cohort | Selected antibiotic regimensg | Strength of recommendation and quality of evidenceh | Reference(s) |
|---|---|---|---|---|
| Mediterranean spotted fever | Adults | Doxycycline, two oral 200-mg doses separated by a 12-h interval | A I | 30 |
| Doxycycline, 200 mg single dose or 100 mg twice a day for 2 to 5 daysa,b | A III | 81, 298 | ||
| Josamycin, 2 oral doses of 1 g every 8 h for 5 daysc | A II | 29 | ||
| Ciprofloxacin, 750 mg every 12 h for 7 days | A II | 298, 299 | ||
| Children | Doxycycline, 2.2 mg/kg every 12 h for children weighing <99 lb (45 kg) or adult dosage if ≥100 lb, for 5 to 10 daysa,b | A III | 137, 265 | |
| Clarythromycin, 15/mg/kg/day in two divided doses for 7 days | A I | 63, 64 | ||
| Azythromicin, 10 mg/kg/day in one dose for 3 days | A III | 208 | ||
| Josamycin, 50 mg/kg every 12 h for 5 days | A III | 29 | ||
| Pregnant women | Josamycin, 50 mg/kg every 12 h for 5 daysb,c | A III | 29 | |
| Rocky Mountain spotted fever | Adults | Doxycycline, 100 mg every 12 h for 5 to 10 daysa | A III | 137 |
| Children | Doxycycline, 2.2 mg/kg every 12 h for children weighing <99 lb (45 kg) or adult dosage if ≥100 lb, for 5 to 10 daysa,d | A III | 137, 265 | |
| Chloramphenicol, 12.5 to 25 mg/kg every 6 h for 5 to 10 dayse | A III | 356 | ||
| Pregnant women | Doxycycline, 100 mg every 12 h for 5 to 10 daysf | A III | 356 |
Oral or intravenous. Intravenous formulation was generally used for patients with vomiting or severe disease. Longer courses of doxycycline treatment may be warranted for patients with severe disease.
Chloramphenicol could be an alternative if it is the sole available drug for empirical treatment of severe cases, as may be the situation in developing countries (B III).
Josamycin is not available in the United States. Based on the results in children and in vitro studies, azythromycin or clarythromycin could represent alternatives (CIV).
Tetracycline antibiotics are generally contraindicated in children under 8 years because of the dose-dependent risk of staining of permanent teeth; however, these antibiotics are superior therapy for RMSF, and doxycycline is the drug of choice for therapy of this life-threatening disease in patients of all ages. Shorter treatment courses may be warranted in some pediatric settings; however, therapy should be continued for at least 48 hours following lysis of fever and evidence of clinical improvement.
Alternate therapy reserved for those patients for whom there is an absolute contraindication for receiving doxycycline. Oral formulation (palmitate) is not available in the United States. Optimal intravenous dosage is determined by measurement of serum concentrations to achieve peak level particularly in newborns, children <2 years of age, pregnant women, patients with hepatic disease, or patients receiving therapy for >5 days. Gray baby syndrome (abdominal distention, pallor, cyanosis, and vasomotor collapse) has been reported when chloramphenicol is given to neonates. Although it has not been reported in neonates after maternal administration of chloramphenicol, it is theoretically possible since drug levels reach 30 to 80% of the maternal serum level. Thus, some authorities advise against using chloramphenicol in women at or near term. New macrolide drugs have not been adequately evaluated as alternate therapies for RMSF.
Tetracycline antibiotics are generally contraindicated during pregnancy because of the risks associated with interference in the development of teeth and long bones in the fetus. However, because RMSF is a life-threatening illness, and because of the demonstrated superiority of doxycycline as therapy for this infection, this drug represents the antibiotic of choice.
Current recommended therapies are in bold.
Strength of recommendation and quality of evidence is used as reported in (reference 157). The author used the letter A to stand for good evidence, B for moderate evidence, and C for limited evidence to support a recommendation for use. Also by this author, I stood for evidence from ≥1 properly randomized controlled trial; II for evidence from ≥1 well-designed clinical trial without randomization or from cohort or case-controlled analytic studies; III for evidence from opinions of respected authorities, based on clinical experience, descriptive studies, or report of expert committees; and IV for evidence based on in vitro studies or anecdotal case reports of treatment success in patients with confirmed disease.
In patients with severe hypersensitivity to tetracyclines, 50 to 75 mg/kg of body weight/day of chloramphenicol can be considered as an alternate therapy, but its use is limited by side effects. In general, use of this drug as therapy for rickettsioses should be considered as empirical treatment of severe cases only if it is the sole available drug, such as in developing countries. Josamycin (50 mg/kg/day) has also been used to treat some patients with certain SFG rickettsioses (276). In pregnant women with MSF, josamycin can be used at a dose of 3 g per day for 7 days (276). Newer macrolides, such as azithromycin and clarithromycin (63, 308), are also of interest. In a recent open-label controlled trial, these two drugs were compared in the treatment of children with MSF in Italy. They were equally tolerated, and no major side effects were observed. All patients recovered, as fever disappeared in less than 7 days in each case. No statistical difference between the times to defervescence of the drugs was found (63). Azithromycin seems to be of particular interest, as it is administered once a day and presents a shorter duration of therapy (3 days, compared to 7 days with clarithromycin). Some fluoroquinolones may have efficacy against spotted fever rickettsiae, although these data are largely anecdotal and require careful clinical evaluation (227, 290, 298, 299).
Many classes of broad-spectrum antibiotics, including penicillins, cephalosporins, and aminoglycosides, are ineffective as therapies for rickettsial diseases. In vivo animal studies, anecdotal clinical experience, and limited evidence from a small clinical trials suggest that sulfa-containing antimicrobials not only are ineffective but may actually exacerbate spotted fever rickettsioses, including RMSF and MSF (131, 133, 299a, 336).
The exact duration of appropriate antibiotic therapy for SFG rickettsioses is generally related more to clinical response than a precise number of days; however, for most of these infections, recommended therapy should continue for at least 3 days after the patient's fever has abated. A single dose of 200 mg of doxycycline has been shown to be sufficient for MSF (276), although for patients with severe forms, such as malignant MSF, doxycycline should be administered intravenously up to 24 h after apyrexia. The use of corticosteroids in severe forms is controversial (160).
CONCLUSION
In 1940, R. R. Parker, commenting on the disease-causing potential of an Amblyomma-associated rickettsia he had identified a year earlier, wrote, “Final decision as to whether maculatum infection is or is not a new disease entity must await further research” (R. R. Parker, Proc. 3rd Int. Congr. Microbiol., p. 390-391, 1940). In the case of R. parkeri, the wait was 65 years. In fact, many of the rickettsiae we now identify as human pathogens were first identified in ticks several years or even decades before a conclusive association with human disease was demonstrated, including 7 of the 11 species or subspecies of tick-borne SFG rickettsiae confirmed as pathogens since 1984.
Various circumstances have contributed to the expansion of distinct tick-borne rickettsioses recognized during the last decades of the 20th century. Heightened awareness of rickettsial illnesses, the careful obtaining of histories, and thorough physical and laboratory examinations by primary physicians have been crucial factors leading to the discovery of several recently described tick-borne rickettsial diseases, including Flinders Island spotted fever, Japanese spotted fever, and Astrakhan fever. Contemporary techniques in molecular biology have greatly facilitated the description of novel rickettsial agents and provided new insights into the epidemiology of emerging rickettsioses on several continents. These include infections caused by R. sibirica subsp. mongolitimonae, R. slovaca, R. aeschlimannii, and R. heilongjiangensis. Classical and improved methods in cell culture methods, coupled with molecular assays, have provided critical confirmatory information to incriminate various species of Rickettsia, including R. parkeri, as pathogens of humans. Finally, an increase in the number of people traveling to foreign countries to participate in recreational activities, including hiking, camping, and hunting, in nondeveloped or rural areas can result in increased contact with ticks and tick-borne pathogens endemic to that region. Increasing reports of imported African tick bite fever among travelers returning to Europe and the United States from southern Africa is illustrative of this process.
The recent history of rickettsial diseases is similar in many ways to that of arboviral diseases, particularly those caused by flaviviruses. Many newly recognized flaviviral diseases have been identified only during the last several decades, and various other flaviviruses have been isolated or detected from arthropod hosts and await linkage to human disease. Advances in viral molecular genetics, phylogenetic studies, genome sequencing, computational techniques have recently provided new tools starting to give some answers on pathogenicity, interactions with vectors, and host and/or vector association specificity and to resolve taxonomy problems (360).
Efforts to characterize distinct tick-borne rickettsioses are in progress on many continents for which a single pathogenic rickettsial species has been previously described, including Central and South America and Asia. Renewed interest and collaborative endeavors with new and established investigators in the tropics, combined with powerful diagnostic methods, will likely herald the identification of several newly recognized rickettsial pathogens in these regions and contribute to an exciting period of discovery in the science of rickettsiology (37, 138, 164, 167, 170, 240, 286, 347). In some Asian countries, although no SFG rickettsiae have been to date detected from ticks or people, serosurveys or serology testing of patients with nonspecified fevers indicates the prevalence of spotted fever group rickettsioses. For example, in the Central Province of Sri Lanka, 10 out of 118 clinically investigated patients were recently shown to have IgM antibodies against SFG rickettsiae, suggestive of an acute SF rickettsiosis (164).
In 2002, rickettsiologist D. H. Walker noted that “old, unresolved problems do not receive the attention that new, interesting issues do. For instance, compare the efforts placed on researching West Nile virus or hantavirus pulmonary syndrome with the application of medical science to RMSF… There is room for more scientists in rickettsiology, and tools of molecular biology and cell biology allow more to be accomplished” (350). Myriad applied and basic research questions remain in the study of spotted fever rickettsiae and the diseases that these agents cause. A sampling of unresolved issues include the need for reliable, early diagnostic tests; development of serological assays that discriminate among various SFG rickettsial infections and provide an agent-specific diagnosis; better characterization of the natural histories of newly recognized SFG rickettsial pathogens; elucidating the pathogenic mechanisms (including rickettsial reactivation in the tick, the role and effect of tick saliva on the early infection, and the identification of rickettsial virulence genes); and prospective active surveillance studies that better the magnitude and distribution of various spotted fever rickettsioses.
There exist many unique tick-associated rickettsiae for which a role in human disease has yet to be determined. Several of these rickettsiae satisfy the first component necessary for a potential tick-borne pathogen, i.e., they reside in a tick species with a natural proclivity to bite humans. The combined efforts of investigators around the world have reduced the concept of “one continent, one pathogenic tick-borne rickettsia” to an anachronism, and subsequent investigations will undoubtedly lead to the discovery of new tick-borne rickettsial diseases in the future.
FIG. 8.
Tick-borne rickettsiae in the Americas. Colored symbols indicate pathogenic rickettsiae. White symbols indicate rickettsiae of possible pathogenicity and rickettsiae of unknown pathogenicity.
FIG. 9.
Tick-borne rickettsiae in Asia and Australia. Colored symbols indicate pathogenic rickettsiae. White symbols indicate rickettsiae of possible pathogenicity and rickettsiae of unknown pathogenicity.
Acknowledgments
We are grateful to Pierre-Edouard Fournier for his help in making Fig. 1.
REFERENCES
- 1.Amaro, M., F. Bacellar, and A. Franca. 2003. Report of eight cases of fatal and severe Mediterranean spotted fever in Portugal. Ann. N. Y. Acad. Sci. 990:331-343. [DOI] [PubMed] [Google Scholar]
- 2.Ammerman, N. C., K. L. Swanson, J. M. Anderson, T. R. Schwartz, E. C. Seaberg, E. G. Glass, and D. E. Norris. 2004. Spotted-fever group Rickettsia in Dermacentor variabilis, Maryland. Emerg. Infect. Dis. 10:1478-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, J. F., L. A. Magnerelli, R. N. Philip, and W. Burgdorfer. 1986. Rickettsia rickettsii and Rickettsia montana from ixodid ticks in Connecticut. Am. J. Trop. Med. Hyg. 35:187-191. [DOI] [PubMed] [Google Scholar]
- 4.Andrew, R., J. M. Bonnin, and S. William. 1946. Tick typhus in north Queensland. Med. J. Aust. 2:253-258. [PubMed] [Google Scholar]
- 5.Anigstein, L., and D. Anigstein. 1975. A review of the evidence in retrospect for a rickettsial etiology in Bullis fever. Texas Rep. Biol. Med. 33:201-211. [PubMed] [Google Scholar]
- 6.Anigstein, L., and M. N. Bader. 1943. Investigations on rickettsial diseases in Texas. Part 4. Experimental study of Bullis fever. Texas Rep. Biol. Med. 1:389-409. [Google Scholar]
- 7.Anton, E., B. Font, T. Munoz, I. Sanfeliu, and F. Segura. 2003. Clinical and laboratory characteristics of 144 patients with mediterranean spotted fever. Eur. J. Clin. Microbiol. Infect. Dis. 22:126-128. [DOI] [PubMed] [Google Scholar]
- 8.Aquilini, D., P. Parola, E. Salvo, and A. Paladini. 2001. Seroepidemiology of rickettsioses, human granulocytic ehrlichioses, Lyme disease, Q fever, and tularaemia in forestry workers in Tuscany, Italy. J. Spirochetal Tick-Borne Bacterial Dis. 7:35-41. [Google Scholar]
- 9.Araki, M., K. Takatsuka, J. Kawamura, and Y. Kanno. 2002. Japanese spotted fever involving the central nervous system: two case reports and a literature review. J. Clin. Microbiol. 40:3874-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azad, A. F., and C. B. Beard. 1998. Rickettsial pathogens and their arthropod vectors. Emerg. Infect. Dis. 4:179-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babalis, T., Y. Tselentis, V. Roux, A. Psaroulaki, and D. Raoult. 1994. Isolation and identification of a rickettsial strain related to Rickettsia massiliae in Greek ticks. Am. J. Trop. Med. Hyg. 50:365-372. [DOI] [PubMed] [Google Scholar]
- 12.Bacellar, F., L. Beati, A. Franca, J. Pocas, R. Regnery, and A. Filipe. 1999. Israeli spotted fever rickettsia (Rickettsia conorii complex) associated with human disease in Portugal. Emerg. Infect. Dis. 5:835-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bacellar, F., R. L. Regnery, M. S. Nuncio, and A. R. Filipe. 1995. Genotypic evaluation of rickettsial isolates recovered from various species of ticks in Portugal. Epidemiol. Infect. 114:169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bacellar, F., R. Sousa, A. Santos, M. Santos-Silva, and P. Parola. 2003. Boutonneuse fever in Portugal: 1995-2000. Data of a state laboratory. Eur. J. Epidemiol. 18:275-277. [DOI] [PubMed] [Google Scholar]
- 15.Baird, R. W., M. Lloyd, J. Stenos, B. C. Ross, R. S. Stewart, and B. Dwyer. 1992. Characterization and comparison of Australian human spotted fever group rickettsiae. J. Clin. Microbiol. 30:2896-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baird, R. W., J. Stenos, R. Stewart, B. Hudson, M. Lloyd, S. Aiuto, and B. Dwyer. 1996. Genetic variation in Australian spotted fever group rickettsiae. J. Clin. Microbiol. 34:1526-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balayeva, N. M., M. E. Eremeeva, V. F. Ignatovich, N. V. Rudakov, T. A. Reschetnikova, I. E. Samoilenko, V. K. Yastrebov, and D. Raoult. 1996. Biological and genetic characterization of Rickettsia sibirica strains isolated in the endemic area of the north Asian tick typhus. Am. J. Trop. Med. Hyg. 55:685-692. [DOI] [PubMed] [Google Scholar]
- 18.Baldridge, G. D., N. Y. Burkhardt, J. A. Simser, T. J. Kurtti, and U. G. Munderloh. 2004. Sequence and expression analysis of the ompA gene of Rickettsia peacockii, an endosymbiont of the Rocky Mountain wood tick, Dermacentor andersoni. Appl. Environ. Microbiol. 70:6628-6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barré, N., G. Garris, and E. Camus. 1995. Propagation of the tick Amblyomma variegatum in the Caribbean. Rev. Sci. Tech. Off. Int. Epizoot. 14:841-855. [DOI] [PubMed] [Google Scholar]
- 20.Baumann, D., N. Pusterla, O. Peter, F. Grimm, P. E. Fournier, G. Schar, W. Bossart, H. Lutz, and R. Weber. 2003. Fever after a tick bite: clinical manifestations and diagnosis of acute tick bite-associated infections in northeastern Switzerland. Dtsch. Med. Wochenschr. 128:1042-1047. (In German.) [DOI] [PubMed] [Google Scholar]
- 21.Beati, L., J. P. Finidori, B. Gilot, and D. Raoult. 1992. Comparison of serologic typing, sodium dodecyl sulfate-polyacrylamide gel electrophoresis protein analysis, and genetic restriction fragment length polymorphism analysis for identification of rickettsiae: characterization of two new rickettsial strains. J. Clin. Microbiol. 30:1922-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beati, L., P. J. Kelly, L. A. Matthewman, P. R. Mason, and D. Raoult. 1995. Prevalence of rickettsia-like organisms and spotted fever group rickettsiae in ticks (Acari: Ixodidae) from Zimbabwe. J. Med. Entomol. 32:787-792. [DOI] [PubMed] [Google Scholar]
- 23.Beati, L., M. Meskini, B. Thiers, and D. Raoult. 1997. Rickettsia aeschlimannii sp. nov., a new spotted fever group rickettsia associated with Hyalomma marginatum ticks. Int. J. Syst. Bacteriol. 47:548-554. [DOI] [PubMed] [Google Scholar]
- 24.Beati, L., O. Peter, W. Burgdorfer, A. Aeschlimann, and D. Raoult. 1993. Confirmation that Rickettsia helvetica sp. nov. is a distinct species of the spotted fever group of rickettsiae. Int. J. Syst. Bacteriol. 43:521-526. [DOI] [PubMed] [Google Scholar]
- 25.Beati, L., and D. Raoult. 1993. Rickettsia massiliae sp. nov., a new spotted fever group Rickettsia. Int. J. Syst. Bacteriol. 43:839-840. [DOI] [PubMed] [Google Scholar]
- 26.Beati, L., V. Roux, A. Ortuno, J. Castella, F. S. Porta, and D. Raoult. 1996. Phenotypic and genotypic characterization of spotted fever group Rickettsiae isolated from Catalan Rhipicephalus sanguineus ticks. J. Clin. Microbiol. 34:2688-2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bell, E. J., G. M. Kohls, H. G. Stoenner, and D. B. Lackman. 1963. Nonpathogenic rickettsias related to the spotted fever group isolated from ticks Dermacentor variabilis and Dermacentor andersoni from eastern Montana. J. Immunol. 90:770-781. [PubMed] [Google Scholar]
- 28.Bella, F., E. Espejo, S. Uriz, J. A. Serrano, M. D. Alegre, and J. Tort. 1991. Randomized trial of 5-day rifampin versus 1-day doxycycline therapy for Mediterranean spotted fever. J. Infect. Dis. 164:433-434. [DOI] [PubMed] [Google Scholar]
- 29.Bella, F., B. Font, S. Uriz, T. Munoz, E. Espejo, J. Traveria, J. A. Serrano, and F. Segura. 1990. Randomized trial of doxycycline versus josamycin for Mediterranean spotted fever. Antimicrob. Agents Chemother. 34:937-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bella-Cueto, F., B. Font-Creus, F. Segura-Porta, E. Espejo-Arenas, P. Lopez-Pares, and T. Munoz-Espin. 1987. Comparative, randomized trial of one-day doxycycline versus 10-day tetracycline therapy for Mediterranean spotted fever. J. Infect. Dis. 155:1056-1058. [DOI] [PubMed] [Google Scholar]
- 31.Reference deleted.
- 32.Beninati, T., N. Lo, H. Noda, F. Esposito, A. Rizzoli, G. Favia, and C. Genchi. 2002. First detection of spotted fever group rickettsiae in Ixodes ricinus from Italy. Emerg. Infect. Dis. 8:983-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernasconi, M. V., S. Casati, S. O. Peter, and J. C. Piffaretti. 2002. Rhipicephalus ticks infected with Rickettsia and Coxiella in southern Switzerland (Canton Ticino). Infect. Genet. Evol. 2:111-120. [DOI] [PubMed] [Google Scholar]
- 34.Billings, A. N., G. J. Teltow, S. C. Weaver, and D. H. Walker. 1998. Molecular characterization of a novel Rickettsia species from Ixodes scapularis in Texas. Emerg. Infect. Dis. 4:305-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Billings, A. N., X. J. Yu, F. D. Teel, and D. H. Walker. 1998. Detection of a spotted fever group rickettsia in Amblyomma cajennense (Acari: Ixodidae) in south Texas. J. Med. Entomol. 35:474-478. [DOI] [PubMed] [Google Scholar]
- 36.Blair, P. J., J. Jiang, G. B. Schoeler, C. Moron, E. Anaya, M. Cespedes, C. Cruz, V. Felices, C. Guevara, L. Mendoza, P. Villaseca, J. W. Sumner, A. Richards, and J. G. Olson. 2004. Characterization of spotted fever group rickettsiae in flea and tick specimens from northern Peru. J. Clin. Microbiol. 42:4961-4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blair, P. J., G. B. Schoeler, C. Moron, E. Anaya, R. Caceda, M. Cespedes, C. Cruz, V. Felices, C. Guevara, A. Huaman, R. Luckett, L. Mendoza, A. L. Richards, Z. Rios, J. W. Sumner, P. Villaseca, and J. G. Olson. 2004. Evidence of rickettsial and Leptospira infections in Andean northern Peru. Am. J. Trop. Med. Hyg. 70:357-363. [PubMed] [Google Scholar]
- 38.Blanc, G., and J. Caminopetros. 1932. Epidemiological and experimental studies on Boutonneuse fever done at the Pasteur Institute in Athens. Arch. Inst. Pasteur Tunis 20:394. [Google Scholar]
- 39.Bozeman, F. M., B. L. Elisberg, J. W. Humphries, and D. B. Palmer, Jr. 1970. Serologic evidence of Rickettsia canada infection of man. J. Infect. Dis. 121:367-371. [DOI] [PubMed] [Google Scholar]
- 40.Bozeman, F. M., A. Shirai, J. W. Humphries, and H. S. Fuller. 1967. Ecology of Rocky Mountain spotted fever. II. Natural infection of wild mammals and birds in Virginia and Maryland. Am. J. Trop. Med. Hyg. 16:48-59. [PubMed] [Google Scholar]
- 40a.Breed, R. S., E. G. D. Murray, and P. A. Hitchens (ed.). 1948. Bergey’s manual of determinative bacteriology, 6th ed., xvi-1291. The Williams & Wilkins Company, Baltimore, Md.
- 41.Brennan, J. M. 1945. Field investigations pertinent to Bullis fever. Texas Rep. Biol. Med. 3:204-226. [Google Scholar]
- 42.Brouqui, P., F. Bacellar, G. Baranton, R. J. Birtles, A. Bjoersdorff, J. R. Blanco, G. Caruso, M. Cinco, P. E. Fournier, E. Francavilla, M. Jensenius, J. Kazar, H. Laferl, A. Lakos, S. Lotric-Furlan, M. Maurin, J. A. Oteo, P. Parola, C. Perez-Eid, O. Peter, D. Postic, D. Raoult, A. Tellez, Y. Tselentis, and B. Wilske. 2004. Guidelines for the diagnosis of tick-borne bacterial diseases in Europe. Clin. Microbiol. Infect. 10:1108-1132. [DOI] [PubMed] [Google Scholar]
- 43.Brumpt, E. 1932. Longevité du virus de la fièvre boutonneuse (Rickettsia conorii, n. sp.) chez la tique Rhipicephalus sanguineus. C. R. Soc. Biol. 110:1119. [Google Scholar]
- 44.Burgdorfer, W. 1969. Ecology of tick vectors of American spotted fever. Bull. W. H. O. 40:375-381. [PMC free article] [PubMed] [Google Scholar]
- 45.Burgdorfer, W. 1977. Tick-borne diseases in the United States: Rocky Mountain spotted fever and Colorado tick fever. A review. Acta Trop. 34:103-126. [PubMed] [Google Scholar]
- 46.Burgdorfer, W., A. Aeschlimann, O. Peter, S. F. Hayes, and R. N. Philip. 1979. Ixodes ricinus vector of a hitherto undescribed spotted fever group agent in Switzerland. Acta Trop. 36:357-367. [PubMed] [Google Scholar]
- 47.Burgdorfer, W., and L. P. Brinton. 1975. Mechanisms of transovarial infection of spotted fever Rickettsiae in ticks. Ann. N. Y. Acad. Sci. 266:61-72. [DOI] [PubMed] [Google Scholar]
- 48.Burgdorfer, W., L. P. Brinton, W. L. Krinsky, and R. N. Philip. 1978. Rickettsia rhipicephali: a new spotted fever group rickettsia from the brown dog tick Rhipicephalus sanguineus, p. 307-316. In J. Kazar, R. A. Ormsbee, and I. Tarasevich (ed.), Rickettsiae and rickettsial diseases. House of the Slovak Academy of Sciences, Bratislava, Slovakia.
- 49.Burgdorfer, W., J. C. Cooney, and L. A. Thomas. 1974. Zoonotic potential (Rocky Mountain spotted fever aed tularemia) in the Tennessee Valley region. II. Prevalence of Rickettsia rickettsii and Francisella tularensis in mammals and ticks from Land Between the Lakes. Am. J. Trop. Med. Hyg. 23:109-117. [DOI] [PubMed] [Google Scholar]
- 50.Burgdorfer, W., K. T. Friedhoff, and J. L. Lancaster. 1966. Natural history of tick-borne spotted fever in the USA. Bull. W. H. O. 35:149-153. [PMC free article] [PubMed] [Google Scholar]
- 51.Burgdorfer, W., S. F. Hayes, and A. J. Mavros. 1981. Nonpathogenic rickettsiae in Dermacentor andersoni: a limiting factor for the distribution of Rickettsia rickettsii, p. 585-594. In W. Burgdorfer and R. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, New York, N.Y.
- 52.Burgdorfer, W., S. F. Hayes, L. A. Thomas, and J. L. Lancaster. 1980. A new spotted fever group rickettsia from the lone star tick, Amblyomma americanum, p. 595-602. In W. Burgdorfer and R. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, Inc., New York, N.Y.
- 53.Burgdorfer, W., V. F. Newhouse, E. G. Pickens, and D. B. Lackman. 1962. Ecology of Rocky Mountain spotted fever in western Montana. I. Isolation of Rickettsia rickettsii from wild mammals. Am. J. Hyg. 76:293-301. [DOI] [PubMed] [Google Scholar]
- 54.Burgdorfer, W., R. A. Ormsbee, M. L. Schmidt, and H. Hoogstraal. 1973. A search for the epidemic typhus agent in Ethiopian ticks. Bull. W. H. O. 48:563-569. [PMC free article] [PubMed] [Google Scholar]
- 55.Burgdorfer, W., and M. G. Varma. 1967. Trans-stadial and transovarial development of disease agents in arthropods. Annu. Rev. Entomol. 12:376. [DOI] [PubMed] [Google Scholar]
- 56.Bustamente, M. E., and G. Varela. 1943. Una nueva rickettsiosis en Mexico. Existencia de la fiebre manchada americana en los estados de Sinoloa y Sonora. Rev. Inst. Salub. Enferm. Trop. 4:189-210. [Google Scholar]
- 57.Bustamente, M. E., and G. Varela. 1947. Distribucion de las rickettsiasis en Mexico. Rev. Inst. Salub. Enferm. Trop. 8:3-14. [Google Scholar]
- 58.Bustamente, M. E., and G. Varela. 1947. Estudios de fiebre manchada en Mexico. Papel del Rhipicephalus sanguineus en la transmission de la fiebre manchada en la Republica Mexicana. Rev. Inst. Salub. Enferm. Trop. 8:139-141. [Google Scholar]
- 59.Bustamente, M. E., G. Varela, and C. O. Mariotte. 1946. Estudios de fiebre manchada en Mexico. Fiebre manchada en la Laguna. Rev. Inst. Salub. Enferm. Trop. 7:39-49. [Google Scholar]
- 60.Campbell, R. W., and R. Domrow. 1974. Rickettsioses in Australia: isolation of Rickettsia tsutsugamushi and R. australis from naturally infected arthropods. Trans. R. Soc. Trop. Med. Hyg. 68:397-402. [DOI] [PubMed] [Google Scholar]
- 61.Cardenosa, N., F. Segura, and D. Raoult. 2003. Serosurvey among Mediterranean spotted fever patients of a new spotted fever group rickettsial strain (Bar29). Eur. J. Epidemiol. 18:351-356. [DOI] [PubMed] [Google Scholar]
- 62.Caruso, G., C. Zasio, F. Guzzo, C. Granata, V. Mondardini, E. Guerra, E. Macri, and P. Benedetti. 2002. Outbreak of African tick-bite fever in six Italian tourists returning from South Africa. Eur. J. Clin. Microbiol. Infect. Dis. 21:133-136. [DOI] [PubMed] [Google Scholar]
- 63.Cascio, A., C. Colomba, S. Antinori, D. L. Paterson, and L. Titone. 2002. Clarithromycin versus azithromycin in the treatment of Mediterranean spotted fever in children: a randomized controlled trial. Clin. Infect. Dis. 34:154-158. [DOI] [PubMed] [Google Scholar]
- 64.Cascio, A., C. Colomba, D. Di Rosa, L. Salsa, L. di Martino, and L. Titone. 2001. Efficacy and safety of clarithromycin as treatment for Mediterranean spotted fever in children: a randomized controlled trial. Clin. Infect. Dis. 33:409-411. (Erratum, 33:749.) [DOI] [PubMed] [Google Scholar]
- 65.Cazorla, C., M. Enea, F. Lucht, and D. Raoult. 2003. First isolation of Rickettsia slovaca from a patient, France. Emerg. Infect. Dis. 9:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Centers for Disease Control and Prevention. 1998. African tick-bite fever among international travelers—Oregon, 1998. Morb. Mortal. Wkly. Rep. 47:950-952. [PubMed] [Google Scholar]
- 67.Chapman, S., S. M. Murphy, L. J. Demma, R. C. Holman, A. T. Curns, J. H. McQuiston, J. W. Krebs, and D. L. Swerdlow. Rocky Mountain spotted fever in the United States, 1997-2002. Am. J. Trop. Med. Hyg., in press.
- 68.Childs, J. E., and C. D. Paddock. 2002. Passive surveillance as an instrument to identify risk factors for fatal Rocky Mountain spotted fever: is there more to learn? Am. J. Trop. Med. Hyg. 66:450-457. [DOI] [PubMed] [Google Scholar]
- 69.Childs, J. E., and C. D. Paddock. 2003. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu. Rev. Entomol. 48:307-337. [DOI] [PubMed] [Google Scholar]
- 70.Chin, R. H., and I. D. Jennens. 1995. Rickettsial spotted fever in Tasmania. Med. J. Aust. 162:669. [DOI] [PubMed] [Google Scholar]
- 71.Christova, I., J. van de Pol, S. Yazar, E. Velo, and L. Schouls. 2003. Identification of Borrelia burgdorferi sensu lato, Anaplasma and Ehrlichia species, and spotted fever group Rickettsiae in ticks from southeastern Europe. Eur. J. Clin. Microbiol. Infect. Dis. 22:535-542. [DOI] [PubMed] [Google Scholar]
- 72.Conor, A., and A. Bruch. 1910. Une fièvre éruptive observée en Tunisie. Bull. Soc. Pathol. Exot. Filial. 8:492-496. [Google Scholar]
- 73.Conti-Diaz, I. A., I. Rubio, R. E. Somma Moreira, and G. Perez Bormida. 1990. Rickettsiosis cutaneo ganglionar por Rickettsia conorii en el Uruguay. Rev. Inst. Med. Trop. Sao Paulo 32:313-318. [DOI] [PubMed] [Google Scholar]
- 74.Cook, I., and R. W. Campbell. 1965. Rickettsiosis—North Queensland tick typhus. Rep. Queensland Inst. Med. Res. 20:4. [Google Scholar]
- 75.Cory, J., C. E. Yunker, J. A. Howarth, Y. Hokama, L. E. Hughes, L. A. Thomas, and C. M. Clifford. 1975. Isolation of spotted fever group and Wolbachia-like agents from field-collected materials by means of plaque formation in mammalian and mosquito cells. Acta Virol. 19:443-445. [PubMed] [Google Scholar]
- 76.Cox, G. M., and D. J. Sexton. 1995. Photo quiz: Rocky Mountain spotted fever. Clin. Infect. Dis. 21:315, 429. [PubMed] [Google Scholar]
- 77.Dasch, G. A., and L. M. Jackson. 1998. Genetic analysis of isolates of the spotted fever group of rickettsiae belonging to the R. conorii complex. Ann. N. Y. Acad. Sci. 29:11-20. [DOI] [PubMed] [Google Scholar]
- 78.Davis, G. E. 1943. Experimental transmission of the spotted fevers of the United States, Colombia, and Brazil by the argasid tick Ornithodoros parkeri. Public Health Rep. 58:1201-1208. [Google Scholar]
- 79.De Lemos, E. R. S., F. B. F. Alvarenga, M. L. Cintra, M. C. Ramos, C. D. Paddock, T. L. Ferebee, S. R. Zaki, F. C. C. Ferreira, R. C. Ravagnani, R. D. Machado, M. A. A. M. Guimaraes, and J. R. Coura. 2001. Spotted fever in Brazil: a seroepidemiological study and description of clinical cases in an endemic area in the state of Sao Paulo. Am. J. Trop. Med. Hyg. 65:329-334. [DOI] [PubMed] [Google Scholar]
- 80.De Lemos, E. R. S., R. D. Machado, and J. R. Coura. 1994. Rocky Mountain spotted fever in an endemic area in Minas Gerais, Brazil. Mem. Inst. Oswaldo Cruz 89:497-501. [DOI] [PubMed] [Google Scholar]
- 81.Demartino, G., P. Narciso, C. Struglia, F. Zechini, and G. Visco. 1981. Therapeutic experience in the treatment of Rickettsia conorii infections with a single dose of doxycycline. Clin. Ter. 97:59-62. (In Italian.) [PubMed] [Google Scholar]
- 82.Demma, L. J., M. S. Traeger, W. L. Nicholson, C. D. Paddock, D. M. Blau, M. E. Eremeeva, G. A. Dasch, M. L. Levin, J. Singleton, S. R. Zaki, J. E. Cheek, D. L. Swerdlow, and J. H. McQuiston. 2005. Rocky Mountain spotted fever from an unexpected tick vector in Arizona. N. Engl. J. Med. 353:587-594. [DOI] [PubMed] [Google Scholar]
- 83.de Rodaniche, E. C., and A. Rodaniche. 1950. Spotted fever in Panama: isolation of the etiologic agent from a fatal case. Am. J. Trop. Med. 30:511-517. [DOI] [PubMed] [Google Scholar]
- 84.de Rodaniche, E. C. 1953. Natural infection of the tick, Amblyomma cajenense with Rickettsia rickettsii in Panama. Am. J. Trop. Med. Hyg. 2:696-699. [DOI] [PubMed] [Google Scholar]
- 85.de Sousa, R., S. D. Nobrega, F. Bacellar, and J. Torgal. 2003. Mediterranean spotted fever in Portugal: risk factors for fatal outcome in 105 hospitalized patients. Ann. N. Y. Acad. Sci. 990:285-294. [DOI] [PubMed] [Google Scholar]
- 86.Dias, E., and A. V. Martins. 1939. Spotted fever in Brazil. A summary. Am. J. Trop. Med. Hyg. 19:103-108. [Google Scholar]
- 87.Diaz, I. A. 2003. Rickettsiosis caused by Rickettsia conorii in Uruguay. Ann. N. Y. Acad. Sci. 990:264-266. [DOI] [PubMed] [Google Scholar]
- 88.Drancourt, M., L. Beati, I. Tarasevich, and D. Raoult. 1992. Astrakhan fever rickettsia is identical to Israel tick typhus rickettsia, a genotype of the Rickettsia conorii complex. J. Infect. Dis. 165:1167-1168. [DOI] [PubMed] [Google Scholar]
- 89.Drancourt, M., P. J. Kelly, R. Regnery, and D. Raoult. 1992. Identification of spotted fever group rickettsiae using polymerase chain reaction and restriction-endonuclease length polymorphism analysis. Acta Virol. 36:1-6. [PubMed] [Google Scholar]
- 90.Drancourt, M., L. Tran-Hung, J. Courtin, H. Lumley, and D. Raoult. 2005. Bartonella quintana in a 4000-year-old human tooth. J. Infect. Dis. 191:607-611. [DOI] [PubMed] [Google Scholar]
- 91.Duh, D., M. Petrovec, T. Trilar, V. Punda-Polic, N. Bradaric, Z. Klismanic, and T. Avsic-Zupanc. 2003. A follow-up study on newly recognized spotted fever group rickettsiae in ticks collected in southern Croatia. Ann. N. Y. Acad. Sci. 990:149-151. [DOI] [PubMed] [Google Scholar]
- 92.Dupont, H. T., J. P. Cornet, and D. Raoult. 1994. Identification of rickettsiae from ticks collected in the Central African Republic using the polymerase chain reaction. Am. J. Trop. Med. Hyg. 50:373-380. [DOI] [PubMed] [Google Scholar]
- 93.Dwyer, B. W., S. R. Graves, M. I. McDonald, A. P. Yung, R. R. Doherty, and J. K. McDonald. 1991. Spotted fever in East Gippsland, Victoria: a previously unrecognised focus of rickettsial infection. Med. J. Aust. 154:121-125. [DOI] [PubMed] [Google Scholar]
- 94.Eremeeva, M., N. Balayeva, V. Roux, V. Ignatovich, M. Kotsinjan, and D. Raoult. 1995. Genomic and proteinic characterization of strain S, a rickettsia isolated from Rhipicephalus sanguineus ticks in Armenia. J. Clin. Microbiol. 33:2738-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Eremeeva, M. E., N. M. Balayeva, V. F. Ignatovich, and D. Raoult. 1993. Proteinic and genomic identification of spotted fever group rickettsiae isolated in the former USSR. J. Clin. Microbiol. 31:2625-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eremeeva, M. E., N. M. Balayeva, V. F. Ignatovich, and D. Raoult. 1995. Serologic response to rickettsial antigens in patients with Astrakhan fever. Eur. J. Epidemiol. 11:383-387. [DOI] [PubMed] [Google Scholar]
- 97.Eremeeva, M. E., L. Beati, V. A. Makarova, N. F. Fetisova, I. V. Tarasevich, N. M. Balayeva, and D. Raoult. 1994. Astrakhan fever rickettsiae: antigenic and genotypic analysis of isolates obtained from human and Rhipicephalus pumilio ticks. Am. J. Trop. Med. Hyg. 51:697-706. [DOI] [PubMed] [Google Scholar]
- 98.Fan, M. Y., D. H. Walker, S. R. Yu, and Q. H. Liu. 1987. Epidemiology and ecology of rickettsial diseases in the People's Republic of China. Rev. Infect. Dis. 9:823-840. [PubMed] [Google Scholar]
- 99.Fan, M. Y., J. Z. Zhang, M. Chen, and X. J. Yu. 1999. Spotted fever group rickettsioses in China, p. 247-257. In D. Raoult and P. Brouqui (ed.), Rickettsiae and rickettsial diseases at the turn of the third millenium. Elsevier, Paris, France.
- 100.Feng, W. C., E. S. Murray, W. Burgdorfer, J. M. Spielman, G. Rosenberg, K. Dang, C. Smith, C. Spickert, and J. L. Waner. 1980. Spotted fever group rickettsiae in Dermacentor variabilis from Cape Cod, Massachusetts. Am. J. Trop. Med. Hyg. 29:691-694. [DOI] [PubMed] [Google Scholar]
- 101.Fernández-Soto, P., A. Encinas-Grandes, and R. Pérez-Sánchez. 2003. Rickettsia aeschlimannii in Spain: molecular evidence in Hyalomma marginatum and five other tick species that feed on humans. Emerg. Infect. Dis. 9:889-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Font-Creus, B., F. Bella-Cueto, E. Espejo-Arenas, R. Vidal-Sanahuja, T. Munoz-Espin, M. Nolla-Salas, A. Casagran-Borrell, J. Mercade-Cuesta, and F. Segura-Porta. 1985. Mediterranean spotted fever: a cooperative study of 227 cases. Rev. Infect. Dis. 7:635-642. [DOI] [PubMed] [Google Scholar]
- 103.Fournier, P. E., C. Allombert, Y. Supputamongkol, G. Caruso, P. Brouqui, and D. Raoult. 2004. Aneruptive fever associated with antibodies to Rickettsia helvetica in Europe and Thailand. J. Clin. Microbiol. 42:816-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fournier, P. E., J. S. Dumler, G. Greub, J. Zhang, Y. Wu, and D. Raoult. 2003. Gene sequence-based criteria for identification of new rickettsia isolates and description of Rickettsia heilongjiangensis sp. nov. J. Clin. Microbiol. 41:5456-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fournier, P. E., J. P. Durand, J. M. Rolain, J. L. Camicas, H. Tolou, and D. Raoult. 2003. Detection of Astrakhan fever rickettsia from ticks in Kosovo. Ann. N. Y. Acad. Sci. 990:158-161. [DOI] [PubMed] [Google Scholar]
- 106.Fournier, P. E., H. Fujita, N. Takada, and D. Raoult. 2002. Genetic identification of rickettsiae isolated from ticks in Japan. J. Clin. Microbiol. 40:2176-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fournier, P. E., F. Gouriet, P. Brouqui, F. Lucht, and D. Raoult. 2005. Lymphangitis-associated rickettsiosis (LAR), a new rickettsiosis caused by Rickettsia sibirica mongolotimonae. Seven new cases and review of the literature. Clin. Infect. Dis. 40:1435-1444. [DOI] [PubMed] [Google Scholar]
- 108.Fournier, P. E., F. Grunnenberger, B. Jaulhac, G. Gastinger, and D. Raoult. 2000. Evidence of Rickettsia helvetica infection in humans, eastern France. Emerg. Infect. Dis. 6:389-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fournier, P. E., D. Raoult. 2004. Suicide PCR on skin biopsy specimens for diagnosis of rickettsioses. J. Clin. Microbiol. 42:3428-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fournier, P. E., V. Roux, E. Caumes, M. Donzel, and D. Raoult. 1998. Outbreak of Rickettsia africae infections in participants of an adventure race in South Africa. Clin. Infect. Dis. 27:316-323. [DOI] [PubMed] [Google Scholar]
- 111.Fournier, P. E., V. Roux, and D. Raoult. 1998. Phylogenetic analysis of spotted fever group rickettsiae by study of the outer surface protein rOmpA. Int. J. Syst. Bacteriol. 48:839-849. [DOI] [PubMed] [Google Scholar]
- 112.Fournier, P. E., H. Tissot-Dupont, H. Gallais, and D. R. Raoult. 2000. Rickettsia mongolotimonae: a rare pathogen in France. Emerg. Infect. Dis. 6:290-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fournier, P. E., B. Xeridat, and D. Raoult. 2003. Isolation of a rickettsia related to Astrakhan fever rickettsia from a patient in Chad. Ann. N. Y. Acad. Sci. 990:152-157. [DOI] [PubMed] [Google Scholar]
- 114.Fournier, P. E., Y. Zhu, H. Ogata, and D. Raoult. 2004. Use of highly variable intergenic spacer sequences for multispacer typing of Rickettsia conorii strains. J. Clin. Microbiol. 42:5757-5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fuentes, L. G. 1979. Primer caso de fiebre de las Montanas Rocasas en Costa Rica, America Central. Rev. Latinoam. Microbiol. 21:167-172. [PubMed] [Google Scholar]
- 116.Gage, K., M. E. Schrumpf, R. H. Karstens, W. Burgdorfer, and T. G. Schwan. 1994. DNA typing of rickettsiae in naturally infected ticks using a polymerase chain reaction/restriction fragment length polymorphism system. Am. J. Trop. Med. Hyg. 50:260. [DOI] [PubMed] [Google Scholar]
- 117.Galvao, M. A., J. S. Dumler, S. B. Calic, C. B. Chamone, G. Cesarino Filho, J. P. Olano, and D. H. Walker. 2003. Fatal spotted fever rickettsiosis, Minas Gerais, Brazil. Emerg. Infect. Dis. 9:1402-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Galvao, M. A., C. L. Mafra, C. Moron, E. Anaya, and D. H. Walker. 2003. Rickettsiosis of the genus Rickettsia in South America. Ann. N. Y. Acad. Sci. 990:57-61. [DOI] [PubMed] [Google Scholar]
- 119.Gear, J. H. S. 1938. South African typhus. S. Afr. J. Med. Sci. 3:134-160. [Google Scholar]
- 120.Giammanco, G. M., S. Mansueto, P. Ammatuna, and G. Vitale. 2003. Israeli spotted fever Rickettsia in Sicilian Rhipicephalus sanguineus ticks. Emerg. Infect. Dis. 9:892-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gilot, B., M. L. Laforge, J. Pichot, and D. Raoult. 1990. Relationships between the Rhipicephalus sanguineus complex ecology and Mediterranean spotted fever epidemiology in France. Eur. J. Epidemiol. 6:357-362. [DOI] [PubMed] [Google Scholar]
- 122.Gimenez, D. F. 1964. Staining rickettsiae in yolk-sac cultures. Stain Technol. 39:135-140. [DOI] [PubMed] [Google Scholar]
- 123.Goddard, J. 1988. Was Bullis fever actually ehrlichiosis? JAMA 260:3006-3007. [PubMed] [Google Scholar]
- 124.Goddard, J. 2003. Experimental infection of lone star ticks, Amblyomma americanum (L.), with Rickettsia parkeri and exposure of guinea pigs to the agent. J. Med. Entomol. 40:686-689. [DOI] [PubMed] [Google Scholar]
- 125.Goddard, J., and B. R. Norment. 1986. Spotted fever group Rickettsiae in the Lone Star tick, Amblyomma americanum (Acari, Ixodidae). J. Med. Entomol. 23:465-472. [DOI] [PubMed] [Google Scholar]
- 126.Gouin, E., C. Egile, P. Dehoux, V. Villiers, J. Adams, F. Gertler, R. Li, and P. Cossart. 2004. The RickA protein of Rickettsia conorii activates the Arp2/3 complex. Nature 27:457-461. [DOI] [PubMed] [Google Scholar]
- 127.Graves, S., and J. Stenos. 1999. Rickettsioses in Australia, p. 244-246. In D. Raoult and P. Brouqui (ed.), Rickettsiae and rickettsial diseases at the turn of the third millenium. Elsevier, Paris, France.
- 128.Graves, S., and J. Stenos. 2003. Rickettsia honei—a spotted fever group rickettsia on three continents. Ann. N. Y. Acad. Sci. 990:62-66. [DOI] [PubMed] [Google Scholar]
- 129.Graves, S. R., L. Stewart, J. Stenos, R. S. Stewart, E. Schmidt, J. Banks, Z. Huang, and B. Dwyer. 1993. Spotted fever group rickettsial infection in south-eastern Australia: isolation of rickettsiae. Comp. Immunol. Microbiol. Infect. Dis. 16:223-233. [DOI] [PubMed] [Google Scholar]
- 130.Gross, E. M., and P. Yagupsky. 1987. Israeli rickettsial spotted fever in children. A review of 54 cases. Acta Trop. 44:91-96. [PubMed] [Google Scholar]
- 131.Harrell, G. T. 1949. Rocky Mountain spotted fever. Medicine (Baltimore) 28:333-370. [DOI] [PubMed] [Google Scholar]
- 132.Hayes, S. F., and W. Burgdorfer. 1979. Ultrastructure of Rickettsia rhipicephali, a new member of the spotted fever group rickettsiae in tissues of the host vector Rhipicephalus sanguineus. J. Bacteriol. 137:605-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hazard, G. W., R. N. Ganz, R. W. Nevin, A. H. Nauss, E. Curtis, W. J. Bell, and E. S. Murray. 1969. Rocky Mountain spotted fever in the eastern United States. Thirteen cases from the Cape Cod area of Massachusetts. N. Engl. J. Med. 280:57-62. [DOI] [PubMed] [Google Scholar]
- 134.Hechemy, K. E., T. Avsic-Zupanc, J. E. Childs, and D. A. Raoultd. 2003. Rickettsiology: present and future directions—preface. Ann. N. Y. Acad. Sci. 990:XVII-XVXX. [DOI] [PubMed] [Google Scholar]
- 135.Hemmersbach-Miller, M., P. Parola, P. Brouqui, and D. Raoult. 2004. A 51-year-old homeless man who died in Marseille, Southern France. Clin. Infect. Dis. 38:1412, 1493-1494. [DOI] [PubMed] [Google Scholar]
- 136.Hirunkanokpun, S., P. Kittayapong, J. P. Cornet, and J. P. Gonzalez. 2003. Molecular evidence for novel tick-associated spotted fever group rickettsiae from Thailand. J. Med. Entomol. 40:230-237. [DOI] [PubMed] [Google Scholar]
- 137.Holman, R. C., C. D. Paddock, A. T. Curns, J. W. Krebs, J. H. McQuiston, and J. E. Childs. 2001. Analysis of risk factors for fatal rocky mountain spotted fever: evidence for superiority of tetracyclines for therapy. J. Infect. Dis. 184:1437-1444. [DOI] [PubMed] [Google Scholar]
- 138.Horta, M. C., M. B. Labruna, L. A. Sanguioni, M. C. Vianna, S. M. Gennari, M. A. Galvao, C. L. Mafra, O. Vidotto, T. T. Schumaker, and D. H. Walker. 2004. Prevalence of antibodies to spotted fever group rickettsiae in humans and domestic animals in a Brazilian spotted fever-endemic area in the state of Sao Paulo, Brazil: serologic evidence for infection by Rickettsia rickettsii and another spotted fever group Rickettsia. Am. J. Trop. Med. Hyg. 71:93-97. [PubMed] [Google Scholar]
- 139.Hugues, L. E., C. M. Clifford, R. Gresbrink, L. A. Thomas, and J. E. Keirans. 1976. Isolation of a spotted fever group rickettsia from the Pacific Coast tick, Ixodes pacificus, in Oregon. Am. J. Trop. Med. Hyg. 25:513-516. [DOI] [PubMed] [Google Scholar]
- 140.Ignatovich, V. F. 1977. Antigenic relations of Rickettsia prowazekii and Rickettsia canada, established in the study of sera of patients with Brill's disease. J. Hyg. Epidemiol. Microbiol. Immunol. 21:55-60. [PubMed] [Google Scholar]
- 141.Inokuma, H., H. Takahata, P. E. Fournier, P. Brouqui, D. Raoult, M. Okuda, T. Onishi, K. Nishioka, and M. Tsukahara. 2003. Tick paralysis by Ixodes holocyclus in a Japanese traveler returning from Australia associated with Rickettsia helvetica infection. J. Travel Med. 10:61-63. [DOI] [PubMed] [Google Scholar]
- 142.Isaac, R., G. M. Varghese, E. Mathai, J. Manjula, and I. Joseph. 2004. Scrub typhus: prevalence and diagnostic issues in rural Southern India. Clin. Infect. Dis. 39:1395-1396. [DOI] [PubMed] [Google Scholar]
- 143.Jayaseelan, E., S. C. Rajendran, S. Shariff, D. Fishbein, and J. S. Keystone. 1991. Cutaneous eruptions in Indian tick typhus. Int. J. Dermatol. 30:790-794. [DOI] [PubMed] [Google Scholar]
- 144.Jelinek, T., and T. Löscher. 2001. Clinical features and epidemiology of tick typhus in travelers. J. Travel Med. 8:57-59. [DOI] [PubMed] [Google Scholar]
- 145.Jensenius, M., P. E. Fournier, P. Kelly, B. Myrvang, and D. Raoult. 2003. African tick bite fever. Lancet Infect. Dis. 3:557-564. [DOI] [PubMed] [Google Scholar]
- 146.Jensenius, M., P. E. Fournier, S. Vene, T. Hoel, G. Hasle, A. Z. Henriksen, K. B. Hellum, D. Raoult, and B. Myrvang. 2003. African tick bite fever in travelers to rural sub-Equatorial Africa. Clin. Infect. Dis. 36:1411-1417. [DOI] [PubMed] [Google Scholar]
- 147.Jensenius, M., T. Hoel, D. Raoult, P. E. Fournier, H. Kjelshus, A. L. Bruu, and B. Myrvang. 2002. Seroepidemiology of Rickettsia africae infection in Norwegian travellers to rural Africa. Scand. J. Infect. Dis. 34:93-96. [DOI] [PubMed] [Google Scholar]
- 148.Katayama, T., Y. Yoshida, and I. Kaiho. 1996. Spotted fever group rickettsiosis and vectors in Kanagawa prefecture. Kansenshogaku Zasshi 70:561-568. (In Japanese.) [DOI] [PubMed] [Google Scholar]
- 149.Kelly, D. J., A. L. Richards, J. Temenak, D. Strickman, and G. A. Dasch. 2002. The past and present threat of rickettsial diseases to military medicine and international public health. Clin. Infect. Dis. 34:S145-S169. [DOI] [PubMed] [Google Scholar]
- 150.Kelly, P., L. Matthewman, L. Beati, D. Raoult, P. Mason, M. Dreary, and R. Makombe. 1992. African tick-bite fever: a new spotted fever group rickettsiosis under an old name. Lancet 340:982-983. [DOI] [PubMed] [Google Scholar]
- 151.Kelly, P. J., L. Beati, P. R. Mason, L. A. Matthewman, V. Roux, and D. Raoult. 1996. Rickettsia africae sp. nov., the etiological agent of African tick bite fever. Int. J. Syst. Bacteriol. 46:611-614. [DOI] [PubMed] [Google Scholar]
- 152.Kelly, P. J., L. Beati, L. A. Matthewman, P. R. Mason, G. A. Dasch, and D. Raoult. 1994. A new pathogenic spotted fever group rickettsia from Africa. J. Trop. Med. Hyg. 97:129-137. [PubMed] [Google Scholar]
- 153.Kelly, P. J., P. E. Fournier, P. Parola, and D. Raoult. 2003. A survey for spotted fever group rickettsiae and ehrlichiae in Amblyomma variegatum from St. Kitts and Nevis. Am. J. Trop. Med. Hyg. 69:58-59. [PubMed] [Google Scholar]
- 154.Kelly, P. J., and P. Mason. 1991. Transmission of a spotted fever group rickettsia by Amblyomma hebraeum (Acari: Ixodidae). J. Med. Entomol. 28:596-600. [DOI] [PubMed] [Google Scholar]
- 155.Kelly, P. J., D. Raoult, and P. R. Mason. 1991. Isolation of spotted fever group rickettsias from triturated ticks using a modification of the centrifugation-shell vial technique. Trans. R. Soc. Trop. Med. Hyg. 85:397-398. [DOI] [PubMed] [Google Scholar]
- 156.King, W. W. 1906. Experimental transmission of Rocky Mountain spotted fever by means of the tick. Public Health Rep. 21:863-864. [PubMed] [Google Scholar]
- 157.Kish, M. A. 2001. Guide to development of practice guidelines. Clin. Infect. Dis. 32:851-854. [DOI] [PubMed] [Google Scholar]
- 158.Kodama, K., T. Senba, H. Yamauchi, Y. Chikahira, and H. Fujita. 2001. A patient with Japanese spotted fever complicated by meningoencephalitis. Kansenshogaku Zasshi 75:812-814. [DOI] [PubMed] [Google Scholar]
- 159.Kodama, K., T. Senba, H. Yamauchi, Y. Chikahira, and H. Fujita. 2001. Japanese spotted fever associated with multiorgan failure. J. Infect. Chemother. 7:247-250. [DOI] [PubMed] [Google Scholar]
- 160.Kodama, K., T. Senba, H. Yamauchi, Y. Chikahira, T. Katayama, Y. Furuya, H. Fujita, and S. Yamamoto. 2002. Fulminant Japanese spotted fever definitively diagnosed by the polymerase chain reaction method. J. Infect. Chemother. 8:266-268. [DOI] [PubMed] [Google Scholar]
- 161.Kodama, K., T. Senba, H. Yamauchi, T. Nomura, and Y. Chikahira. 2003. Clinical study of Japanese spotted fever and its aggravating factors. J. Infect. Chemother. 9:83-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kollars, T. M., Jr., B. Tippayachai, and D. Bodhidatta. 2001. Short report: Thai tick typhus, Rickettsia honei, and a unique rickettsia detected in Ixodes granulatus (Ixodidae: Acari) from Thailand. Am. J. Trop. Med. Hyg. 65:535-537. [DOI] [PubMed] [Google Scholar]
- 163.Komitova, R., A. Lakos, A. Aleksandrov, I. Christova, and M. Murdjeva. 2003. A case of tick-transmitted lymphadenopathy in Bulgaria associated with Rickettsia slovaca. Scand. J. Infect. Dis. 35:213. [DOI] [PubMed] [Google Scholar]
- 164.Kularatne, S. A., J. S. Edirisingha, I. B. Gawarammana, H. Urakami, M. Chenchittikul, and I. Kaiho. 2003. Emerging rickettsial infections in Sri Lanka: the pattern in the hilly Central Province. Trop. Med. Int. Health 8:803-811. [DOI] [PubMed] [Google Scholar]
- 165.Kuloglu, F., J. M. Rolain, P. E. Fournier, F. Akata, M. Tugrul, and D. Raoult. 2004. First isolation of Rickettsia conorii from human in the Trakya region (European part) of Turkey. Eur. J. Clin. Microbiol. Infect. Dis. 23:609-614. [DOI] [PubMed] [Google Scholar]
- 166.Labruna, M. B., T. Whitworth, M. C. Horta, D. H. Bouyer, J. W. McBride, A. Pinter, V. Popov, S. M. Gennari, and D. H. Walker. 2004. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J. Clin. Microbiol. 42:90-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Labruna, M. B., D. H. Bouyer, J. W. McBride, L. M. A. Camargo, E. P. Camargo, and D. H. Walker. 2004. Molecular evidence for a spotted fever group Rickettsia species in the tick Amblyomma longirostre in Brazil. J. Med. Entomol. 41:533-537. [DOI] [PubMed] [Google Scholar]
- 168.Labruna, M. B., C. D. Paula, T. F. Lima, and D. A. Sana. 2002. Ticks (Acari: Ixodidae) on wild animals from the Porto-Primavera hydroelectric power station area, Brazil. Mem. Inst. Oswaldo Cruz 97:1133-1136. [DOI] [PubMed] [Google Scholar]
- 169.Labruna, M. B., T. Whitworth, D. H. Bouyer, J. W. McBride, L. M. A. Camargo, E. P. Camargo, V. Popov, and D. H. Walker. 2004. Rickettsia bellii and Rickettsia amblyommii in Amblyomma ticks from the state of Rondonia, Western Amazon, Brazil. J. Med. Entomol. 41:1073-1081. [DOI] [PubMed] [Google Scholar]
- 170.Lackman, D. B., E. J. Bell, H. G. Stoenner, and E. G. Pickens. 1965. The Rocky Mountain spotted fever group rickettsias. Health Lab. Sci. 2:135-141. [PubMed] [Google Scholar]
- 171.Lackman, D. B., R. N. Philip, E. A. Casper, J. F. Bell, J. B. Enright, and E. G. Pickens. 1967. Q fever immunity in man. Health Lab. Sci. 4:236-244. [PubMed] [Google Scholar]
- 172.Lambert, M., T. Dugernier, G. Bigaignon, J. Rahier, and P. Piot. 1984. Mediterranean spotted-fever in Belgium. Lancet ii:1038. [DOI] [PubMed] [Google Scholar]
- 173.Lane, R. S., R. N. Philip, and E. A. Casper. 1981. Ecology of tick-borne agents in California. III. Further observations on rickettsiae, p. 575-584. In W. Burgdorfer and R. L. Anacker (ed.), Rickettsiae and rickettsial diseases. Academic Press, New York, N.Y.
- 174.La Scola, B., and D. Raoult. 1997. Laboratory diagnosis of rickettsioses: current approaches to diagnosis of old and new rickettsial diseases. J. Clin. Microbiol. 35:2715-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Leitner, M., S. Yitzhaki, S. Rzotkiewicz, and A. Keysary. 2002. Polymerase chain reaction-based diagnosis of Mediterranean spotted fever in serum and tissue samples. Am. J. Trop. Med. Hyg. 67:166-169. [DOI] [PubMed] [Google Scholar]
- 176.Letaief, A., J. Souissi, H. Trabelsi, H. Ghannem, and L. Jemni. 2003. Evaluation of clinical diagnosis scores for Boutonneuse fever. Ann. N. Y. Acad. Sci. 990:327-330. [DOI] [PubMed] [Google Scholar]
- 177.Lewin, M. R., D. H. Bouyer, D. H. Walker, and D. M. Musher. 2003. Rickettsia sibirica infection in members of scientific expeditions to northern Asia. Lancet 362:1201-1202. [DOI] [PubMed] [Google Scholar]
- 178.Li, H., and D. H. Walker. 1998. rOmpA is a critical protein for the adhesion of Rickettsia rickettsii to host cells. Microb. Pathog. 24:289-298. [DOI] [PubMed] [Google Scholar]
- 179.Linnemann, C. C., Jr., C. I. Pretzman, and E. D. Peterson. 1989. Acute febrile cerebrovasculitis. A non-spotted fever group rickettsial disease. Arch. Intern. Med. 149:1682-1684. [DOI] [PubMed] [Google Scholar]
- 180.Livesay, H. R., and M. Pollard. 1943. Laboratory report on a clinical syndrome referred to as “Bullis fever.” Am. J. Trop. Med. 23:475-479. [Google Scholar]
- 181.Macaluso, K. R., J. Davis, U. Alam, A. Korman, J. S. Rutherford, R. Rosenberg, and A. F. Azad. 2003. Spotted fever group rickettsiae in ticks from the Masai Mara region of Kenya. Am. J. Trop. Med. Hyg. 68:551-553. [DOI] [PubMed] [Google Scholar]
- 182.Macaluso, K. R., D. E. Sonenshine, S. M. Ceraul, and A. F. Azad. 2002. Rickettsial infection in Dermacentor variabilis (Acari: Ixodidae) inhibits transovarial transmission of a second Rickettsia. J. Med. Entomol. 39:809-813. [DOI] [PubMed] [Google Scholar]
- 183.Mahara, F. 1984. Three Weil-Felix reaction OX2 positive cases with skin eruptions and high fever. J. Anan Med. Assoc. 68:4-7. [Google Scholar]
- 184.Mahara, F. 1997. Japanese spotted fever: report of 31 cases and review of the literature. Emerg. Infect. Dis. 3:105-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Mahara, F. 1999. Rickettsioses in Japan, p. 233-239. In D. Raoult and P. Brouqui (ed.), Rickettsiae and rickettsial diseases at the turn of the third millenium. Elsevier, Paris, France.
- 186.Mahara, F., K. Koga, S. Sawada, T. Taniguchi, F. Shigemi, T. Suto, Y. Tsuboi, A. Ooya, H. Koyama, T. Uchiyama, and T. Uchida. 1985. The first report of the rickettsial infections of spotted fever group in Japan; three clinical cases. Jpn. J. Assoc. Infect. Dis. 59:1165-1172. [DOI] [PubMed] [Google Scholar]
- 187.Malek, J. A., J. M. Wierzbowski, W. Tao, S. A. Bosak, D. J. Saranga, L. Doucette-Stamm, D. R. Smith, P. J. Mcewan, and K. J. McKernan. 2004. Protein interaction mapping on a functional shotgun sequence of Rickettsia sibirica. Nucleic Acids Res. 32:1059-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mansueto, S., G. Tringali, and D. H. Walker. 1986. Widespread, simultaneous increase in the incidence of spotted fever group rickettsioses. J. Infect. Dis. 154:539-540. [DOI] [PubMed] [Google Scholar]
- 189.Mariotte, C. O., M. E. Bustamente, and G. Varela. 1944. Hallazago del Rhipicephalus sanguineus Latreille infectado naturalmente con fiebre manchada de las Montanas Rocosas, en Sonora (Mexico) 1944. Rev. Inst. Salub. Enferm. Trop. 5:297-300. [Google Scholar]
- 190.Marquez, F. J., V. Ibarra, J. A. Oteo, and M. A. Muniain. 2003. Which spotted fever group Rickettsia are present in Dermacentor marginatus ticks in Spain? Ann. N. Y. Acad. Sci. 990:141-142. [DOI] [PubMed] [Google Scholar]
- 191.Marquez, F. J., M. A. Muniain, R. C. Soriguer, G. Izquierdo, J. Rodriguez-Bano, and M. V. Borobio. 1998. Genotypic identification of an undescribed spotted fever group rickettsia in Ixodes ricinus from southwestern Spain. Am. J. Trop. Med. Hyg. 58:570-577. [DOI] [PubMed] [Google Scholar]
- 192.Marshall, G. S., G. G. Stout, R. F. Jacobs, G. E. Schutze, H. Paxton, S. C. Buckingham, J. P. DeVincenzo, M. A. Jackson, V. H. San Joaquin, S. M. Standaert, C. R. Woods, and and the Tick-Borne Infections in Children Study Group. 2003. Antibodies reactive to Rickettsia rickettsii among children living in the southeast and south central regions of the United States. Arch. Pediatr. Adolesc. Med. 157:443-448. [DOI] [PubMed] [Google Scholar]
- 193.Massung, R. F., L. E. Davis, K. Slater, D. B. McKechnie, and M. Puerzer. 2001. Epidemic typhus meningitis in the southwestern United States. Clin. Infect. Dis. 32:979-982. [DOI] [PubMed] [Google Scholar]
- 194.Masters, E. J., G. S. Olson, S. J. Weiner, and C. D. Paddock. 2003. Rocky mountain spotted fever—a clinician's dilemma. Arch. Intern. Med. 163:769-774. [DOI] [PubMed] [Google Scholar]
- 195.Mathai, E., G. Lloyd, T. Cherian, O. C. Abraham, and A. M. Cherian. 2001. Serological evidence for the continued presence of human rickettsioses in southern India. Ann. Trop. Med. Parasitol. 95:395-398. [DOI] [PubMed] [Google Scholar]
- 196.Matsumoto, K., P. Brouqui, D. Raoult, and P. Parola. 2005. Transmission of Rickettsia massiliae in the tick, Rhipicephalus turanicus. Med. Vet. Entomol. 19:263-270. [DOI] [PubMed] [Google Scholar]
- 197.Matsumoto, K., P. Parola, P. Brouqui, and D. Raoult. 2004. Rickettsia aeschlimannii in Hyalomma ticks from Corsica. Eur. J. Clin. Microbiol. Infect. Dis. 23:732-734. [DOI] [PubMed] [Google Scholar]
- 198.Maxey, E. E. 1899. Some observations of the so-called spotted fever of Idaho. Med. Sentinel 10:433-438. [Google Scholar]
- 199.McCall, C. L., A. T. Curns, L. D. Rotz, J. A. Singleton, Jr., T. Treadwell, J. A. Comer, W. L. Nicholson, J. G. Olson, and J. E. Childs. 2001. Fort Chaffee revisited: the epidemiology of tick-borne rickettsial and ehrlichial diseases at a natural focus. Vector Borne Zoonotic Dis. 1:119-127. [DOI] [PubMed] [Google Scholar]
- 200.McDade, J. E., and V. F. Newhouse. 1986. Natural history of Rickettsia rickettsii. Annu. Rev. Microbiol. 40:287-309. [DOI] [PubMed] [Google Scholar]
- 201.McKiel, J. A. 1950. The rodent and avian-borne diseases in Canada. Can. J. Public Health 51:220-225. [Google Scholar]
- 202.McKiel, Y. A., E. J. Bell, and D. B. Lackman. 1967. Rickettsia canada: a new member of the typhus group of rickettsiae isolated from Haemaphysalis leporispalustris ticks in Canada. Can. J. Microbiol. 13:503-510. [DOI] [PubMed] [Google Scholar]
- 203.Mcleod, M. P., X. Qin, S. E. Karpathy, J. Gioia, S. K. Highlander, G. E. Fox, T. Z. McNeill, H. Y. Jiang, D. Muzny, L. S. Jacob, A. C. Hawes, E. Sodergren, R. Gill, J. Hume, M. Morgan, G. W. Fan, A. G. Amin, R. A. Gibbs, C. Hong, X. J. Yu, D. H. Walker, and G. M. Weinstock. 2004. Complete genome sequence of Rickettsia typhi and comparison with sequences of other rickettsiae. J. Bacteriol. 186:5842-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.McNaught, J. G. 1911. A tick-borne fever in the Union of South Africa. J. R. Army Med. Corps 16:505. [Google Scholar]
- 205.McQuiston, J. H., C. D. Paddock, J. Singleton, J. T. Wheeling, S. R. Zaki, and J. E. Childs. 2004. Imported spotted fever rickettsioses in United States travelers returning form Africa: a summary of cases confirmed by laboratory testing at the Centers for Disease Control and Prevention, 1999-2002. Am. J. Trop. Med. Hyg. 70:98-101. [PubMed] [Google Scholar]
- 206.Mediannikov, O., T. Sidelnikov, L. Ivanov, E. Mokretsova, P. E. Fournier, I. Tarasevich, and D. Raoult. 2004. Acute tick-borne rickettsiosis, caused by Rickettsia heilongjiangensis in the Russian Far East. Emerg. Infect. Dis. 10:810-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Melles, H. H., S. Colombo, and M. V. da Silva. 1992. Spotted fever: isolation of Rickettsia from a skin biopsy sample. Rev. Inst. Med. Trop. Sao Paulo 34:37-41. (In Portuguese.) [PubMed] [Google Scholar]
- 208.Meloni, G., and T. Meloni. 1996. Azithromycin vs. doxycycline for Mediterranean spotted fever. Pediatr. Infect. Dis. J. 15:1042-1044. [DOI] [PubMed] [Google Scholar]
- 209.Morel, P. C. 1959. Les Hyalomma (Acariens, Ixodidae) de France. Ann. Parasitol. 34:552-555. [PubMed] [Google Scholar]
- 210.Mulenga, A., K. R. Macaluso, J. A. Simser, and A. F. Azad. 2003. Dynamics of Rickettsia-tick interactions: identification and characterization of differentially expressed mRNAs in uninfected and infected Dermacentor variabilis. Insect Mol. Biol. 12:185-193. [DOI] [PubMed] [Google Scholar]
- 211.Murali, N., S. Pillai, T. Cherian, P. Raghupathy, V. Padmini, and E. Mathai. 2001. Rickettsial infections in South India—how to spot the spotted fever. Indian Pediatr. 38:1393-1396. [PubMed] [Google Scholar]
- 212.Ndip, L. M., D. H. Bouyer, A. P. A. T. Da Rosa, V. P. K. Titanji, R. B. Tesh, and D. H. Walker. 2004. Acute spotted fever rickettsiosis among febrile patients, Cameroon. Emerg. Infect. Dis. 10:432-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ndip, L. M., E. B. Fokam, D. H. Bouyer, R. N. Ndip, V. P. Titanji, D. H. Walker, and J. W. McBride. 2004. Detection of Rickettsia africae in patients and ticks along the coastal region of Cameroon. Am. J. Trop. Med. Hyg. 71:363-366. [PubMed] [Google Scholar]
- 214.Niebylski, M. L., M. G. Peacock, and T. G. Schwan. 1999. Lethal effect of Rickettsia rickettsii on its tick vector (Dermacentor andersoni). Appl. Environ. Microbiol. 65:773-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Niebylski, M. L., M. E. Schrumpf, W. Burgdorfer, E. R. Fischer, K. L. Gage, and T. G. Schwan. 1997. Rickettsia peacockii sp. nov., a new species infecting wood ticks, Dermacentor andersoni, in Western Montana. Int. J. Syst. Bacteriol. 47:446-452. [DOI] [PubMed] [Google Scholar]
- 216.Nilsson, K., O. Lindquist, A. J. Liu, T. G. Jaenson, G. Friman, and C. Pahlson. 1999. Rickettsia helvetica in Ixodes ricinus ticks in Sweden. J. Clin. Microbiol. 37:400-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Nilsson, K., O. Lindquist, and C. Pahlson. 1999. Association of Rickettsia helvetica with chronic perimyocarditis in sudden cardiac death. Lancet 354:1169-1173. [DOI] [PubMed] [Google Scholar]
- 218.Nilsson, K., C. Pahlson, A. Lukinius, L. Eriksson, L. Nilsson, and O. Lindquist. 2002. Presence of Rickettsia helvetica in granulomatous tissue from patients with sarcoidosis. J. Infect. Dis. 185:1128-1138. [DOI] [PubMed] [Google Scholar]
- 219.Norment, B. R., and W. Burgdorfer. 1984. Susceptibility and reservoir potential of the dog to spotted fever-group rickettsiae. Am. J. Vet. Res. 45:1710. [PubMed] [Google Scholar]
- 220.Ogata, H., S. Audic, P. Renesto-Audiffren, P. E. Fournier, V. Barbe, D. Samson, V. Roux, P. Cossart, J. Weissenbach, J. M. Claverie, and D. Raoult. 2001. Mechanisms of evolution in Rickettsia conorii and R. prowazekii. Science 293:2093-2098. [DOI] [PubMed] [Google Scholar]
- 221.Ogata, H., S. Audic, C. Abergel, P. E. Fournier, and J. M. Claverie. 2002. Protein coding palindromes are a unique but recurrent feature in Rickettsia. Genome Res. 12:808-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ogata, H., S. Audic, V. Barbe, F. Artiguenave, P. E. Fournier, D. Raoult, and J. M. Claverie. 2000. Selfish DNA in protein-coding genes of Rickettsia. Science 290:347-350. [DOI] [PubMed] [Google Scholar]
- 223.Olmer, D. 1925. Sur une infection épidémique, avec exanthème de nature indéterminée. Mars. Med. 22:1291-1293. [Google Scholar]
- 224.O'Reilly, M., C. Paddock, B. Elchos, J. Goddard, J. Childs, and M. Currie. 2003. Physician knowledge of the diagnosis and management of Rocky Mountain spotted fever—Mississippi, 2002. Ann. N. Y. Acad. Sci. 990:295-301. [DOI] [PubMed] [Google Scholar]
- 225.Oteo, J. A., V. Ibarra, J. R. Blanco, V. Martinez de Artola, A. Portillo, D. Raoult, and P. Anda. 2004. Dermacentor-borne necrosis erythema and lymphadenopathy: clinical and epidemiological features of a new tick-borne disease. Clin. Microbiol. Infect. 10:327-331. [DOI] [PubMed] [Google Scholar]
- 226.Oteo, J. A., V. Ibarra, J. R. Blanco, M. Vallejo, and V. M. De Artola. 2003. Epidemiological and clinical differences among Rickettsia slovaca rickettsiosis and other tick-borne diseases in Spain. Ann. N. Y. Acad. Sci. 990:355-356. [DOI] [PubMed] [Google Scholar]
- 227.Paddock, C. D., O. Brenner, C. Vaid, D. B. Boyd, J. M. Berg, R. J. Joseph, S. R. Zaki, and J. E. Childs. 2002. Short report: concurrent Rocky Mountain spotted fever in a dog and its owner. Am. J. Trop. Med. Hyg. 66:197-199. [DOI] [PubMed] [Google Scholar]
- 228.Paddock, C. D., P. W. Greer, T. L. Ferebee, J. Singleton, Jr., D. B. McKechnie, T. A. Treadwell, J. W. Krebs, R. C. Holman, J. G. Olson, J. E. Childs, and S. R. Zaki. 1999. Hidden mortality attributable to Rocky Mountain spotted fever: immunohistochemical detection of fatal, serologically unconfirmed disease. J. Infect. Dis. 179:1469-1476. [DOI] [PubMed] [Google Scholar]
- 229.Paddock, C. D., R. C. Holman, J. W. Krebs, and J. E. Childs. 2002. Assessing the magnitude of fatal Rocky Mountain spotted fever in the United States: comparison of two national data sources. Am. J. Trop. Med. Hyg. 67:349-354. [DOI] [PubMed] [Google Scholar]
- 230.Paddock, C. D., J. W. Sumner, J. A. Comer, S. R. Zaki, C. C. Goldsmith, J. Goddard, S. L. F. McLellan, C. L. Tamminga, and C. A. Ohl. 2004. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 38:805-811. [DOI] [PubMed] [Google Scholar]
- 231.Parker, R. R., J. F. Bell, W. S. Chalgren, F. B. Thrailkill, and M. T. McKee. 1952. The recovery of starians of Rocky Mountain spotted fever and tularemia from ticks of the eastern United States. J. Infect. Dis. 91:231-237. [DOI] [PubMed] [Google Scholar]
- 232.Parker, R. R., and G. E. Davis. 1933. Protective value of covalescent sera of Sao Paulo exanthematic typhus against virus of Rocky Mountain spotted fever. Public Health Rep. 48:501-507. [Google Scholar]
- 233.Parker, R. R., G. M. Kohls, G. W. Cox, and G. E. Davis. 1939. Observations on an infectious agent from Amblyomma maculatum. Public Health Rep. 54:1482-1484. [Google Scholar]
- 234.Parker, R. R., G. M. Kohls, and E. A. Steinhaus. 1943. Rocky Mountain spotted fever: spontaneous infection in the tick Amblyomma americanum. Public Health Rep. 58:721-729. [Google Scholar]
- 235.Parker, R. R., C. B. Philip, and W. L. Jellison. 1933. Rocky Mountain spotted fever. Potentialities of tick transmission in relation to geographical occurrence in the United States. Am. J. Trop. Med. Hyg. 8:341-379. [Google Scholar]
- 236.Parker, R. R., and R. R. Spencer. 1926. Rocky mountain spotted fever. A study of the relationship between the presence of rickettsia-like organisms in tick smears and the infectiveness of the same ticks. Public Health Rep. 41:461-469. [Google Scholar]
- 237.Parola, P., J. Attali, and D. Raoult. 2003. First detection of Rickettsia africae on Martinique, in the French West Indies. Ann. Trop. Med. Parasitol. 97:535-537. [DOI] [PubMed] [Google Scholar]
- 238.Parola, P., and N. Barré. 2004. Rickettsia africae, the agent of African tick-bite fever: an emerging pathogen in the West Indies and Reunion Island (Indian Ocean). Bull. Soc. Pathol. Exot. 97:193-198. (In French.) [PubMed] [Google Scholar]
- 239.Parola, P., L. Beati, M. Cambon, and D. Raoult. 1998. First isolation of Rickettsia helvetica from Ixodes ricinus ticks in France. Eur. J. Clin. Microbiol. Infect. Dis. 17:95-100. [DOI] [PubMed] [Google Scholar]
- 240.Parola, P., J. P. Cornet, Y. O. Sanogo, R. S. Miller, H. V. Thien, J. P. Gonzalez, D. Raoult, S. R. Telford III, and C. Wongsrichanalai. 2003. Detection of Ehrlichia spp., Anaplasma spp., Rickettsia spp., and other eubacteria in ticks from the Thai-Myanmar border and Vietnam. J. Clin. Microbiol. 41:1600-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Parola, P., F. Fenollar, S. Badiaga, P. Brouqui, and D. Raoult. 2001. First documentation of Rickettsia conorii infection (strain Indian tick typhus) in a traveler. Emerg. Infect. Dis. 7:909-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Parola, P., H. Inokuma, J. L. Camicas, P. Brouqui, and D. Raoult. 2001. Detection and identification of spotted fever group Rickettsiae and Ehrlichiae in African ticks. Emerg. Infect. Dis. 7:1014-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Parola, P., J. Jourdan, and D. Raoult. 1998. Tick-borne infection caused by Rickettsia africae in the West Indies. N. Engl. J. Med. 338:1391. [DOI] [PubMed] [Google Scholar]
- 244.Parola, P., R. S. Miller, P. McDaniel, S. R. Telford III, C. Wongsrichanalai, and D. Raoult. 2003. Emerging rickettsioses of the Thai-Myanmar border. Emerg. Infect. Dis. 9:592-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Parola, P., and D. Raoult. 2001. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin. Infect. Dis. 32:897-928. (Erratum, Clin. Infect. Dis. 33:749.) [DOI] [PubMed] [Google Scholar]
- 246.Parola, P., G. Vestris, D. Martinez, B. Brochier, V. Roux, and D. Raoult. 1999. Tick-borne rickettiosis in Guadeloupe, the French West Indies: isolation of Rickettsia africae from Amblyomma variegatum ticks and serosurvey in humans, cattle, and goats. Am. J. Trop. Med. Hyg. 60:888-893. [DOI] [PubMed] [Google Scholar]
- 247.Patino, L., A. Afanador, and J. H. Paul. 1937. A spotted fever in Tobia, Columbia. Preliminary report. Am. J. Trop. Med. 17:639-653. [Google Scholar]
- 248.Peter, O., W. Burgdorfer, A. Aeschlimann, and P. Chatelanat. 1984. Rickettsia conorii isolated from Rhipicephalus sanguineus introduced into Switzerland on a pet dog. Z. Parasitenkd. 70:265-270. [DOI] [PubMed] [Google Scholar]
- 249.Philip, C. B. 1950. Miscellaneous human rickettsioses, p. 781-788. In R. L. Pullen (ed.), Communicable diseases. Lea & Febiger, Philadelphia, Pa.
- 250.Philip, C. B. 1959. Some epidemiological considerations in Rocky Mountain spotted fever. Public Health Rep. 74:595-600. [PMC free article] [PubMed] [Google Scholar]
- 251.Philip, R. N., E. A. Casper, R. L. Anacker, J. Cory, S. F. Hayes, W. Burgdorfer, and C. E. Yunker. 1983. Rickettsia bellii sp. nov.—a tick-borne Rickettsia, widely distributed in the United States, that is distinct from the spotted fever and typhus biogroups. Int. J. Syst. Bacteriol. 33:94-106. [Google Scholar]
- 252.Philip, R. N., E. A. Casper, W. Burgdorfer, R. K. Gerloff, L. E. Hughes, and E. J. Bell. 1978. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J. Immunol. 121:1961-1968. [PubMed] [Google Scholar]
- 253.Philip, R. N., R. S. Lane, and E. A. Casper. 1981. Serotypes of tick-borne spotted fever group rickettsiae from western California. Am. J. Trop. Med. Hyg. 30:722-727. [DOI] [PubMed] [Google Scholar]
- 254.Pijper, A. 1934. Tick-bite fever. S. Afr. Med. J. 11:551-556. [Google Scholar]
- 255.Pijper, A. 1936. Etude expérimentale comparée de la Fièvre boutonneuse et de la tick-bite-fever. Arch. Inst. Pasteur Tunis 25:388-401. [Google Scholar]
- 256.Plotz, H., J. E. Smadel, and B. I. Bennet. 1946. North Queensland tick typhus: studies of the aetiological agent and its relation to other rickettsial diseases. Med. J. Aust. 263-268. [PubMed]
- 257.Pope, J. H. 1955. The isolation of a rickettsia resembling Rickettsia australis in south-east Queensland. Med. J. Aust. 1:763. [PubMed] [Google Scholar]
- 258.Pretorius, A. M., and R. Birtles. 2004. Rickettsia mongolotimonae infection in South Africa. Emerg. Infect. Dis. 10:125-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Pretorius, A. M., and R. J. Birtles. 2002. Rickettsia aeschlimannii: a new pathogenic spotted fever group rickettsia, South Africa. Emerg. Infect. Dis. 8:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Pretzman, C., N. Daugherty, K. Poetter, and D. Ralph. 1990. The distribution and dynamics of rickettsia in the tick population of Ohio. Ann. N. Y. Acad. Sci. 590:227-236. [DOI] [PubMed] [Google Scholar]
- 261.Price, E. G. 1948. Fighting spotted fever in the Rockies, p. 1-269. Naegle Printing, Helena, Mont.
- 262.Psaroulaki, A., I. Spyridaki, A. Ioannidis, T. Babalis, A. Gikas, and Y. Tselentis. 2003. First isolation and identification of Rickettsia conorii from ticks collected in the region of Fokida in central Greece. J. Clin. Microbiol. 41:3317-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Punda-Polic, V., Z. Klismanic, V. Capkun, and N. Bradaric. 2003. Demographic and epidemiologic features of Mediterranean spotted fever cases in the region of Split, Croatia. Ann. N. Y. Acad. Sci. 990:143-148. [DOI] [PubMed] [Google Scholar]
- 264.Punda-Polic, V., M. Petrovec, T. Trilar, D. Duh, N. Bradaric, Z. Klismanic, and T. Avsic-Zupanc. 2002. Detection and identification of spotted fever group rickettsiae in ticks collected in southern Croatia. Exp. Appl. Acarol. 28:169-176. [DOI] [PubMed] [Google Scholar]
- 265.Purvis, J. J., and M. S. Edwards. 2000. Doxycycline use for rickettsial disease in pediatric patients. Pediatr. Infect. Dis. J. 19:871-874. [DOI] [PubMed] [Google Scholar]
- 266.Ralph, D., C. Pretzman, N. Daugherty, and K. Poetter. 1990. Genetic-relationships among the members of the family Rickettsiaceae as shown by DNA restriction fragment polymorphism analysis. Ann. N. Y. Acad. Sci. 590:541-552. [DOI] [PubMed] [Google Scholar]
- 267.Raoult, D. 2004. A new tick-borne rickettsiosis in the USA. Clin. Infect. Dis. 38:812-813.14999623 [Google Scholar]
- 268.Raoult, D., and C. D. Paddock. 2005. Rickettsia parkeri and other spotted fever infections in the United States. N. Engl. J. Med. 353:626-627. [DOI] [PubMed] [Google Scholar]
- 269.Raoult, D., G. Aboudharam, E. Crubezy, G. Larrouy, B. Ludes, and M. Drancourt. 2000. Molecular identification by “suicide PCR” of Yersinia pestis as the agent of medieval black death. Proc. Natl. Acad. Sci. USA 97:12800-12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Raoult, D., P. Berbis, V. Roux, W. Xu, and M. Maurin. 1997. A new tick-transmitted disease due to Rickettsia slovaca. Lancet. 350:112-113. [DOI] [PubMed] [Google Scholar]
- 271.Raoult, D., P. Brouqui, and V. Roux. 1996. A new spotted-fever-group rickettsiosis. Lancet 348:412. [DOI] [PubMed] [Google Scholar]
- 272.Raoult, D., P. E. Fournier, P. Abboud, and F. Caron. 2002. First documented human Rickettsia aeschlimannii infection. Emerg. Infect. Dis. 8:748-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Raoult, D., P. E. Fournier, F. Fenollar, M. Jensenius, T. Prioe, J. J. de Pina, G. Caruso, N. Jones, H. Laferl, J. E. Rosenblatt, and T. J. Marrie. 2001. Rickettsia africae, a tick-borne pathogen in travelers to sub-Saharan Africa. N. Engl. J. Med. 344:1504-1510. [DOI] [PubMed] [Google Scholar]
- 274.Raoult, D., H. Gallais, A. Ottomani, J. P. Resch, D. Tichadou, P. De Micco, and P. Casanova. 1983. Malignant form of Mediterranean boutonneuse fever. 6 cases. Presse Med. 12:2375-2378. (In French.) [PubMed] [Google Scholar]
- 275.Raoult, D., A. Lakos, F. Fenollar, J. Beytout, P. Brouqui, and P. E. Fournier. 2002. Spotless rickettsiosis caused by Rickettsia slovaca and associated with Dermacentor ticks. Clin. Infect. Dis. 34:1331-1336. [DOI] [PubMed] [Google Scholar]
- 276.Raoult, D., and V. Roux. 1997. Rickettsioses as paradigms of new or emerging infectious diseases. Clin. Microbiol. Rev. 10:694-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Raoult, D., D. H. Tissot, P. Caraco, P. Brouqui, M. Drancourt, and C. Charrel. 1992. Mediterranean spotted fever in Marseille: descriptive epidemiology and the influence of climatic factors. Eur. J. Epidemiol. 8:192-197. [DOI] [PubMed] [Google Scholar]
- 278.Regev-Yochay, G. E., E. Segal, and E. Rubinstein. 2000. Glucose-6-phosphate dehydrogenase deficiency: possible determinant for a fulminant course of Israeli spotted fever. Isr. Med. Assoc. 2:781-782. [PubMed] [Google Scholar]
- 279.Rehacek, J. 1984. Rickettsia slovaca, the organism and its ecology. Acta Sci. Nat. Brno. 18:1-50. [Google Scholar]
- 280.Rehacek, J., and I. Tarasevich. 1988. Acari-borne rickettsiae and rickettsioses in Eurasia, p. 128-145. Veda, Publishing House of the Slovak Academy of Sciences, Bratislava, Slovakia.
- 281.Renesto, P., P. Dehoux, E. Gouin, L. Touqui, P. Cossart, and D. Raoult. 2003. Identification and characterization of a phospholipase D-superfamily gene in rickettsiae. J. Infect. Dis. 188:1276-1283. [DOI] [PubMed] [Google Scholar]
- 282.Renesto, P., H. Ogata, S. Audic, J. M. Claverie, and D. Raoult. 2005. Some lessons from Rickettsia genomics. FEMS Microbiol. Rev. 29:99-117. [DOI] [PubMed] [Google Scholar]
- 283.Ricketts, H. T. 1906. The transmission of Rocky Mountain spotted fever by the bite of the wood tick (Dermacentor occidentalis). JAMA 47:458. [Google Scholar]
- 284.Ricketts, H. T. 1909. Some aspects of Rocky Moutain spotted fever as shown by recent investigations. Med. Rec. 76:843-855. [DOI] [PubMed] [Google Scholar]
- 285.Ricketts, H. T., and L. Gomez. 1908. Studies on immunity in Rocky Mountain spotted fever. J. Infect. Dis. 5:221-244. [Google Scholar]
- 286.Ripoll, C. M., C. E. Remondegui, G. Ordonez, R. Arazamendi, H. Fusaro, M. J. Hyman, C. D. Paddock, S. R. Zaki, J. G. Olson, and C. A. Santos-Buch. 1999. Evidence of rickettsial spotted fever and ehrlichial infections in a subtropical territory of Jujuy, Argentina. Am. J. Trop. Med. Hyg. 61:350-354. [DOI] [PubMed] [Google Scholar]
- 287.Roberts, F. H. S. 1960. A systematic study of the Australian species of the genus Ixodes (Acarine: Ixodidae). Aust. J. Zool. 8:392-485. [Google Scholar]
- 288.Roberts, F. H. S. 1970. Australian ticks, p. 267. Commonwealth Scientific and Industrial Research Organisation, Australia, Melbourne, Australia.
- 289.Robertson, R. G., and C. L. Wisseman, Jr. 1973. Tick-borne rickettsiae of the spotted fever group in west Pakistan. II. Serological classification of isolates from west Pakistan and Thailand: evidence for two new species. Am. J. Epidemiol. 97:55-64. [DOI] [PubMed] [Google Scholar]
- 290.Rolain, J. M., M. Maurin, G. Vestris, and D. Raoult. 1998. In vitro susceptibilities of 27 rickettsiae to 13 antimicrobials. Antimicrob. Agents Chemother. 42:1537-1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Rolain, J. M., S. Shpynov, and D. Raoult. 2003. Spotted-fever-group rickettsioses in north Asia. Lancet 362:1939. [DOI] [PubMed] [Google Scholar]
- 292.Roux, V., P. E. Fournier, and D. Raoult. 1996. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J. Clin. Microbiol. 34:2058-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Roux, V., and D. Raoult. 1995. Phylogenetic analysis of the genus Rickettsia by 16S rDNA sequencing. Res. Microbiol. 146:385-396. [DOI] [PubMed] [Google Scholar]
- 294.Roux, V., and D. Raoult. 2000. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB). Int. J. Syst. Evol. Microbiol. 50:1449-1455. [DOI] [PubMed] [Google Scholar]
- 295.Roux, V., E. Rydkina, M. Eremeeva, and D. Raoult. 1997. Citrate synthase gene comparison, a new tool for phylogenetic analysis, and its application for the rickettsiae. Int. J. Syst. Bacteriol. 47:252-261. [DOI] [PubMed] [Google Scholar]
- 296.Rovery, C., P. Renesto, N. Crapoulet, K. Matsumoto, P. Parola, H. Ogata, and D. Raoult. 2005. Transcriptional response of Rickettsia conorii exposed to temperature variation and stress starvation. Res. Microbiol. 156:211-218. [DOI] [PubMed] [Google Scholar]
- 297.Rudakov, N., I. Samoilenko, V. V. Yakimemko, T. A. Reshetnikova, S. Shpynov, D. H. Walker, and M. Tankibaev. 1999. The re-emergence of Siberian tick typhus: field and experimental observations, p. 269-273. In D. Raoult and P. Brouqui (ed.), Rickettsiae and rickettsial diseases at the turn of the third millenium. Elsevier, Paris, France.
- 298.Ruiz Beltran, R., and J. I. Herrero Herrero. 1991. New quinolones in the treatment of Mediterranean spotted fever (MSF): a comparative study with other antibiotics regimen, p. 714-717. In J. Kazar and D. Raoult (ed.), Rickettsiae and rickettsial diseases. Publishing House of the Slovak Academy of Sciences, Bratislava, Slovakia.
- 299.Ruiz Beltran, R., and J. I. Herrero Herrero. 1992. Evaluation of ciprofloxacin and doxycycline in the treatment of Mediterranean spotted fever. Eur. J. Clin. Microbiol. Infect. Dis. 11:427-431. [DOI] [PubMed] [Google Scholar]
- 299a.Ruiz Beltran, R., and J. I. Herrero Herrero. 1992. Deleterious effect of trimethoprim-sulfamethoxazole in Mediterranean spotted fever. Antimicrob. Agents Chemother. 36:1342-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Rutherford, J. S., K. R. Macaluso, N. Smith, S. R. Zaki, C. D. Paddock, J. Davis, E. D. Peterson, A. F. Azad, and R. Rosenberg. 2004. Fatal spotted fever rickettsiosis, Kenya. Emerg. Infect. Dis. 10:910-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Rydkina, E., V. Roux, N. Rudakov, M. Gafarova, I. Tarasevich, and D. Raoult. 1999. New Rickettsiae in ticks collected in territories of the former Soviet Union. Emerg. Infect. Dis. 5:811-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Salgo, M. P., E. E. Telzak, B. Currie, D. C. Perlman, N. Litman, M. Levi, G. Nathenson, J. L. Benach, R. Alhafidh, and J. Casey. 1988. A focus of Rocky Mountain spotted fever within New York City. N. Engl. J. Med. 318:1345-1348. [DOI] [PubMed] [Google Scholar]
- 303.Sanguioni, L. A., M. C. Horta, M. C. B. Vianna, S. M. Gennari, R. M. Soares, M. A. M. Galvao, T. T. S. Schumaker, F. Ferreira, O. Vidotto, and M. B. Labruna. 2005. Rickettsial infections in animals and Brazilian spotted fever endemicity. Emerg. Infect. Dis. 11:265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sanogo, Y. O., P. Parola, S. Shpynov, J. L. Camicas, P. Brouqui, G. Caruso, and D. Raoult. 2003. Genetic diversity of bacterial agents detected in ticks removed from asymptomatic patients in northeastern Italy. Ann. N. Y. Acad. Sci. 990:182-190. [DOI] [PubMed] [Google Scholar]
- 305.Sant'Anna, J. F. 1911. On a disease in man following tick-bites and occurring in Lourenco Marques. Parasitology 4:87-88. [Google Scholar]
- 306.Sarov, B., A. Galil, E. Sikuler, P. Yagupsky, A. Saah, A. Gilad, L. Naggan, and I. Sarov. 1990. Prospective study on symptomatic versus asymptomatic infections and serological response to spotted fever group rickettsiae in 2 rural sites in the Negev (Southern Israel). Ann. N. Y. Acad. Sci. 590:243-245. [DOI] [PubMed] [Google Scholar]
- 307.Schriefer, M. E., and A. F. Azad. 1994. Changing ecology of Rocky Mountain spotted fever, p. 314-326. In D. E. Sonenshine and T. N. Mather (ed.), Ecological dynamics of tick-borne zoonoses. Oxford University Press, New York, N.Y.
- 308.Segura, F., and E. Anton. 2002. Clarithromycin for the treatment of Mediterranean spotted fever. Clin. Infect. Dis. 34:560. [DOI] [PubMed] [Google Scholar]
- 309.Sekeyova, Z., P. E. Fournier, J. Rehacek, and D. Raoult. 2000. Characterization of a new spotted fever group rickettsia detected in Ixodes ricinus (Acari: Ixodidae) collected in Slovakia. J. Med. Entomol. 37:707-713. [DOI] [PubMed] [Google Scholar]
- 310.Sekeyova, Z., V. Roux, and D. Raoult. 2001. Phylogeny of Rickettsia spp. inferred by comparing sequences of ‘gene D’, which encodes an intracytoplasmic protein. Int. J. Syst. Evol. Microbiol. 51:1353-1360. [DOI] [PubMed] [Google Scholar]
- 311.Sekeyova, Z., V. Roux, W. Xu, J. Rehacek, and D. Raoult. 1998. Rickettsia slovaca sp. nov., a member of the spotted fever group rickettsiae. Int. J. Syst. Bacteriol. 48:1455-1462. [DOI] [PubMed] [Google Scholar]
- 312.Senneville, E., F. Ajana, P. Lecocq, C. Chidiac, and Y. Mouton. 1991. Rickettsia conorii isolated from ticks introduced to Northern France by a dog. Lancet 337:676. [DOI] [PubMed] [Google Scholar]
- 313.Sexton, D. J. 2001. Rocky Mountain spotted fever, p. 437-442. In M. W. Service (ed.), The encyclopedia of arthropod-transmitted infections. CABI Publishing, New York, N.Y.
- 314.Sexton, D. J., J. Banks, S. Graves, K. Hughes, and B. Dwyer. 1991. Prevalence of antibodies to spotted fever group rickettsiae in dogs from Southeastern Australia. Am. J. Trop. Med. Hyg. 45:243-248. [DOI] [PubMed] [Google Scholar]
- 315.Sexton, D. J., and B. Dwyer. 1990. Fatal Queensland tick typhus. J. Infect. Dis. 162:779-780. [DOI] [PubMed] [Google Scholar]
- 316.Sexton, D. J., B. Dwyer, R. Kemp, and S. Graves. 1991. Spotted fever group rickettsial infections in Australia. Rev. Infect. Dis. 13:876-886. [DOI] [PubMed] [Google Scholar]
- 317.Sexton, D. J., and K. S. Kaye. 2002. Rocky mountain spotted fever. Med. Clin. N. Am. 86:351-360. [DOI] [PubMed] [Google Scholar]
- 318.Sexton, D. J., M. Muniz, G. R. Corey, E. B. Breitschwerdt, B. C. Hegarty, J. S. Dumler, D. H. Walker, P. M. Pecanha, and R. Dietze. 1993. Brazilian spotted fever in Espirito Santo, Brazil: description of a focus of infection in a new endemic region. Am. J. Trop. Med. Hyg. 49:222-226. [DOI] [PubMed] [Google Scholar]
- 319.Shazberg, G., J. Moise, N. Terespolsky, and H. Hurvitz. 1999. Family outbreak of Rickettsia conorii infection. Emerg. Infect. Dis. 5:723-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Shpynov, S., P. E. Fournier, N. Rudakov, and D. Raoult. 2003. “Candidatus Rickettsia tarasevichiae” in Ixodes persulcatus ticks collected in Russia. Ann. N. Y. Acad. Sci. 990:162-172. [DOI] [PubMed] [Google Scholar]
- 321.Shpynov, S., P. E. Fournier, N. Rudakov, M. Tankibaev, I. Tarasevich, and D. Raoult. 2004. Detection of a rickettsia closely related to Rickettsia aeschlimannii, “Rickettsia heilongjiangensis,” Rickettsia sp. strain RpA4, and Ehrlichia muris in ticks collected in Russia and Kazakhstan. J. Clin. Microbiol. 42:2221-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Shpynov, S., P. Parola, N. Rudakov, I. Samoilenko, M. Tankibaev, I. Tarasevich, and D. Raoult. 2001. Detection and identification of spotted fever group rickettsiae in Dermacentor ticks from Russia and central Kazakhstan. Eur. J. Clin. Microbiol. Infect. Dis. 20:903-905. [DOI] [PubMed] [Google Scholar]
- 323.Simser, J. A., A. T. Palmer, V. Fingerle, B. Wilske, T. J. Kurtti, and U. G. Munderloh. 2002. Rickettsia monacensis sp. nov., a spotted fever group Rickettsia, from ticks (Ixodes ricinus) collected in a european city park. Appl. Environ. Microbiol. 68:4559-4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Simser, J. A., A. T. Palmer, U. G. Munderloh, and T. J. Kurtti. 2001. Isolation of a spotted fever group rickettsia, Rickettsia peacockii, in a Rocky Mountain wood tick, Dermacentor andersoni cell line. Appl. Environ. Microbiol. 67:546-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Spencer, R. R., and R. R. Parker. 1923. Rocky mountain spotted fever: infectivity of fasting and recently fed ticks. Public Health Rep. 38:339. [Google Scholar]
- 326.Stackebrandt, E., W. Frederiksen, G. M. Garrity, P. A. D. Grimont, P. Kampfer, M. C. J. Maiden, X. Nesme, R. Rossello-Mora, J. Swings, H. G. Truper, L. Vauterin, A. C. Ward, and W. B. Whitman. 2002. Report of the ad hoc committee for the re-evaluation of the species definition in bacteriology. Int. J. Syst. Evol. Microbiol. 52:1043-1047. [DOI] [PubMed] [Google Scholar]
- 327.Stenos, J., V. Roux, D. Walker, and D. Raoult. 1998. Rickettsia honei sp. nov., the aetiological agent of Flinders Island spotted fever in Australia. Int. J. Syst. Bacteriol. 48:1399-1404. [DOI] [PubMed] [Google Scholar]
- 328.Stewart, R. S. 1991. Flinders Island spotted fever: a newly recognised endemic focus of tick typhus in Bass Strait. Part 1. Clinical and epidemiologiocal features. Med. J. Aust. 154:94-99. [DOI] [PubMed] [Google Scholar]
- 329.Stoenner, H. G., R. Holdenreid, D. Lackman, and J. S. Orsborn, Jr. 1959. The occurrence of Coxiella burnetii, Brucella and other pathogens among fauna of the Great Lake desert in Utah. Am. J. Trop. Med. Hyg. 8:590. [DOI] [PubMed] [Google Scholar]
- 330.Sundhindra, B. K., S. Vijayakumar, K. A. Kutty, S. R. Tholpadi, R. S. Rajan, E. Mathai, D. Raoult, and T. J. John. 2004. Rickettsial spotted fever in Kerala. Natl. Med. J. India 17:51-52. [PubMed] [Google Scholar]
- 331.Tanskul, P., H. E. Stark, and I. Inlao. 1983. A checklist of ticks of Thailand (Acari: Metastigmata: Ixodoidea). J. Med. Entomol. 20:330-341. [DOI] [PubMed] [Google Scholar]
- 332.Tarasevich, I., and P. Somov. 1966. Comparative serological study of tick-borne typhus of northern Asia and tsutsugamushi fever. J. Microbiol. Epidemiol. Immunol. 1:83-86. [Google Scholar]
- 333.Tarasevich, I. V., V. A. Makarova, N. F. Fetisova, A. V. Stepanov, E. D. Miskarova, N. Balayeva, and D. Raoult. 1991. Astrakhan fever, a spotted-fever rickettsiosis. Lancet 337:172-173. [DOI] [PubMed] [Google Scholar]
- 334.Tarasevich, I. V., V. A. Makarova, N. F. Fetisova, A. V. Stepanov, E. D. Miskarova, and D. Raoult. 1991. Studies of a “new” rickettsiosis “Astrakhan” spotted fever. Eur. J. Epidemiol. 7:294-298. [DOI] [PubMed] [Google Scholar]
- 335.Taylor, J. P., W. B. Tanner, J. A. Rawlings, J. Buck, L. B. Elliott, H. J. Dewlett, B. Taylor, and T. G. Betz. 1985. Serological evidence of subclinical Rocky Mountain spotted fever infections in Texas. J. Infect. Dis. 151:367-369. [DOI] [PubMed] [Google Scholar]
- 336.Topping, N. H. 1939. Experimental Rocky Mountain spotted fever and epidemic typhus treated with prontosil sulfapyridine. Public Health Rep. 54:1143-1147. [Google Scholar]
- 337.Treadwell, T. A., R. C. Holman, M. J. Clarke, J. W. Krebs, C. D. Paddock, and J. E. Childs. 2000. Rocky Mountain spotted fever in the United States, 1993-1996. Am. J. Trop. Med. Hyg. 63:21-26. [DOI] [PubMed] [Google Scholar]
- 338.Tzianabos, T., B. E. Anderson, and J. E. McDade. 1989. Detection of Rickettsia rickettsii in clinical specimens by using polymerase chain reaction technology. J. Clin. Microbiol. 27:2866-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Uchida, T., F. Tashiro, T. Funato, and Y. Kitamura. 1986. Immunofluorescence test with Rickettsia montana for serologic diagnosis of rickettsial infection of the spotted fever group in Shikoku, Japan. Microbiol. Immunol. 30:1061-1066. [DOI] [PubMed] [Google Scholar]
- 340.Uchida, T., F. Tashiro, T. Funato, and Y. Kitamura. 1986. Isolation of a spotted fever group Rickettsia from a patient with febrile exanthematous illness in Shikoku, Japan. Microbiol. Immunol. 30:1323-1326. [DOI] [PubMed] [Google Scholar]
- 341.Uchida, T., T. Uchiyama, and A. H. Koyama. 1988. Isolation of spotted fever group rickettsiae from humans in Japan. J. Infect. Dis. 158:664-665. [DOI] [PubMed] [Google Scholar]
- 342.Uchida, T., T. Uchiyama, K. Kumamo, and D. H. Walker. 1992. Rickettsia japonica sp. nov., the etiological agent of spotted fever group rickettsiosis in Japan. Int. J. Syst. Bacteriol. 42:303-305. [DOI] [PubMed] [Google Scholar]
- 343.Uchida, T., X. J. Yu, T. Uchiyama, and D. Walker. 1989. Identification of a unique spotted fever group rickettsia from humans in Japan. J. Infect. Dis. 159:1122-1126. [DOI] [PubMed] [Google Scholar]
- 344.Uchiyama, T. 2003. Adherence to and invasion of Vero cells by recombinant Escherichia coli expressing the outer membrane protein rOmpB of Rickettsia japonica. Ann. N. Y. Acad. Sci. 990:585-590. [DOI] [PubMed] [Google Scholar]
- 345.Valero, A. 1949. Rocky Mountain spotted fever in Palestine. Harefuah 36:99. [PubMed] [Google Scholar]
- 346.Vandamme, P., B. Pot, M. Gillis, P. Devos, K. Kersters, and J. Swings. 1996. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol. Rev. 60:407-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Venzal, J. M., A. Portillo, A. Estrada-Pena, O. Castro, P. A. Cabrera, and J. A. Oteo. 2004. Rickettsia parkeri in Amblyomma triste from Uruguay. Emerg. Infect. Dis. 10:1493-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Vestris, G., J. M. Rolain, P. E. Fournier, M. L. Birg, M. Enea, J. Y. Patrice, and D. Raoult. 2003. Seven years' experience of isolation of Rickettsia spp. from clinical specimens using the shell vial cell culture assay. Ann. N. Y. Acad. Sci. 990:371-374. [DOI] [PubMed] [Google Scholar]
- 349.Vitale, G., S. Mansueto, J. M. Rolain, and D. Raoult. 2005. Rickettsia massiliae: first isolation from the blood of a patient. Emerg. Infect. Dis., in press. [DOI] [PMC free article] [PubMed]
- 350.Walker, D. H. 2002. Rickettsia rickettsii: as virulent as ever. Am. J. Trop. Med. Hyg. 66:448-449. [DOI] [PubMed] [Google Scholar]
- 351.Walker, D. H., H. M. Feng, and V. L. Popov. 2001. Rickettsial phospholipase A(2) as a pathogenic mechanism in a model of cell injury by typhus and spotted fever group rickettsiae. Am. J. Trop. Med. Hyg. 65:936-942. [DOI] [PubMed] [Google Scholar]
- 352.Walker, D. H., H. M. Feng, J. I. Saada, P. Crocquetvaldes, S. Radulovic, V. L. Popov, and E. Manor. 1995. Comparative antigenic analysis of spotted fever group Rickettsiae from Israel and other closely related organisms. Am. J. Trop. Med. Hyg. 52:569-576. [DOI] [PubMed] [Google Scholar]
- 353.Walker, D. H., and D. B. Fishbein. 1991. Epidemiology of rickettsial diseases. Eur. J. Epidemiol. 7:237-245. [DOI] [PubMed] [Google Scholar]
- 354.Walker, D. H., R. M. Gay, and M. Valdesdapena. 1981. The occurrence of eschars in Rocky Mountain spotted fever. J. Am. Acad. Dermatol. 4:571-576. [DOI] [PubMed] [Google Scholar]
- 355.Walker, D. H., Q. H. Liu, X. J. Yu, H. Li, C. Taylor, and H. M. Feng. 1992. Antigenic diversity of Rickettsia conorii. Am. J. Trop. Med. Hyg. 47:78-86. [DOI] [PubMed] [Google Scholar]
- 356.Walker, D. H., and D. J. Sexton. 2002. Rickettsia rickettsii (Rocky Mountain spotted fever), p. 899-906. In V. L. Yu, R. Weber, and D. Raoult (ed.), Antimicrobial therapy and vaccines, 2nd ed, vol. 1. Microbes. Apple Trees Productions, LLC, New York, N.Y. [Google Scholar]
- 357.Walker, D. H., G. A. Valbuena, and J. P. Olano. 2003. Pathogenic mechanisms of diseases caused by Rickettsia. Ann. N. Y. Acad. Sci. 990:1-11. [DOI] [PubMed] [Google Scholar]
- 358.Watt, G., and P. Parola. 2003. Scrub typhus and tropical rickettsioses. Curr. Opin. Infect. Dis. 16:429-436. [DOI] [PubMed] [Google Scholar]
- 359.Wayne, L. G., D. J. Brenner, R. R. Colwell, P. A. D. Grimont, O. Kandler, M. I. Krichevsky, L. H. Moore, W. E. C. Moore, R. G. E. Murray, E. Stackebrandt, M. P. Starr, and H. G. Truper. 1987. Report of the Ad hoc committee on reconciliation of approaches to bacterial systematics. Int. J. Syst. Bacteriol. 37:463-464. [Google Scholar]
- 360.Weaver, S. C., and D. Barrett. 2004. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2:789-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Weller, S. J., G. D. Baldridge, U. G. Munderloh, H. Noda, J. Simser, and T. J. Kurtti. 1998. Phylogenetic placement of rickettsiae from the ticks Amblyomma americanum and Ixodes scapularis. J. Clin. Microbiol. 36:1305-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Whitworth, T., V. Popov, V. Han, D. Bouyer, J. Stenos, S. Graves, L. Ndip, and D. Walker. 2003. Ultrastructural and genetic evidence of a reptilian tick, Aponomma hydrosauri, as a host of Rickettsia honei in Australia—possible transovarial transmission. Ann. N. Y. Acad. Sci. 990:67-74. [DOI] [PubMed] [Google Scholar]
- 363.Wike, D. A., and W. Burgdorfer. 1972. Plaque formation in tissue cultures by Rickettsia rickettsi isolated directly from whole blood and tick hemolymph. Infect. Immun. 6:736-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Wike, D. A., R. A. Ormsbee, G. Tallent, and M. G. Peacock. 1972. Effects of various suspending media on plaque formation by rickettsiae in tissue culture. Infect. Immun. 6:550-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Wolbach, S. B. 1919. Studies on Rocky Mountain spotted fever. J. Med. Res. 41:1-197. [PMC free article] [PubMed] [Google Scholar]
- 366.Reference deleted.
- 367.Woodland, J. C., M. M. McDowell, and J. T. Richards. 1943. Bullis fever (lone star fever-tick fever). JAMA 122:1156-1160. [Google Scholar]
- 368.Wu, Y. M., S. R. Yu, and D. Lou. 1994. Western-blot analysis of Rickettsia heilongjiangi. J. Prev. Med. P. L. A. 12:28-30. [Google Scholar]
- 369.Yagupsky, P., and B. Wolach. 1993. Fatal Israeli spotted fever in children. Clin. Infect. Dis. 17:850-853. [DOI] [PubMed] [Google Scholar]
- 370.Yevich, S. J., J. L. Sanchez, R. F. DeFraites, C. C. Rives, J. E. Dawson, I. J. Uhaa, B. J. Johnson, and D. B. Fishbein. 1995. Seroepidemiology of infections due to spotted fever group rickettsiae and Ehrlichia species in military personnel exposed in areas of the United States where such infections are endemic. J. Infect. Dis. 171:1266-1273. [DOI] [PubMed] [Google Scholar]
- 371.Yu, X., Y. Jin, M. Fan, G. Xu, Q. Liu, and D. Raoult. 1993. Genotypic and antigenic identification of two new strains of spotted fever group rickettsiae isolated from China. J. Clin. Microbiol. 31:83-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Zhang, J. Z., and M. Y. Fan. 1995. Detection of spotted fever group rickettsiae in ticks and rodents by polymerase chain reaction technique in People's Republic of China. Acta Virol. 39:263-267. [PubMed] [Google Scholar]
- 373.Zhang, J. Z., M. Y. Fan, Y. M. Wu, P. E. Fournier, V. Roux, and D. Raoult. 2000. Genetic classification of “Rickettsia heilongjiangii” and “Rickettsia hulinii,” two Chinese spotted fever group rickettsiae. J. Clin. Microbiol. 38:3498-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Zhu, Y., P. E. Fournier, M. Eremeeva, and D. Raoult. 2005. Proposal to create subspecies of Rickettsia conorii based on multi-locus sequence typing and an emended description of Rickettsia conorii. BMC Microbiol. 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Zinsser, H., and S. Bayne-Jones. 1939. A textbook of bacteriology: the application of bacteriology and immunology to the etiology, diagnosis, specific therapy and prevention of infectious diseases for students and practitioners of medicine and public health, p. 1-990. D. Appleton-Century Company, Inc., London, United Kingdom.