Abstract
Among animal viruses, arboviruses are unique in that they depend on arthropod vectors for transmission. Field research and laboratory investigations related to the three components of this unique mode of transmission, virus, vector, and vertebrate host, have produced an enormous amount of valuable information that may be found in numerous publications. However, despite many reviews on specific viruses, diseases, or interests, a systematic approach to organizing the available information on all facets of biological transmission and then to interpret it in the context of the evolutionary process has not been attempted before. Such an attempt in this review clearly demonstrates tremendous progress made worldwide to characterize the viruses, to comprehend disease transmission and pathogenesis, and to understand the biology of vectors and their role in transmission. The rapid progress in molecular biologic techniques also helped resolve many virologic puzzles and yielded highly valuable data hitherto unavailable, such as characterization of virus receptors, the genetic basis of vertebrate resistance to viral infection, and phylogenetic evidence of the history of host range shifts in arboviruses. However, glaring gaps in knowledge of many critical subjects, such as the mechanism of viral persistence and the existence of vertebrate reservoirs, are still evident. Furthermore, with the accumulated data, new questions were raised, such as evolutionary directions of virus virulence and of host range. Although many fundamental questions on the evolution of this unique mode of transmission remained unresolved in the absence of a fossil record, available observations for arboviruses and the information derived from studies in other fields of the biological sciences suggested convergent evolution as a plausible process. Overall, discussion of the diverse range of theories proposed and observations made by many investigators was found to be highly valuable for sorting out the possible mechanism(s) of the emergence of arboviral diseases.
INTRODUCTION
Among animal viruses, arboviruses are unique in that they are transmitted by blood-sucking arthropods (vectors) to vertebrates, a mode of transmission commonly known as biological transmission. This peculiar mode of transmission involving the three essential components (virus, vector, and vertebrate) has intrigued many medical entomologists, epidemiologists, and virologists alike, and raised fundamental questions ranging from the advantages of such a complicated mode of transmission to its impact on the genetics of viruses.
The importance of arboviral infections has been illustrated by the dramatically increasing frequency and magnitude of old and newly emerging arboviral disease problems. In the West Nile fever outbreak in North America that is currently in progress, in 2003 in the United States alone 9,858 confirmed cases with 262 deaths were reported. The annual incidence and fatalities by Japanese encephalitis in Asia are estimated to be 30,000 to 50,000 and 10,000, respectively. Furthermore, dengue afflicts more than 50 million people worldwide every year.
The transmission of arboviruses has constituted an indispensable core knowledge in the discussions or reviews organized according to particular disease problem, epidemiology, disease control, virus, host, ecological factor, or other specific topic of interest. Because arboviral research encompasses several major branches of science, assembling and systematically organizing the data and observations of biological transmission published in many disciplines has been difficult. This may partly explain why a comprehensive review of all facets of the transmission mechanism has been rarely attempted, despite its importance.
However, for the growing number of new scientists and students interested in research on arboviruses and the diseases they transmit, the availability of more comprehensive reviews on carefully selected subjects that provide systematically organized information and relevant source references is highly desirable.
In this review, the determinants that facilitated establishment of the biological transmission found in each of the three major components of biological transmission (vector, vertebrate, and virus) are characterized. In particular, the factors that facilitate contact among the three components and perpetual natural transmission or viral survival, as well as the factors that act antagonistically to the development of biological transmission, are emphasized. Ecological factors are also emphasized to illustrate the importance of the interactions among the three components under natural conditions. Then, unique traits of this mode of transmission are examined. The relevant information drawn from the aforementioned subjects, in combination with various hypotheses proposed in the past and new molecular biologic data, were then used to discuss the signs of evolutionary processes that facilitated the establishment of the transmission mechanisms. This review was also designed to present research questions by identifying the important subjects that still lack relevant data as well as the unresolved topics with discrepant data or conflicting views.
Limitation of the Scope
In this review, selected factors that are of fundamental importance in the establishment and maintenance of biological transmission are examined. The relevant information from the older contributions that have not received adequate attention and more recent data are the major sources of information compiled. Many important subjects that have been comprehensively reviewed multiple times in the past are not covered. For those subjects, the following publications provide rich sources of basic information: epidemiology (214); general biology of vectors and virus-vector-vertebrate relationships (74, 159, 238, 318, 335); vertical transmission in vector (237, 270, 318); vector competence (102, 110, 212); and competent host (203, 356). Characterization of arboviruses or diseases, viral replication in hosts, diagnosis, prevention, and control are beyond the scope of this review.
The signs of evolutionary trends in each component conducive to the establishment of biological transmission but not the evolution of arboviruses is one of the major themes. However, meaningful discussion of the passages to the evolution of biological transmission without any reference to the evolution of viruses is not possible because the two subjects are inseparable depending on topic. Thus, viral evolution is commented on in a small number of sections to enrich the discussion when it is absolutely necessary or relevant, but the extent of its coverage is limited to a minimum. Although biological transmission is the theme of this review, two modes of nonbiological transmission (direct and mechanical) are briefly described. Inclusion of these modes of transmission is important, since many arboviruses are also transmitted occasionally by these mechanisms, and, furthermore, they provide useful information for the discussion.
Definitions
The term arbovirus that derived from laboratory jargon used in early 1940s among the investigators in California (255) refers to an animal virus that is transmitted to vertebrate hosts by blood-sucking arthropod vectors. The basic requirements for arboviruses defined by the World Health Organization (350) were viral replication in both phyla of hosts and viral transmission by blood-sucking arthropod on vertebrate host demonstrating viremia. Subsequently, the definition was modified to include direct transmission as an alternative mode of transmission (351).
Insect virus and arthropod virus are used in this review to denote viruses that can infect only insects (including mosquitoes) or any arthropod, respectively. These viruses cannot replicate in vertebrates and hence are not arboviruses. Vertebrate virus is used to refer to a virus that can infect only vertebrates but not arthropods. Virus lineage in this review refers to a group of closely related or monophyletically related viruses. In the original definition in microbiology, the word vector is used for all organisms, including vertebrates, that function as carriers of infectious agents to another organisms. However, in this review it is used exclusively for hematophagous arthropods involved in biological transmission of arboviruses and is not used for the vertebrate hosts involved in direct transmission between vertebrates. Unique examples of infected humans' playing the role of vector by the original definition but still in the context of biological transmission are described in the section on reservoirs. On the other hand, host is used to mean both vectors and vertebrate hosts.
Traditionally, human diseases have been classified according to the source of infectious agent. Thus, human diseases transmitted from animals to humans have been called zoonoses. However, others have defined the term zoonosis more narrowly by restricting its usage to vertebrate-to-human transmissions only (198), a source of confusion. Accordingly, if one follows this tradition, arboviral diseases that involve both arthropods and vertebrates for ultimate transmission to humans should be called anthroponoses or anthropozoonoses, depending on the virus. However, because these terms have been used rarely, in this review the term zoonosis is applied to an arboviral disease as well, following the recommendation of Hubálek (131).
Family, generic, and species names of taxonomically assigned viruses are italicized at first use and many are abbreviated, but the names of viruses in tentative status or not listed are not, according to the conventions of the International Committee on the Taxonomy on Viruses (139).
NONBIOLOGICAL TRANSMISSION
Animal viruses are generally transmitted between hosts directly, mechanically, and/or vertically. Although biological transmission is uniquely observed only in arboviruses, nonbiologic transmission mechanisms are also observed in arboviruses as well. A few questions are asked regarding the transmission mechanisms involved in the evolution of biological transmission. What was the order of the steps leading to the establishment of biological transmission? Was mechanical transmission a precursor of biological transmission? Did direct transmission influence the evolution of biological transmission?
Direct Transmission
Among the major modes of animal virus transmission, unquestionably direct transmission is shared among all virus groups and considered the fundamental mechanism in all animal virus groups, including the only alphavirus (of fishes) that is not an insect-borne arbovirus (342). Thus, it is not surprising that this mode of transmission is widespread among all major arbovirus groups (164). The common routes are intranasal, oral, venereal, and exposure of skin with abrasion, cornea, reproductive tissue, or any mucous tissue. For many animals, oral and intranasal routes may be more adequately characterized as nasopharyngeal routes of infection. The common sources of contaminants include food, aerosol, bodily secretions, urine, fecal matter, saliva, milk, hair, feathers, and skin (81, 164). The animal behaviors conducive to this mode of transmission include eating (including cannibalism), drinking, sniffing, licking, preening, nuzzling, and any aggressive behaviors resulting in injury. Some viruses, such as Venezuelan equine encephalitis virus, are readily transmissible by aerosol. Under special circumstances of high virus concentration, however, many arboviruses classified at a lower biohazard level (such as Dengue virus [DENV]) also can infect vertebrates by aerosol (168). In a recent report, occurrence of such a direct transmission of West Nile virus (WNV) in flocks of geese was reported (12). Omsk hemorrhagic fever virus is also spread among muskrats by direct contact, as the virus is shed in urine and feces.
Insectivorous animals, such as bats, become infected by ingesting mosquitoes infected with viruses such as Rift Valley fever virus, Yellow fever virus (YFV), and Japanese encephalitis virus (JEV) (164). An additional interest in insectivorous bats is their potential role in the evolution of arboviruses originating from strictly insect viruses through ingestion of infected insects. Recently, at least under laboratory conditions, an indirect mode of oral transmission was proposed to be possible for Vesicular stomatitis virus (VSV) New Jersey serotype. According to the proposal, the chain of events is initiated first by grasshoppers cannibalizing infected grasshoppers and then by cattle accidentally ingesting infected grasshoppers in the pasture (235). Contact transmission of this virus between livestock also has been recognized (204).
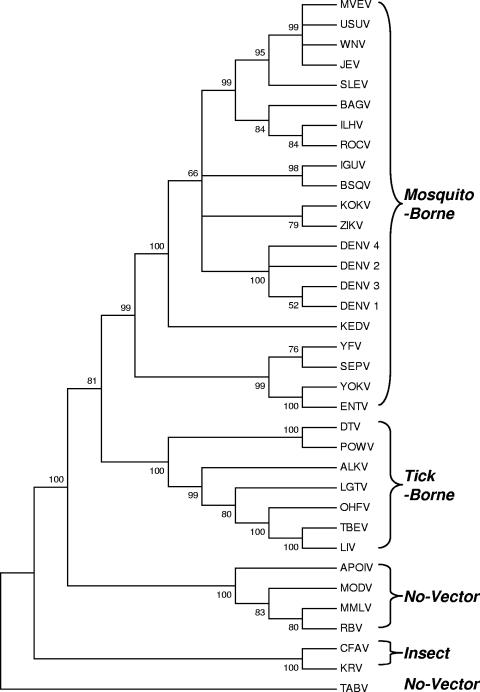
The flaviviruses without a known vector (hereafter called the no-vector group) are an interesting group of vertebrate viruses. Their phylogenetic tree position is much closer to the root than that of vector-borne flavivirus groups, strongly suggesting that direct transmission preceded biological transmission (169) (Fig. 1). The no-vector group of flaviviruses depends on direct transmission for survival in nature (14, 65). Many of those viruses infect small mammals with rapid turnover, which improves the probability of a continuing supply of a large number of immunologically naïve hosts. As theorized by Woolhouse et al. (349), the viruses that depend almost entirely on direct transmission are much less zoonotic. This may explain why the no-vector group of flaviviruses are not zoonotic.
FIG. 1.
Phylogram of flaviviruses using a neighbor-joining inference method (MEGA) based on the complete RNA-dependent RNA polymerase domain of the NS5 gene of 35 viruses deposited in GenBank. The numbers at nodes indicate % branch supports by bootstrap sampling with 500 replicates. Distances were calculated with Poisson correction. Virus abbreviation-virus name (GenBank accession number): ALKV-Alkhurma virus (NC004355); APOIV-Apoi virus (AF160193); BAGV-Bagaza virus (AY632545*); BSQV-Bussuquara virus (AY632536*); CFAV-cell fusing agent virus (M91671); DTV-deer tick virus (NC003218); DENV-1—dengue serotype 1 (U88535); DENV-2—dengue serotype 2 (M20558); DENV-3—dengue serotype 3 (M93130); DENV-4—dengue serotype 4 (M14931); ENTV—Entebbe bat virus (AY632537*); IGUV—Iguape virus (AY632538*); ILHV—Ilhéus virus (AY632539*); JEV—Japanese encephalitis virus (M18370); KRV—Kamiti River virus (NC005064); KEDV—Kédougou virus (AY632540*); KOKV—Kokobera virus (AY632541*); LGTV—Langat virus (NC003690); LIV—louping ill virus (Y07863); MODV—Modoc virus (AJ242984); MMLV—Montana myotis leukoencephalitis virus (NC00419.1); MVEV—Murray Valley encephaliltis virus (NC000943); OHFV—Omsk hemorrhagic fever virus (AY193805); POWV—Powassan virus (L06436); RBV—Rio Bravo virus (AF144692); ROCV—Rocio virus (AY632542*); SEPV—Sepik virus (AY632543*); SLEV—St. Louis encephalitis virus-Argentine 66 (AY632544*); TABV—Tamana bat virus (AF285080); TBEV—tick-borne encephalitis virus (U27495); USUV—Usutu virus (NC006551); WNV—West Nile virus (AF196835); YFV—yellow fever virus (X03700); YOKV—Yokose virus (AB114858); and ZIKV—Zika virus (AY632535*). *, sequence deposited by G. Kuno and G.-J. J. Chang.
Transmission by immature stages of vectors is best recognized in ticks, as they engage in blood feeding at all postembryonic stages. The exceptions are larvae of some soft ticks that are autogenous. In contrast, direct transmission at an immature stage of mosquitoes is generally poorly known, since larvae do not blood-feed. However, direct transmission of arboviruses to mosquitoes occurs in aquatic environments as well. In fact, YFV, JEV, Western equine encephalitis virus, and Rift Valley fever virus in contaminated water, could be transmitted orally to mosquito larvae through feeding activity (L. Thomas, cited in references 47, 122, 321, and 346). Although the significance is unclear, Turell et al. (321) speculated a possibility in seasonally created environments such as dambos in Africa. These environments are shared by a large number of animals for living. Under such conditions, viruses from the infected animals released into water as a result of cannibalism or death due to infection could be ingested by mosquito larvae. Interestingly, Turell et al. (321) determined that Rift Valley fever virus could survive in water at 30°C for more than a few days.
Mechanical Transmission
Most likely, soon after arthropods established dependence on blood feeding on vertebrate hosts, mechanical transmission concomitantly began to occur via contaminated mouthparts of the blood-sucking arthropods that switched vertebrate hosts during feeding activity. Generally, compared with insect vectors, acarines (ticks and mites) are not efficient mechanical transmitters. This is due to the low frequency of interrupted feeding as well as to their feeding behavior to stay on the same host except for drop-off between growth stages.
While most cases of mechanical transmission of animal viruses occur without a biologic significance in natural transmission, some are of significant veterinary importance. The vertebrates involved are typically mid-size to large animals. The DNA viruses involved include Myxoma virus, Rabbit fibroma virus, Lumpy skin disease virus of cattle (51), and African swine fever virus. The mechanically transmitted RNA viruses include Equine infectious anemia virus, Bovine viral diarrhea virus, Bovine leukemia virus, hog cholera virus, and Rift Valley fever virus.
Other factors being equal, for a virus to be mechanically transmitted, higher virus titer in skin or blood is required to make mechanical transmission efficient (121) because the volume of blood contaminating mouthparts is usually less than 20 nanoliters. Furthermore, the viruses involved must be resistant to adverse environmental conditions that render viruses noninfectious (44). Many dipterous insects are implicated in mechanical transmission; and their efficiency increases when the density of virus-infected vertebrates is high in the environment. For example, it was speculated that mechanical transmission by a variety of blood-feeding insects (including black flies) at least partly contributed to major outbreaks of Rift Valley fever in Africa and Venezuelan equine encephalitis in Colombia, respectively (121, 335).
Mechanical transmission has been regarded by many investigators merely as an accidental transport of virus, a biological phenomenon associated with the evolution of scavenging, ectoparasitic, and blood-feeding behaviors of arthropods. Thus, it is not considered a primordial, transient stage of viral transmission preceding biological transmission.
AUTOGENY AND HEMATOPHAGY
Autogeny
Some blood-sucking arthropods may be able to complete a gonotrophic cycle (a physiological cycle of egg maturation in the female vector) once after emergence without engaging in blood feeding and ovipositing. This phenomenon, called autogeny, is recognized in about 60 species and is controlled by multiple genes (294). It is not clear when this phenomenon evolved in some blood-sucking dipterans, such as mosquitoes (family Culicidae), biting midges (family Ceratopogonidae), sand flies (family Psychodidae), black flies (family Simuliidae), and other flies. But it is largely absent in ticks, with an exception of facultative autogeny in some Ornithodoros ticks.
Autogeny is clearly advantageous for survival when blood meal sources are either only seasonally available or when blood feeding is suppressed for some reasons (72, 293, 316). Additionally, it also has a significant survival value for the viruses vertically transmitted by those vectors. Variation in autogeny within a species of vector is well documented. Many geographic strains of Aedes aegypti in Africa are autogenous; however, this phenomenon is not generally observed in most laboratory colonies (55).
With respect to its significance in vertebrate host association, Culex pipiens provides a valuable information. In this mosquito, females of anautogenous populations (which depend on blood meal for every reproductive cycle) have been observed to feed mainly on birds; while females of the hybrid of autogenous and anautogenous populations feed indiscrimately on avian or mammalian hosts (295). Generally, autogeny is considered to reduce the efficacy of those arthropods as disease vectors. Paradoxically, in Culex tarsalis populations in North America, autogeny was reported to reach nearly 95% in midsummer, the peak season for the transmission of western equine encephalitis virus and St. Louis encephalitis virus (293). Interestingly, vertical transmission of St. Louis encephalitis virus through eggs laid by autogenous Aedes mosquitoes was also proven in a laboratory experiment (242).
Hematophagy
For unraveling the evolutionary process leading to the establishment of biological transmission, an accurate chronological order of the emergence of virus, vector, and vertebrate host provides the most reliable information. However, in the absence of fossil record of viruses, reconstruction of the chronological order is impossible. Still, it is highly probable that by the time the extant arboviral lineages evolved arthropods had been blood-feeding on vertebrate hosts for many millions of years.
Survey of hematophagous insects and acarines.
Hematophagy evolved in more than 14,000 species (in about 400 genera in 14 families) of arthropods, including nearly 800 species of soft and hard ticks (262). Most of the hematophagous insects are the members of one of the four orders (Anoplura, Diptera, Hemiptera, and Siphonaptera). It has been observed even in males of a tropical, nocturnal moth species (order Lepidoptera). While some members of lower Diptera (or Nematocera), including biting midges, black flies, mosquitoes, and sand flies as well as fleas (order Siphonaptera), suck blood only in the adult stage, others suck at all postembryonic stages (60). In some genera of the family Calliphoridae (order Diptera), which are not involved in biological transmission of virus, larvae are obligatory bloodsuckers for nourishment.
Ticks, on the other hand, engage in blood-feeding at all postembryonic developmental stages, with exceptions of larvae of some soft ticks. Some of the blood-feeding insects are sexually dimorphic, only adult females in some groups (such as mosquitoes, sand flies, and biting midges) blood-feeding; while in others (i.e., tsetse flies of the family Glossinidae) both sexes engage in blood sucking. Exactly why blood-feeding is restricted to females of these insects, in contrast to the families (such as Tabanidae) of higher dipterous groups, is not well understood. To make the blood-feeding behavior of insects more varied, a unique group of biting midges (genus Trithecoides of the family Ceratopogonidae) in Southeast Asia are known to pierce the abdomen of recently engorged mosquitoes to obtain blood indirectly from vertebrate hosts (140).
With respect to vertebrate host specificity, some arthropods are restricted to mammalian hosts, others (such as members of the family Simuliidae) to mammals and birds, and yet another group (i.e., many phlebotomine sand flies) to cold-blooded vertebrates. Among mosquitoes, species of Culex are predominantly bird-mammalian feeders, while species of the genera Aedes and Anopheles are mostly mammalian feeders. Still, a small number of culicine species (in the genera Aedes and Uranotaenia) feed on poikilothermic vertebrates. Flies of the families Tabanidae (which are involved in mechanical transmission) and Rhagionidae feed on both warm-blooded and cold-blooded vertebrates, such as crocodiles, lizards, and turtles, chiefly in tropical areas. Those biting flies cannot successfully bite avian hosts in daytime due to defensive behaviors of the hosts, except for a rare record of Chrysops biting crows (72).
Evolution of hematophagy.
Evolutionary reconstruction of the development of hematophagy in arthropods is a subject rich in diverse theories and hence controversies. Nevertheless, most experts agree that hematophagy developed independently through convergence in many disparate groups (families and orders) of arthropods at different geologic periods. Apparently, sand flies were blood feeding on vertebrates and transmitting leishmanial parasites in the Lower Cretaceous Period (105 to 100 million years ago) in exactly the same host-parasite relationship observed today. In fact, a recent examination of a blood-engorged sand fly embedded in amber revealed remarkably well-preserved reptilian blood cells and the parasites in the gut (246). This, in turn, strongly supports the speculation that the biological transmission of arboviruses also occurred many million years ago. However, the extant arboviral lineages are not the direct descendants of the speculated, paleontologic lineages.
Regardless of the exact dating of the beginning of hematophagy or the order of morphological modification of mouthparts to blood-sucking forms, it is believed by some that arthropods could not become vectors in biological transmission until they developed antihemostatic mechanisms. According to one theory, blood feeding of ticks evolved about 120 to 92 million years ago, when ticks developed inhibitors of hemostasis, such as blood coagulation inhibitors (i.e., anti-Xa, anti-VIII, anti-X, and apyrase) and antivasoconstrictory factors (prostaglandin E2, prostacyclin, nitric oxide, tachykinin, etc.). Today, the genes involved in the syntheses of these substances are widely distributed among many different species of hematophagous arthropods (262). The anticoagulant released by the Aedes aegypti mosquito, for example, shares similarities to the serpin superfamily of serine protease inhibitors (299). Interestingly, molecular clock studies of the antihemostatic genes of a diverse group of organisms ranging from blood-feeding insects and leeches to mammals (such as vampire bats) revealed that the development of antihemostatic mechanisms in ticks coincided with the evolution of blood coagulation in vertebrates (200).
Hematophagy is speculated by some to be still evolving. As examples, face flies (genus Hematobia) and stable flies (genus Stomoxys) produce a salivary anticoagulant but still lack the vasodilatory or antiplatelet substances found in most hematophagous arthropods. Accordingly, they are considered to be in a transient stage of adaptation to full-fledged hematophagy (263). By a similar line of thought, arthropods such as gamasid acarines and bugs (family Reduviidae) are speculated to be in a transient stage, because they are facultative parasites engaged in both entomophagy and hematophagy.
VECTORS
For biological transmission to evolve, the factors that favor appropriate encounters among virus, competent vector, and susceptible vertebrate are of fundamental importance. As the first of the three-component discussion, in this section, the traits of vectors are described.
Survey of Blood-Sucking Arthropods Involved in Biological Transmission
Both insects and arachnids are involved in biological transmission. In insects, 14,000 to 15,000 species of blood-sucking species representing only about 1.5% of the total number of insect species have been recognized (60). It becomes evident why the biological transmission of viruses evolved unevenly only in selected groups of hematophagous insects when one compares the role in transmission between the two groups of dipterous insects with similar numbers of species worldwide. For example, many mosquitoes (about 3,400 to 3,500 species) are involved in biological transmission, but no tabanid flies (about 3,500 species) play any role in such transmission.
Unfortunately, no satisfactory theory has ever been proposed to explain why those hematophagous, higher dipterous insects have not become biological vectors. At least in tabanid flies, from which California serogroup bunyaviruses have occasionally been isolated, absence of viral replication was confirmed by oral feeding and parenteral inoculation methods (207). Also, despite the large number of flea species, the total absence of flea-borne arboviruses has been puzzling, although limited Tick-borne encephalitis virus (TBEV) replication in fleas and gamasid acarines was demonstrated experimentally (231, 291).
Similarly, the absence of a truly tick-borne alphavirus (family Togaviridae) is enigmatic, even though several viruses have occasionally been isolated from ticks and mites. Among the families Bunyaviridae, Reoviridae, and Rhabdoviridae, ticks are far more involved in transmitting the first two families of viruses than the third family. In contrast to tick-borne and mosquito-borne viruses, very few viruses (all members of the family Togaviridae) are transmitted by lice. They include a new alphavirus isolated from lice infesting a southern elephant seal (184), Fort Morgan virus, Buggy Creek virus, and a subtype of Tonate virus in North America that are transmitted by nest bugs (family Cimicidae). However, the role of the nest bugs from which Kaeng Khoi virus (a bunyavirus with unknown arbovirus status) was isolated is still inconclusive (347).
As for black flies, VSV New Jersey (a member of the family Rhabdoviridae) is the only virus known to be biologically transmitted by them (61), although Eastern equine encephalitis virus (family Togaviridae) has occasionally been isolated from these flies. The Culicoides midges are vectors of more than 50 viruses in three families of viruses (Bunyaviridae, Reoviridae, and Rhabdoviridae) but not in the members of the families Flaviviridae and Togaviridae. Nearly 45% of the viruses isolated from Culicoides midges have not been isolated from other arthropods (206).
Host Selection and Feeding Behavior
Host selection.
Generally, selection of a vertebrate host by vectors is determined by a combination of genetic, behavioral, and ecological factors. Genetic factors are implicated in the anthropophilic behavior of Aedes aegypti and Anopheles gambiae, the vectors of DENV, YFV, Chikungunya virus, and/or O'nyong nyong virus. However, the phenotypic expression, if any, of genetic preference for humans may be transient. In a genetic experiment, although at first the propensity to respond to human was transmitted for a few generations of Aedes aegypti, its magnitude declined in the face of continuing selection for that behavioral trait (273). Gillies (93) concluded that the natural vector population comprised of subpopulations with a range of responsiveness to human. Trpis and Hausermann (315), on the other hand, demonstrated that the house-entering behavior of Aedes aegypti was genetically controlled. Physiologically, the preferential feeding of Aedes aegypti on humans was thought to promote a higher egg production which could be explained by the high isoleucine concentration unique to human blood (111, 281). Between two closely related anthropophilic viruses, Chikungunya virus, transmitted by Aedes spp., and O'nyong nyong virus, transmitted by Anopheles spp., genetic factors in vectors are strongly implicated to account for the difference in vectors involved in transmission.
In the Culex pipiens complex, while some of its populations bite only birds, the others bite mainly humans or other mammals. It was reported that the Nearctic populations feed on both birds and mammals. This was interpreted to explain why the frequencies of infections in human, horse, and bird by WNV were higher in North America (83). This conclusion was disputed on the ground that there existed no strong evidence of difference in vertebrate group preference between the Palearctic and Nearctic poulations of this mosquito (296). As described earlier in the section on autogeny, for this mosquito population, vertebrate host preference is determined on the basis of autogenous versus anautogenous subpopulations (295).
Although genetic and ecological factors set the limits within which a vector selects vertebrate hosts, opportunistic behavior of vectors often modifies actual host selection, depending on the densities of available vertebrate hosts in a given location. As an example, Culex fatigans females collected from houses in Pakistan fed more often on humans than cattle in sheds or in agricultural fields, but the same mosquitoes resting in cattle sheds in winter fed mostly on birds and bovids. However, they changed to humans and bovids during the spring and then back to humans and birds during summer (259). In other study, the principal vectors of JEV obtained blood meals primarily from pigs, cattle, or birds (in the family Ardeidae), depending on the most abundant host in each locality in India (261). Similar opportunistic feeding behavior was also observed for Culex tarsalis, the vector of western equine encephalitis virus (341), and some sand flies as well.
Feeding behaviors.
Evolution of blood-feeding behavior is inseparable from vertebrate-host-seeking mechanisms by the vectors and hence has a crucial importance in the selection of hosts for viruses. Visual and olfactory cues, thermal gradients, and sound frequencies used by vectors in search of vertebrate hosts have been reviewed previously (74).
Daily feeding activity of the vectors must have evolved to synchronize with the activities of their vertebrate hosts to maximize the success of obtaining blood meal. Thus, many indoor mosquitoes feeding humans (such as Anopheles spp.) are nocturnal feeders, as biting success is best when the hosts are asleep, but diurnal feeding activity of the anthropophilic vector of dengue and yellow fever viruses, Aedes aegypti, does not follow such a pattern. Interestingly, it was found that a subpopulation of this mosquito in the savannah of the Ivory Coast was reported to be nocturnal rather than diurnal (70). Similar feeding activity of this mosquito was observed in another subpopulation in Trinidad (46).
It is not known if these unusual reports have any evolutionary significance.
Some mosquitoes that feed first on a vertebrate host have been reported to “remember” the host, and return to the same host in the second attempt even when they are given a choice of hosts. This phenomenon was documented not only in malaria vectors but also in a JEV vector, Culex tritaeniorhynchus (228). However, the conclusions of these reports were challenged by others who could not reproduce the same results (5).
Multiple feeding, which is more common in such vectors as Aedes aegypti, ticks, and sand flies, is also observed, albeit less frequently, in other vectors (i.e., Culex tarsalis) (341). This feeding activity is clearly significant in enhancing transmission of virus among vertebrate hosts and in increasing the probability of concurrent infection and viral genetic mixing (recombination and reassortment).
Between the two major groups of vectors, ticks and mosquitoes, it is believed by some that the associations of some ticks with vertebrate hosts present more primitive forms than those of mosquitoes because ticks' blood feeding even in the absence of a virus causes toxicosis or mortality in some vertebrates. The major physiologic difference in blood digestion between insects and ticks is that in the former it occurs extracellularly at midgut epithelium, while in the latter it occurs within midgut cells. Ticks also differ from mosquitoes for their blood feeding activity at immature stages, longer feeding period, and larger volume of blood imbibed per life span. These are significant for the survival of tick-borne viruses. Other factors being equal, the large volume of blood meal (as much as 2 to 3 ml or more through life) imbibed by ticks favors virus acquisition from infected vertebrates with low virus titer or without demonstrable viremia.
Another behavior that increases vertebrate host range is feeding pattern of many ticks on a different host in each postembryonic life stage. For example, some ticks feed on birds as larva and nymph but on small or larger mammals as adults. According to Hoogstraal and Aeschlimann (129), the multihost pattern which is characteristic of the soft ticks and some hard ticks represents a primitive host association. On the other hand, the one-host life cycle of some hard ticks is the most advanced. As for the Ixodes ticks transmitting TBEV, among three developmental stages engaged in blood feeding, the most important stage for vertebrate host determination was identified to be adult (158).
Virus Receptors
Identification of the virus receptors on the cells in the gut and salivary gland of vectors is critically important, as virus must pass through the gut barrier after ingestion of blood meal and then must replicate in salivary gland before release after extrinsic incubation period (110). Current data have been mostly obtained in vitro, using arthropod cell cultures. Usually, multiple, poorly characterized receptors were identified per virus. Thus, the receptors for DENV were reported to be polypeptides of 40 to 45, 67, and 80 kDa (223, 275). For Venezuelan equine encephalitis virus, the dominant, putative receptor was a 32-kDa polypeptide (191). Those receptors are most likely involved in viral replication in some tissues in vectors.
Strictly speaking, however, the relevance of all the data obtained in vitro remains inconclusive because, unlike mammalian cell cultures, for which tissue origins are well known, the exact tissue origin has never been identified for all currently used mosquito cell lines. This is because homogenates of the entire mosquito embryos were used to start mosquito cell lines. Nevertheless, it was repeatedly demonstrated that the virus-specific rreceptors on mosquito cells were different from those on vertebrate cells (135). When studied in situ, the receptors for Chikungunya virus determined in the midgut brush border membrane of adult and larval Aedes aegypti mosquitoes were glycoproteins of 24, 45, 58, 60, and 62 kDa (222).
Vector-Enhanced Transmission
Vectors facilitate virus replication by injecting a variety of substances in saliva. Some of these substances promote viral replication. Others are immunosuppressants, including antihemostatic substances. Injection of the latter substances results in modified physiologic conditions or behaviors of vertebrate hosts or shortening of blood vessel probing time (and hence enhanced feeding activity) for vectors (271). Such mosquito-enhanced viral replication in laboratory animals was observed for Cache Valley virus, La Crosse encephalitis virus, Semliki Forest virus, Rift Valley fever virus, African swine fever virus, and DENV (75, 100, 118, 211, 361). Virus-infected mosquitoes may assume modified feeding behavior, contributing to enhanced transmission. For example, it was reported that DENV-infected Aedes aegypti probed and fed longer than uninfected mosquitoes (244). However, not all infected vectors demonstrate enhanced feeding. As an example, refeeding rates of Culex pipiens with disseminated infection with Rift Valley fever virus were 21% less than those of the same mosquitoes with nondisseminated viral infection (320).
Selective viral infection of neurons of mosquito vector may have a functional significance. It was found that JEV infects the neurons of the compound eyes but in much less intensity in the neurons for chemoreception. According to the hypothesis of Johnson (144), behavioral modification by viral infection in these neurons would enhance attraction of infected mosquitoes to the source of carbon dioxide (vertebrate hosts). This hypothesis was based on the disproportionately higher ratio of infected mosquitoes captured in light traps baited with CO2 than in the traps without it.
As for tick-borne viruses, the salivary gland extract obtained from ticks was found not only to increase acquisition of TBEV by noninfected ticks cofeeding on an infected vertebrate host but to increase viremia level (4, 170). Although salivary gland extract was reported to be specific to the virus by which the tick species is naturally infected (238), it was also found to promote replication of non-tick-borne VSV, at least in vitro (107). It has been speculated that the pharmacologic substances in the saliva of ticks either immunosuppress vertebrate hosts with anti-inflammatory and antihemostasis molecules or introduce a variety of chemicals to suppress defense mechanisms of vertebrate host by targeting natural killer and interferon synthesis (36). Alternatively, the function of the tick salivary gland extract substances was hypothesized to be maintenance of fluidity of the blood as it passes through the mouthparts and into gut rather than inhibition of blood coagulation at the feeding site (30).
Pathology and Resistance to Viral Infection
Pathology.
Contrary to the old notion that arboviral infection is not detrimental to the vectors, recent closer reexaminations revealed pathologies and reduced functions in mosquito vectors infected with such viruses as Semliki Forest virus, eastern equine encephalitis virus, Sindbis virus, and Rift Valley fever virus (29, 78, 211, 280, 320, 339). Mortality is sometimes considerable in argasid ticks infected with African swine fever virus (77, 118). Obviously, for an arthropod to be a competent vector, the absence or a minimum level of pathology still permitting optimal viral replication is ideal.
However, the impact of pathology on the vector needs to be interpreted at the population level rather than at the individual level. Other factors being equal, when pathology (expressed as mortality and morbidity) is severe, the impact on biological transmission by loss of one host is far smaller (or negligible) in the vector population than in the vertebrate population because, generally, the population of a vector in a given location is far greater than that of the vertebrate. Biological transmission may be temporarily or even permanently interrupted if too many vertebrate hosts are lost. On the other hand, a proportional loss in the vector population does not result in the same magnitude of outcome simply because of the vector's high reproductive rate and the enormous size of the uninfected population that compensates for the loss.
Resistance.
Regarding resistance to viral infection by vectors, there are obvious differences in antiviral defense mechanism between invertebrates and vertebrates. One notable example is lack of humoral antibody responses similar to those in vertebrates. Interestingly, some hard ticks employ an unusual IgG-binding protein to excrete antibody during blood feeding (238). A variety of molecules with antiviral activity also have been occasionally found in mosquito cell cultures infected with arboviruses (175, 232, 265). In more recent studies, Toll-like receptors were found to be responsible in nonadaptive, innate resistance to microbes. These receptors are shared between arthropods (including mosquitoes) and vertebrates (193). Furthermore, although little is known about the molecules in immune responses induced in arthropod cells, activation of several genes encoding anti-microbial peptides was confirmed in mosquito cells infected with WNV (213). RNA interference is a conserved mechanism that pervades the biological world. While RNA-mediated silencing of arboviruses, such as DENV or WNV, in transfected cells in vitro has been reported (277), little is known about the level of the endogenous interference activity in mosquitoes in vivo.
Viral Persistence, Vertical Transmission, and Transmission between Ticks
Once infected, many vectors remain infected for the rest of the life. Generally, the longevity of adult mosquitoes (or viral persistence in adult) is short. For ticks, in contrast, viral persistence should be measured in the context of transstadial transmission, because they can become infected at immature stages. For example, it was reported that Nairobi sheep virus (a tick-borne bunyavirus) survived in larva for 245 days, in nymph for 359 days, and in adult as long as 871 days (179); and ticks infected with Langat virus was able to transmit the virus after more than 3 years (322). Some insect vectors other than mosquitoes with a long life cycle, such as avian nest bugs transmitting Buggy Creek virus (an alphavirus), survive up to 2 years without its host, the cliff swallow (130). Also, avian nest bugs could transmit another alphavirus (Fort Morgan virus) to house sparrows 311 days after acquisition of the virus (272).
Vertical transmission, another mechanism of arboviral persistence in nature, has been documented for an increasing number of arboviruses, although true intrafollicular infection of ovary has not been confirmed in all reports (318). The frequency of vertical transmission is generally low (much less than 1%) for many viruses; however, unusually high frequencies (from 20% to as high as over 90%) of vertical transmission of some bunyaviruses belonging to the California serogroup (of the genus Bunyavirus) and to the genus Phlebovirus were reported.
Both viral persistence and vertical transmission in vector are important not only for viral survival in nature but also for their role in the establishment of biological transmission. However, a mathematical study revealed that viruses could not be maintained in nature with vertical mode of transmission alone no matter how high the rate of vertical transmission was, thus requiring occasional horizontal transmission (82).
Transovarial transmission as a form of intergenerational transfer of virus has been well recognized in ticks too. However, the early observations in Russia that mammalian hosts of TBEV were often infested with both nymphs and adults of Ixodes vectors of different generations led to a speculation that another type of intergenerational transfer of the virus could occur between cofeeding ticks (157). Many tick-borne viruses are probably transmitted intra- as well as intergenerationally between infected (i.e., nymphs or adults) and uninfected (larvae or nymphs) ticks cofeeding on the same vertebrate host (251). This phenomenon thus has unique and important implications on viral persistence, vectorial capacity, and disease transmission dynamics of the tick-borne viruses.
VERTEBRATES
Host Range, Requirements as Hosts, and Virus Receptors
Host range and requirements as host.
The theoretical maximum of vertebrate host range for a virus is genetically determined by virus, vector, and vertebrate host. The actual breadth of host range, however, is reduced by a variety of modifiers, such as presence of ecogeographic barriers preventing viral contact with other vectors and vertebrates. Another form of barrier is unsynchronized seasonal timing between availability of infected vertebrates in viremic stage and feeding activity of vectors or between available uninfected vertebrates and infective vectors in a given environment. One of the two major obstacles for determining natural host range is incomplete field investigation. The other problem is the difficulty of segregating the hosts essential for perpetual biological transmission from nonessential accidental hosts among susceptible vertebrates. Also, degrees of contribution to viral transmission are not the same among competent vertebrates. In TBEV transmission, it was found that 20% of the vertebrate hosts were involved in about three-quarters of transmissions by ticks (252).
Sometimes, maximum susceptible host range is accidentally but only partially revealed when an unusual encounter between a virus and “unnatural” vertebrates occurs as a result of the natural breakdown of ecological barrier or human activities that modify natural conditions. Transportation of vectors or vertebrates and unusual movements of vectors and/or vertebrate hosts either by themselves or by the change of weather patterns are other causes. For example, after the invasion of North America by WNV, the virus was found to infect many vertebrates of both the Old and the New Worlds kept in a zoo or animal breeding facilities, which are otherwise, under normal conditions, not exposed to the virus due to ecogeographic barriers (189, 209).
Transmission of arboviruses largely depends on the availability of a sufficient population of susceptible (or competent) vertebrate hosts in place and time to coincide with biting activity of the vectors. Except for direct or nonviremic transmission, infection of these vertebrates must lead to the development of viremia of sufficient length and viral concentration exceeding threshold. Most mammalian hosts develop immune responses that prevent reinfection. Thus, herd immunity and reduced population size of the susceptibles are the two other interactive negative determinants for transmission. With these constraints, vertebrate host species with a high birth rate or turnover are more suitable for viruses. Unfortunately, available data based on field studies for those negative determinants are scarce.
For a stable, focalized sylvatic YFV transmission to occur in a community of 130 monkeys, it was estimated that a minimum annual birth rate of 400 per 1,000 would be necessary; otherwise, for maintaining the sylvatic cycle, YFV needs to infect many monkey populations geographically separated in a far larger land mass, which results in constant geographic shift in enzootic foci (G. Macdonald, cited by Smith [292]). In fact, in the sylvatic environment, epizootic wave was estimated to move at a rate of 0.5 to 1.0 mile/day (215). Thus, the report of cyclic pattern of sylvatic outbreaks of DENV-2 infections in simian populations (the only vertebrate hosts) characterized by 5 to 8 years of silent interval between outbreaks in the gallery forest in Sénégal (68) is intriguing with respect to herd immunity in primates as a modulating factor in transmission. Interestingly, the sylvatic dengue outbreaks by this serotype have occurred independently of rainfall fluctuation over a period of 28 years there.
Thus, it would be of interest to learn if insufficient population size of any single vertebrate species at any fixed location explains rapid shift of epizootic foci of multihost viruses, such as WNV. On the other hand, for the urban dengue transmission to perpetuate in a fixed urban area, the minimum human population size was estimated to lie between 100,000 and 1 million (162, 163).
Virus receptors.
Thus far, characterization of the virus receptors has been limited to molecular size determination. Extrapolating receptor data obtained in cell culture to viral infection in vivo still requires a cautious interpretation, as described later in the section of shared traits. Some of the better characterized receptors are briefly described.
(i) Fcγ receptors I, II, and III.
Fcγ receptors have been identified in vitro and speculated by some to be responsible for capturing DENV-antibody immune complex to initiate antibody-dependent enhanced viral replication (186, 196). However, non-Fcγ receptors involved in enhancement have also been identified on human lymphoid cells (21).
(ii) Lectins.
Lectins are a group of adhesion molecules within intercellular adhesion molecule (ICAM) found on dendritic cells or on macrophage subpopulation in the dermis of the skin, mucosal surface, lymph nodes, and peripheral tissues. Another group of receptors is DC-SIGN (dendritic cell-specific ICAM-3 grabbing nonintegrins). These molecules have been found to mediate DENV infection of dendritic cells as well as Sindbis virus (153, 311), but are not necessary for WNV and YFV.
(iii) Integrin and laminin receptors.
Integrins are a family of glycoproteins that are heterodimers comprised of α and β chain subunits. There was a speculation that the integrin-binding amino acid motif, RGD, of envelope protein of Murray Valley encephalitis virus was involved in the virus adsorption on cells (176). Similar conclusion was obtained for Bluetongue virus (310). The αvβ3 integrin, a prominent endothelial cell receptor, has been implicated as the functional receptor and associated signaling pathway necessary for WNV entry into vertebrate cells (53).
Laminin receptors (63/67 kDa) are found on cell surfaces characterized by binding to basement membrane laminin with high affinity. Some of the laminin receptors belong to integrin family. High-affinity laminin receptor, which is highly conserved among vertebrates and mosquitoes, was identified as receptor for Sindbis virus (332). The laminin receptors for DENV-1 and DENV-2 identified in hepatic cells were a 37- to 67-kDa protein and the glucose-regulated protein 78 (or GRP 78), respectively (142, 313). The nonintegrin laminin receptor on human embryonic cells utilized by TBEV was found to be a 67-kDa polypeptide (249).
Glycosaminoglycans, such as heparan sulfate, heparin, dermatan sulfate, and chondroitin sulfate, are highly conserved, sulfated, linear polyanionic carbohydrates involved in cellular adhesion. They are ubiquitously expressed in a specifically regulated manner on different tissues and throughout different developmental stages of humans and many vertebrates. Besides DENV, Sindbis virus, JEV, and Ross River virus (42, 48, 352, 359), a few other arboviruses (TBEV, Semliki Forest virus, and Venezuelan equine encephalitis virus) were found to bind to these molecules.
Febrile Condition and Enhanced Attractiveness of Virus-Infected Vertebrates to Blood-Sucking Arthropods
Viral infections of vertebrates often result in febrile conditions. Does higher body temperature serve as a selective thermal cue for blood-feeding activity of some vectors? Is ornithophilic feeding behavior of many Culex mosquitoes explained on the basis of genetically primed attraction to higher body temperature of birds (as high as 41°C)? The available data reveal that, interestingly, higher thermal mutants that replicated well at 40 to 42°C were obtained mostly from the viruses transmitted by ornithophilic Culex mosquitoes (i.e., western equine encephalitis virus, JEV, and WNV) but not from Aedes-borne DENV (138, 240, 288). However, interpretation of the results is difficult because selection of the viruses for experiments has been biased in favor of neurotropic arboviruses that are transmitted by Culex mosquitoes. In other studies, change in ambient temperature or increased carbon dioxide concentration by Sindbis virus- or Rift Valley fever virus-infected hosts was speculated to be the cause of enhanced feeding (197, 319).
Regarding the stimulatory effect of the symptoms of viral infection, one of the questions raised for hemorrhagic viral infections (such as dengue) was if increased vascular permeability as a result of thrombocytopenia increases the probability of transmission to biting mosquitoes (264). The effects for increased transmission were also investigated with respect to increased level of odor in vertebrate hosts infected with TBEV. It was found that the viral infection increased the blood level of testosterone, which, in turn, rendered infected male rodents to be more aggressive and attractive to estrous females, thus enhancing viral dissemination (221).
Vertebrate Resistance to Viral Infection
Innate resistance.
Apart from the age-related resistance to flaviviral infection demonstrated in many mammals, innate resistance of vertebrates to some arboviral infections has been documented, including a report of white-tailed deer populations in North America against Epizootic hemorrhagic disease virus (90). Some rodent species are resistant to at least 13 flaviviruses, such as JEV, louping ill virus, Murray Valley encephalitis virus, St. Louis encephalitis virus, and YFV, mediated by flavivirus resistance genes (33, 34, 325). In more recent studies, the key molecule responsible for resistance to flaviviral infection in rodents in the field as well as in some laboratory-bred mice was identified as 2′-5′-oligoadenylate synthetase (2′-5′-OAS) (201). These resistance genes have been speculated to have evolved in wild rodent populations subjected to the selection pressure by flaviviral infection. However, the evolutionary significance of this genetic resistance is not fully understood, given the fact that rodents are still natural hosts for a nearly dozen vector-borne flaviviruses (i.e., Langat virus, TBEV, Iguape virus, and Saboya virus). Furthermore, it was reported that 2′-5′-OAS played only a minor role in resistance (287). Genetic resistance of rat variants to Rift Valley fever virus was also reported (6).
Another autosomal allele responsible for innate resistance of inbred mice to infection by influenza A virus was designated Mx (for myxovirus). Mx protein has a molecular size of 70 to 80 kDa. It is induced in resistant animals not only by α/β-interferons but also by double-strand RNA. The Mx gene was found highly conserved and has been found in a variety of animals ranging from mammals and birds to fishes; and it is even similar to a gene product of Drosophila melanogaster. The innate resistance factor in mammalian cells against tick-borne myxoviruses (Thogotovirus and Batken virus) and bunyaviruses (Crimean-Congo hemorrhagic fever virus and Dugbe virus) has been determined to be MxA or Mx1 protein (7, 32, 84, 108).
The difference in susceptibility of endothelial cells to infection by bluetongue virus between cattle and sheep determines the disease manifestation, asymptomatic infection in the former but severe infection in the latter. Similarly, the contrasting disease severity in Culicoides-transmitted epizootic hemorrhagic disease virus infections between two subspecies of white-tailed deer in North America could be better explained on genetic difference in susceptibility rather than on difference in acquired humoral immunity. The conclusion was based on the observation that viremia levels and antibody titers were similar between the two subspecies of deer (90).
Cellular and humoral immunity.
It has become increasingly apparent that the important groups of molecules used by mammalian hosts for surveillance to detect invasion of exogenous viruses are Toll-like receptors (TLR). Each TLR recognizes a distinctive pathogen-associated molecular pattern and invokes various antimicrobial innate immunity. The TLR3 recognizes double-stranded RNA, the replicative by-product during RNA virus replication. Some vertebrate cells involved in adaptive immune system (such as B and T cells) are endowed with innate sensing of viruses by means of Toll-like receptors. The Toll-like receptors identified for VSV were TLR7 and TLR8 (24). However, not all TLR functions are protective. TLR3 was speculated to facilitate WNV entry into the brain causing lethal encephalitis (333).
When analyzing the sign of evolutionary trends in the factors, one of the obvious questions asked is the impact of herd immunity induced. Was it absolutely necessary for arboviruses to switch from vertebrate to vector for their survival to avoid rising herd immunity in vertebrate population? Does it explain why no true arbovirus is known to chronically infect vertebrates that are endowed with a full complement of humoral and cellular immune systems?
In most other arboviral infections, however, in the face of rising herd immunity arboviruses had three major strategies for survival. The first strategy was to move to other locations populated with the same host at a less herd immunity level, using the mobility provided by infected vectors. The second strategy was to select vertebrate species with a very high rate of fecundity to ensure perpetual supply of a sufficient number of susceptibles. The third strategy was to develop a mechanism to escape immune reaction and remain within the infected hosts. Adoption of the first strategy was necessary, if the size of vertebrate population per location was small. The second strategy was adopted by choosing the vertebrates with a high reproductive rate, such as rodents and some birds. Both the first and second strategies are considered necessary because arboviruses are the animal viruses that cause primarily acute (but not chronic) infections.
As for the third strategy, many true vertebrate viruses causing chronic infections have developed an immune evasion mechanism (120). Some arboviruses are shielded in particular cells, tissues, or organs and remain infectious and/or are shed for a long period despite high titers of neutralizing antibody in blood (165,166, 238, 312). Some of them even serve to transmit virus to uninfected vectors, as in the cases of TBEV. Accordingly, the traditional concept to regard all immunized vertebrates demonstrating neutralizing antibody as dead-end hosts was questioned (171, 238).
Viremia, Nonviremic Transmission, and Long-Term Infection
Viremia.
Theoretically, the higher the concentration of virus in blood and the longer the duration of viremia, the greater is the probability of a vector acquiring virus from infected vertebrates. According to a theory, some flavivirus (such as DENV and YFV) acquired lymphotropism that is more favorable for generating a high level of viremia to ensure continuous biological transmission (216).
Thus, at least theoretically, vertebrate hosts that are chronically infected with a vertebrate virus and demonstrating a high level of viremia for a long time and that are frequently exposed to the bite of vectors are ideal for supporting biological transmission. For example, hog cholera virus, equine infectious anemia virus, and some rodent-borne arenaviruses, i.e., Lymphocytic choriomeningitis virus, Machupo virus, or Amapari virus, demonstrating almost constant or intermittent viremia of high titer and/or virus shedding in the respective hosts (52) may be considered the ideal candidates to become an arbovirus. Furthermore, these hosts are exposed to the bite of vectors under natural conditions. Other groups of animal viruses, including DNA viruses, are similarly known to establish persistent infection in many of those animals. However, none of those viruses have become arboviruses yet.
Nonviremic transmission.
Development of viremia in vertebrate host has been one of the important requirements for biological transmission (351). Recently, examples of virus transmission between infected and noninfected vectors through cofeeding on the same infected vertebrate host without evidence of viremia (hereafter called nonviremic transmission) have been demonstrated in Thogoto virus, TBEV, Crimean-Congo hemorrhagic fever virus, bluetongue virus, louping ill virus, VSV, and WNV (3, 4, 96, 145, 146, 149, 170, 174, 205). More recently, this mode of transmission was reported for the first time in mosquitoes infected with WNV (119).
Although little is known about the significance or frequency of the occurrence in natural transmission, in terms of viral survival in nature, this phenomenon at least by ticks is likely to be significant. It was also suggested that nonviremic transmission would favor viral survival because of less pathological effects on vertebrate hosts (171, 237). It is noted that, with two exceptions thus far, the majority of the vectors involved in nonviremic transmission have been ticks. Nuttall and Labuda (238) considered ticks to be most suitable vectors for this type of transmission. Ticks tend to feed as a group of many individuals tightly congregated in particular body parts of vertebrates, the ideal condition that facilitates viral transmission in the absence of viremia.
It is also speculated that ticks were involved in the early stage of the evolution for certain lineages of arboviruses; and transmission by mosquitoes, which require a much higher level of viremia evolved only after the virus titers in the blood exceeded the threshold required by mosquito vectors. Regardless of the validity of this hypothesis, at least in flaviviruses, the sequence of vector group association revealed in phylogenetic studies agrees with this speculation (Fig. 1). However, this applies only to the lineage of flaviviruses and cannot be used to generalize for other arbovirus lineages, since most likely not all virus lineages had the same history of host adaptation.
Long-term infection.
The majority of arboviral infections in vertebrate hosts are short. However, exceptionally prolonged infection has been documented experimentally. A report of prolonged infection of birds by western equine encephalitis virus for as long as 234 days (256) generated interests. Other examples of long-term infections include bluetongue virus infection in cattle that lasted as long as 100 days, Omsk hemorrhagic fever virus infection in water voles for 155 days, and western equine encephalitis virus infection in tortoise for 105 days (28, 150, 165, 195). More recently, it was reported that WNV was shed in urine by infected golden hamsters for 8 months (312).
VIRUSES
Genomic Traits
Host range.
The host range of any arbovirus is unquestionably governed by multiple abiotic and biologic factors including genetic traits of viruses and hosts. Thus, no matter how perfectly other basic requirements for the establishment of biological transmission are met, many viruses do not replicate sufficiently in certain hosts because of genetic constraints (126). This may explain why among closely related JEV, Murray Valley encephalitis virus, and WNV, which are all neurotropic in human, significant refractoriness has been observed in crows (JEV), pigs (WNV), and equines (Murray Valley encephalitis virus).
The variation in the breadth of host range among arboviruses is well known, some infecting only a few groups of vectors and/or vertebrates and others infecting many groups (genera and families) of hosts. Generally, it has been recognized that a large proportion of emerging viral infections is caused by multihost zoonotic RNA viruses. These viruses with a higher propensity to switch host are typically characterized by high mutation rate, generating more genetic variants per unit time and have a broader host range. In contrast, the viruses with a very narrow host range were thought less likely to become zoonotic (349). However, application of those generalizations to arboviruses was found difficult because of the lower rates of mutation in those viruses (124, 141). Also, the correlation between restricted host range and reduced zoonotic tendency is questioned, since DENVs in urban areas infect humans, the only vertebrate host. On the other hand, the host shift of DENVs from sylvatic nonhuman primates to humans in urban environments agrees well with the theory that predicts evolution of single host pathogens when the size of the host (humans in this case) population is sufficiently large for viral maintenance (8, 79). Conversely, the viruses with multivertebrate hosts may be hypothesized to be either the consequence of insufficient population size of any single vertebrate species to support transmission or the result of higher rate of mutation unique to those viruses. The former, in turn, leads to the selection of the genetic traits of viruses with a strong propensity of adaptation to multiple vertebrate species. Both possibilities broaden vertebrate host range.
Opinions have been divided regarding the evolutionary significance of host range variation. Some argued that host-parasite relationships with a narrow, specific host range were more advanced, while others thought just the opposite (349). Hurlbut and Thomas (136) speculated an evolutionary progress of flaviviruses towards restricted host range. Mattingly (202) assumed the JEV complex viruses with the widest host range to be the most primitive, Uganda S and Ntaya viruses to be intermediate, and DENV and YFV, with a narrow range, to be most advanced. A part of this hypothesis was also shared by Smith (292), who considered the narrow host ranges of DENV and YFV to be of recent development. On the other hand, a phylogenetic study revealed that the order of divergence of those viruses was just the opposite (169). However, we are of the opinion that, while phylogram reveals the history of host association, it does not reveal if the viruses with a broader vector range are more advanced or more ancestral than the viruses with a narrower host range.
According to a theory primarily developed for multihost helminthic parasites, it has been proposed that increasing number of hosts signifies evolutionary advancement only up to a certain point; thereafter, the reduction of host range is considered a more recent condition. With a limited host range, parasites can enhance their chances of reaching the definitive hosts more directly without a hazardous passage through many intermediate hosts in both aquatic and terrestrial environments (49).
Domain III of the envelope protein gene has been suspected to be involved in regulating the vector range (tick versus mosquito) of flaviviruses. In a replication study of a chimeric infectious clone of DENV-4 whose structural protein genes (partial capsid, premembrane, and envelope) were substituted with the corresponding genes of a tick virus (Langat virus), the chimeric virus nevertheless replicated quite well in mosquito cells, while Langat virus did not (245). This study strongly indicated that, while envelope protein may be involved in vector specificity, nonstructural protein genes and/or noncoding regions are probably more important vector group determinants for flaviviruses.
Rate of mutation.
Regarding the predominance of RNA viruses among arboviruses, their rates of mutation more than a few orders of magnitude higher than those of DNA viruses have been regarded by many to hold a crucial key to unlock the puzzling one-sided distribution of arboviruses by the type of nucleic acid. Was higher rate of mutation (compared with DNA viruses) a requisite for all arboviruses? However, as mentioned earlier, this generalization of high mutation rate among RNA arboviruses has met a problem, because the nonsynonymous/synonymous substitution rates of several alphaviruses and flaviviruses thus far studied were found to be lower than the theoretically expected rates (141). In three studies, the probable cause contributing to this lower rates among arboviruses was identified as vector-borne mode of transmission, since most other RNA viruses directly transmitted by fecal-oral, respiratory, and other contagious routes demonstrated higher rates of substitution (109, 141, 348). Furthermore, in arboviruses, substitution rates were found to be higher during replication in vertebrates than during replication in vectors (125).
Regarding the genetic impact of vector-borne transmission, traditionally genetic constraint has been suspected, two phylogenetically distinct hosts independently selecting virus subpopulation most suitable for replication in each host. For example, Igarashi (137) used a purification theory to explain reduction of genetic variation because arboviruses have to satisfy the requirements for replication in two disparate, alternating hosts. Recent molecular studies using virus population monitored in vivo and in vitro have confirmed that purifying effect or genetic constraint is indeed demonstrable within vector and between two kinds of hosts (125, 183, 337). The occasional, contradictory observations of higher intrahuman rates observed in such viruses as DENV could be attributed mostly to increased frequency of replication in human per unit time, which reflected expanding susceptible human population and dramatically increased frequency of dengue outbreaks in the past half a century (323).
Receptor-binding domain (ligand).
The envelope glycoproteins of some arboviruses involved in adsorption to the host cells have been extensively studied. A genetic reassortant study revealed that glycoproteins encoded in medium segment of the California Serogroup bunyaviruses were responsible for virus-specific adsorption, penetration, disseminated infection in vector mosquito, and transmission (18). For flaviviruses, domain III encoded in the carboxyl-terminal portion of the envelope gene was found to be involved in viral adsorption to host cells (48, 352). In a recent study, an external loop region of domain III of DENV-2 was found to be involved in serotype-specific binding to mosquito cells (135). On the other hand, in other study using human leukocyte cells, involvement of domain II was also observed (21).
One of the integrin-binding motifs, RGD, has also been found to be utilized by many groups of viruses and, thus, considered conserved. The RGD motif was also determined in vitro to be receptor binding for arboviruses such as Murray Valley encephalitis virus (176) and bluetongue virus (310). However, the αvβ3 integrin, the receptor for WNV (and also possibly for JEV), is not highly dependent on the classical RGD binding motif (53). The putative receptor-binding ligand of TBEV includes at least residue 310 of the E protein (199). In other arboviruses, the domains for adsorption and endocytosis of the California serogroup bunyaviruses California encephalitis virus and La Crosse encephalitis virus were determined to be located in G1 glycoprotein (105). For Sindbis virus (an alphavirus), it was located in E2 glycoprotein (25); and specific deletions in the receptor-binding domain of E2 protein severely reduced midgut infectivity in Aedes aegypti mosquitoes (229).
Viral Resistance to Host Defense Mechanisms
Immune escape, immune enhancement, and interference.
African swine fever virus is unique in that it does not even induce neutralizing antibody in infected hosts. However, nearly all animal viruses infecting mammals face induction of protective antibodies in infected hosts. Other factors being equal, the nonpersistent viruses that must evade humoral immune responses, in particular neutralization by antibodies of vertebrate hosts, have a theoretical advantage to become an arbovirus, if they can depend on vectors to escape rising herd immunity. Although neutralization escape mutants were demonstrated many times typically in vitro through repeated exposure of arboviruses to neutralization antibodies (188), evidence of immune escape of arboviruses under natural conditions has been found to be weak (348). In fact, the mechanism of repeated introductions of DENV-1 in parts of the Pacific and Myanmar was frequent lineage extinction and displacement by different genotypes rather than the emergence of neutralization escape mutants (10, 314).
The low possibility of adaptive mutation in arboviruses was considered a trade-off inherent in viral transmission in alternating, phylogenetically disparate hosts (348). Nevertheless, positive selection was phylogenetically demonstrated for VSV New Jersey in Central America (233). This was interpreted ambiguously as the result of adaptation to specific vectors and/or reservoirs at each ecological zone (269). Puzzlingly, however, immune escape mutants of this virus demonstrated loss of fitness in mammalian hosts, at least in vitro (234).
The significance of very weak positive selection in the envelope gene demonstrated for DENV-2 -3, and −4 was interpreted by others to reflect higher frequency of replication in human due to the dramatically increased frequency of outbreaks in the past few decades (324). Interestingly, however, the genes involved in positive selection included not only envelope gene but also nonstructural protein (NS) genes (NS2A, NS2B, and NS5) (19, 324). Positive selection was also documented in purely insect viruses (polydnaviruses) that have nothing to do with replication in vertebrate hosts (113).
Interpretation of the evolutionary significance of multiple-serotype viruses, such as DENV that comprises four serotypes, as the consequence of immune escape has been difficult, because all four DENV serotypes coexist in highly endemic areas without evidence of extinction of any serotype. Based on a computer model, Ferguson et al. (80) interpreted that immune enhancement allowed the coexistence of multiple serotypes and that in its absence only one or a subset of dengue serotype would persist. However, in that study, the possible impact of interference among serotypes (155) was not factored in.
That immunity-driven selection of arboviruses over time can be traced phylogenetically by examining the shift of the prevalent cytotoxic T-cell (CTL) epitope of virus subpopulations (or genotypes) is an interesting new proposition. According to Hughes (134), extinction and emergence of human class I major histocompatibility antigen-restricted CTL epitopes could be recognized in DENV-1 and DENV-3 but not in other dengue virus serotypes, JEV, or WNV. However, more research is necessary before drawing a conclusion because of very small number of virus strain sequences employed in this study.
Interference based on defective interfering particles was hypothesized as one of the mechanisms of genetic constraints of arboviruses (137). The generation of defective interfering particles by several arboviruses (i.e., SINDV, Semliki Forest virus) (73, 302) as well as VSV, Banzi virus, and WNV has been reported. Furthermore, defective interfering particles were reported to have protected mice from lethal infection with VSV and Semliki Forest virus. However, the importance of those data in nature is unknown, since little is known about in vivo production of defective interfering particles of arboviruses in vectors or vertebrates under natural conditions.
More recently, possible evidence suggestive of genotype-specific interference mediated by neutralizing antibody between dengue virus serotypes was presented. According to this report, DENV-1-immune monkeys, upon subsequent secondary infection by an American genotype of DENV-2, were protected. On the other hand, the monkeys secondarily infected with an Asian genotype of DENV-2 were not (155). Most likely, this genotype-restricted interference by the antibody to DENV-1 is not limited to the American genotype of DENV-2, since it had been shown earlier that the prototype from New Guinea could be neutralized similarly by anti-DENV-1 antibody, at least in vitro (354).
Viral genes involved in resistance.
Although viral genes for resistance to host defense have been recognized for many years in large DNA viruses, the evidence of similar genes in arboviruses has accumulated only recently (69). Such viral genes were found in Rift Valley fever virus (26), Bunyamwera virus (340), VSV New Jersey (1), DENV (147; 224; 225), JEV (182), WNV (103), alphaviruses (85), and Thogoto virus (106). All involved genes were found to be NS genes, but the mode of action varies among genes. While some of these antagonists block the transcriptional activation of interferons (IFN-α/β but not IFN-γ), thus increasing the virulence of virus, in others (such as VSV New Jersey) M-protein induces inhibition of host RNA synthesis and nuclear cytoplasmic RNA transport. Also, Kunjin virus NS2A gene was reported to inhibit IFN-β promoter-driven transcription (187). The only DNA arbovirus, African swine fever virus, inhibits expression of proinflammatory cytokines, such as tumor necrosis factor and chemokine (interleukin-8), while inducing production of transforming growth factor β from infected macrophages (317).
The functional significance of the induction of apoptosis reported for an increasing number of arboviruses has been interpreted in two ways. From the point of viruses, it was a pathological effect of viral infection. The development of Councilman bodies in hepatocytes infected with YFV is a good example. Its development in neurologic tissues by neurotropic viruses results in severe consequences. On the other hand, from the view of hosts, it could also be interpreted to be a result of defensive mechanism to rid themselves of virus-infected cells. Apparently, some arboviruses have evolved genes to suppress development of apoptosis to facilitate unimpeded viral replication. As an example, African swine fever virus has two genes encoding for two proteins that inhibit development of apoptosis in the hosts (71).
Virulence
What role the virulence of viruses has played in the evolution of biological transmission is an intriguing subject. Did biological transmission develop only after the virulence of the virus on the host dropped significantly in the course of a long relationship between a virus and a host? Or did it evolve from the beginning of the virus-host relationship only in viruses that could replicate efficiently in the hosts without or with a minimum of pathological effects? Does increased virulence enhance or reduce the transmissibility of arboviruses?
Problems associated with the term “virulence.”
Responding to the above questions regarding arboviruses is difficult without a short introduction to on-going debates, because many virulence-related topics have been the subjects of continuing controversy and disagreement in many branches of microbiology, including arbovirology. The sources of the problems derive from the disagreement over (i) the definition of and (ii) yardstick used for measurement of virulence, (iii) proper level (individual versus population) at which the significance should be interpreted, and (iv) validity of the generalization of the concept developed initially for direct host-parasite relationship (without vector or reservoir) to indirect relationships involving reservoirs and/or vectors (45, 253). Another group of contentious issues include a question over the relevance of the application of the term virulence. The term was coined originally for phenotypic expression of pathogen in vivo. The dispute concerns if the term could be adequately applied to the data obtained in vitro or in unnatural, laboratory animals.
Another fundamental controversy is the debate over the validity of the traditional concept that the evolutionary direction of the host-parasite relationship moves from severely deleterious relations to more stable, least pathological, mutual coexistence (hereafter called the virulence dogma). One of the popular interpretations of the original concept is that virus-host relationships demonstrating severe pathological effects on the host evolved more recently or are primitive; and, conversely, relationships demonstrating a lack of detrimental effects evolved a long time ago and hence are more stable.
Virulence and transmissibility.
Opinion has been divided regarding the relation between the level of viral replication in vertebrate host and extent of transmission in the host population (185). According to Woolhouse et al. (349), although positive relation has been substantiated in some observations, no simple rule has been found to predict the positive correlation between the pathogens with a higher replicative trait (higher virulence) and increased transmission to host, strongly suggesting that the correlation was virus- or even genotype-dependent.
In support of the positive correlation for arboviruses it was demonstrated that the viruses involved in epizootic and/or epidemic transmission of Venezuelan equine encephalitis produced higher levels of viremia; and, furthermore, the vectors involved were different from those in enzootic transmission (306, 355). From these observations developed a concept of dual cycle (enzootic and epizootic) transmission for Venezuelan equine encephalitis virus, enzootic cycle functioning for viral maintenance and epizootic virus strains emerging from enzootic strains by mutation (330, 338). In the similar dual cycle (endemic and epidemic) transmission proposed for dengue, difference in vector species involved in each cycle was considered important (101). Furthermore, in the western equine encephalitis virus complex viruses in the New World, increased neuroinvasiveness of the epizootic western equine encephalitis virus strains was correlated with higher viremia titers and replication in brain tissues.
On the other hand, enzootic strains or subtypes of western equine encephalitis virus (such as an Argentinian strain of western equine encephalitis virus, Fort Morgan virus, and Aura virus) were neither neurovirulent nor neuroinvasive. Like Venezuelan equine encephalitis virus, it was speculated that epizootic strains of western equine encephalitis virus would emerge from nonvirulent strains (20). Also, between the two lineages of WNV, the Lineage I has dispersed extensively causing repeated epizootics and high avian and equine mortalities in Europe and North America (172). On the other hand, lineage II remained in enzootic foci in Africa mostly with inapparent infection in birds and horses (41, 104).
Regarding the observations of the lack of correlation, for example, virulence among the strains of epizootic hemorrhagic disease virus or African swine fever virus could not be gauged by viremia level in deer or swine, respectively, because the viremia levels induced were similar among virulent and attenuated virus strains despite significant difference in the severity of pathology (90, 317). Also, in the 1993 equine outbreak of Venezuelan equine encephalitis in Mexico, an enzootic genotype, IE (which had been traditionally considered less virulent and of limited transmissibility), was involved, causing a 30% attack rate and 50% case fatality rate. Interestingly, viremia by the isolated strains in experimentally inoculated horses was either absent or very low (95). Furthermore, for TBEV transmission it was found that due to the high mortality associated with a higher level of viremia in feral rodents, the rodent hosts that developed a lower level of viremia would contribute most to the viral maintenance (171).
Some investigators favored arbovirus evolution moving towards attenuation in vectors and increased virulence in vertebrate hosts based on the assumption of independent evolution in the two kinds of hosts. When this thought was found to be unrealistic, an alternative theory was developed that predicts evolution towards compromised fitness in both kinds of hosts, replication in vector suppressing viral virulence in vertebrate hosts (335). It is not known, however, if this compromised theory would predict moderation of viral transmission.
Virulence and impact of pathology on the host.
Most of these reports highly critical of the virulence dogma were based on direct transmission of vertebrate pathogens. In contrast, when vector-borne pathogens were studied, the conclusion has not been necessarily the same. For example, in a mathematical model, it was concluded that vector-borne pathogens would remain highly virulent so long as the requirement of a large susceptible host population was met but that ultimately they would evolve toward a less virulent state (151).
Despite recent criticism of virulence dogma, there are many observations of arboviral infections demonstrating low virulence (or inapparent infection) of indigenous virus in indigenous vertebrates in contrast to higher virulence in introduced exogenous hosts (or vice versa) (indigenous-exogenous mismatch). The examples of such mismatch include African horse sickness virus infection between zebras and introduced horses in southern Africa; Omsk hemorrhagic fever virus infection between Russian muskrats and imported North American muskrats in Siberia, Russia; African swine fever virus infection between bushpigs and imported pigs in Africa; bluetongue virus infection between indigenous sheep and imported European breeds in many sheep-breeding countries; Rift Valley fever virus infection between West African dwarf sheep and imported sheep in Africa; YFV infection between African monkeys and New World monkeys; higher viremia titers of eastern equine encephalitis virus in European starlings compared with lower titers in North American robins; and more severe syndrome of indigenous neurotropic arbovirus infection in imported birds than in indigenous birds in North America (87, 156, 165, 189, 215, 241, 298).
ECOLOGIC FACTORS
No single group of organisms demonstrates more extensive and diverse associations with animal viruses than the phylum Arthropoda. The facts that many members of this group are blood-sucking and that they occupy practically all terrestrial ecosystems and biomes except in the Arctic and Antarctic regions provided excellent opportunities for the available viruses to be transmitted biologically.
Environmental Attributes
Tropical forest.
The impact of ecological factors on the diversity of arboviruses is most ideally observed in the least disturbed ecosystem. As an example, the data obtained in the Amazon Basin, which represents an ecosystem enormously rich and diverse in flora and fauna, provide fascinating information on host range and arbovirus speciation. According to the study by Dégallier (67) (Table 1), the mean numbers of the host species per virus involved in transmission of flaviviruses and togaviruses are higher than the corresponding figures for the other three families of viruses (Bunyaviridae, Reoviridae, and Rhabdoviridae). From this table alone a few questions are asked. Do the data show that the former two virus families have a wider host range than the latter three families? Or do they show that the latter families of viruses more efficiently share a smaller number of vertebrate hosts to speciate more extensively in vectors?
TABLE 1.
Variation of host range among virus familiesa
| Virus family | No. of virus species | No. of hosts (mean no. per virus)
|
|
|---|---|---|---|
| Vertebrate | Vector | ||
| Flaviviridae | 8 | 37 (4.6) | 20 (2.5) |
| Togaviridae | 8 | 39 (4.9) | 17 (2.1) |
| Bunyaviridae | |||
| Genus Bunyavirus | 45 | 15 (0.33) | 39 (0.87) |
| Genus Phelebovirus | 25 | 9 (0.36) | 3 (0.19) |
| Reoviridae | 63 | 2 (0.03) | 12 (0.19) |
| Rhabdoviridae | 15 | 3 (0.2) | 6 (0.4) |
Reproduced from reference 67 with minor modifications with permission.
Whatever the mechanism or ecological condition involved, it is clear that a considerable variation in vector range, host sharing, and speciation developed among virus lineages in that tropical environment. Furthermore, as shown in Table 2, in each virus family the number of viruses associated with particular groups of hosts varies considerably, depending not only on host group but on daily feeding activity and vertical stratification of forest habitats (ground level [terrestrial] versus canopy).
TABLE 2.
Relationship of the number of virus species with vector group, biting activity of vector, and ecological characteristics of habitat per virus familya
| Arbovirus family | No. of species
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mosquitoes
|
Sand flies | Midges | Ticks | Vertebrates
|
|||||
| Nocturnal | Diurnal | Diurgnal
|
Nocturnal
|
||||||
| Terrestrial | Canopy | Terrestrial | Canopy | ||||||
| Flaviviridae | 4 | 4 | 0 | 0 | 0 | 6 | 6 | 6 | 6 |
| Togaviridae | 6 | 6 | 0 | 0 | 1 | 6 | 7 | 5 | 5 |
| Bunyaviridae | 37 | 19 | 2 | 2 | 2 | 13 | 17 | 21 | 17 |
| Reoviridae | 8 | 5 | 51 | 0 | 0 | 0 | 1 | 2 | 0 |
| Rhabdoviridae | 3 | 3 | 3 | 0 | 0 | 4 | 2 | 1 | 1 |
Reproduced from reference 67 with minor modifications with permission.
Montane environments.
Understanding of arboviral transmission at high altitudes has been limited in the past with exceptions of some tick-borne viruses. However, interesting observations have been accumulating. Anthropophilic Aedes aegypti is known to inhabit indoors at high altitudes, for example in parts of Colombia, where the mosquito is found at 2,200 m above sea level (305). A recent epidemiologic investigation revealed dengue-specific immunoglobulin M in residents without a recent history of travel outside the community (at >1,700 m above sea level), confirming dengue virus transmission (268). A larger dengue outbreak also occurred in a region at about the same altitude in Mexico (116).
A genetic analysis of the YFV strains isolated in various montane communities (at 1,000 to 3,000 m above sea level) in Peru revealed the absence of genetic exchange between communities. This demonstrated that the virus remained primarily in each enzootic focus without much dispersal (37). Thus, the dispersal of YFV strains in these montane environments contrasted to rapidly moving (wandering) pattern of epizootic foci observed in lowland tropical environments.
For many vectors climatologic conditions are unfavorable at high altitudes. For example, in Sri Lanka, the vectors of JEV are not found beyond 1,200 m above sea level. In the highland of Kenya at 6,200 ft above sea level, ticks were not found in the burrows of warthogs, the vertebrate hosts for African swine fever virus. However, serological evidence of the virus transmission was evident (117), strongly suggesting the occurrence of direct transmission. On the other hand, changing weather pattern may affect vector distribution. In parts of Central Europe, the principal vector of TBEV (Ixodes ricinus) used to be found only up to 700 m above sea level in early 1980s. In another survey conducted in 2001, the tick was found up to 1,000 m (62). Global warming was speculated to be the cause.
Marine and other aquatic environments.
Nearly all arboviruses infect only terrestrial animals, and larval stage of many vectors is spent in aquatic conditions. However, very little has been generally known about arboviral association with other aquatic environments. In fact, arboviruses are not entirely dissociated from marine, riverine, and other aquatic environments. Two alphaviruses of the family Togaviridae that are found in aquatic environments are a virus infecting salmon and trout (342) that is transmitted directly and an arbovirus transmitted by lice infesting southern elephant seals (184). This group of lice are morphologically well adapted to withstand enormous pressure deep in water during the seal's prolonged diving activities. Migratory seabirds are also recognized to be frequently infested with ticks carrying a variety of tick-borne arboviruses, and their role in long-distance dissemination of viruses has been known (56, 357). Even in the sub-Antarctic region, two flaviviruses (Saumarez Reef virus and Gadgets Gully virus) have been isolated from the ticks that infest penguins (220, 300).
Many groups of insects live in marine environments. Certain mosquitoes are known to feed on freshwater or marine fishes (226, 239, 290), but the origin, mechanism, and direction (aquatic to terrestrial or terrestrial to aquatic) of infection that led to the evolution of the aforementioned fish alphavirus without a vector are enigmatic. Some fishes feed on immature vectors in fresh or brackish water. Conversely, terrestrial vertebrates (in particular birds) near wetlands or in coastal areas are known to feed on fishes. Similar to many other monophyletic animal virus groups that have one group of the members exclusively in terrestrial and the other group only in marine environments, food chain is considered one of the possible mechanisms by which arboviral transfer could have occurred either from aquatic to terrestrial environments or vice versa. On the other hand, the unusual St. Louis encephalitis virus isolation from a whale was most likely the result of direct exposure to infective mosquitoes near shore (38).
As mentioned earlier in the section of direct transmission, water voles in the lakes in western Siberia were reported to be infected directly by the Omsk hemorrhagic fever virus released into water from the corpses of diseased muskrats. Normal muskrats were, in turn, infected while wading in water previously virus contaminated by the water voles that shed the virus in urine and feces. Interestingly, it was determined that the virus survived in water for up to 2 weeks in the summer and up to 3 months in the winter in Siberia (87, 150). Including the aforementioned speculation of Rift Valley fever virus transmission in dambos (321), collectively, those reports raised a possible mechanism by which virus transfer could occur between vertebrates and arthropods by direct transmission in such aquatic environments.
Human-modified environments and contact with virus.
A variety of human activities inadvertently facilitated viral contact of vertebrates (including human) that are, otherwise under normal conditions, not exposed to the vectors carrying arboviruses. Subpopulations of vectors near human habitation also gradually changed habitat or feeding behavior accordingly to take advantage of new source of bloodmeal. Many anopheline mosquitoes are primate feeders. Some researchers believe that anthropophilic Anopheles funestus diverged from Anopheles gambiae about 4 to 6 million years ago, which corresponds to the estimated period when human diverged from subhuman primates (39). According to this theory, the increased human density in population centers facilitated Anopheles funestus to specialize in humans. This vector of O'nyong nyong virus rests inside human dwellings and feeds on humans.
Most experts agree that Aedes aegypti similarly originated from a sylvatic ancestor in Africa and became domesticated later through association with human activities (308). In fact, in many parts of Africa, sylvatic Aedes aegypti populations are still zoophilic, rarely biting humans, and play little or no role in DENV and YFV transmission (68, 215). In parts of Asia, subpopulations of mosquitoes normally considered outdoor species, such as Culex tritaeniorhynchus, have become endophilic (indoor living) (148), and in the Reunion Island (Indian Ocean), two dengue vectors, Aedes aegypti and Aedes albopictus, reversed the their typical habitats, the former becoming more exophilic (outdoor living) and not anthropophilic and the latter becoming more endophilic and anthropophilic (276).
It is also strongly speculated that DENV in Asia switched vector from sylvatic mosquitoes (such as Aedes niveus) to Aedes albopictus in peridomestic environments and finally to domesticated Aedes aegypti in urban environments. According to a theory, only after human population centers reached a minimum threshold within the past few centuries, DENV established itself in urban areas (323). Because anthropophilic Aedes aegypti is well recognized to follow human movement, it was even found breeding in gold mine shafts deep underground in Australia (76). Culex pipiens, like Culex quinquefasciatus, is another vector that evolved two genetic groups, cosmopolitan and noncosmopolitan, that flourish inside as well as outside the house (50).
Pig breeding had a profound impact on the distribution of JEV in Asia (289) because of rapid turnover for commercial purpose that ensures abundant supply of nonimmune swine population. Rice paddies provide another environment favorable for breeding of JEV vectors (Culex tritaeniorhynchus, Culex vishnui, and Culex gelidus). The combination of the Green Revolution in Asia and pig breeding probably further created an ideal condition that promoted the expansion of JE problem. It has also been suspected that the transient establishments of JEV in the northern parts of Australia were related to increased, local feral pig population (194).
The dissemination of some Culicoides-borne viruses in Australia has been speculated to be intimately linked to the importation of cattle, as the larvae of Culicoides brevitarsis (midge) depend on cattle dung for growth (241). Omsk hemorrhagic fever became a serious human disease in western Siberia, Russia, after the introduction of muskrats from North America in 1925 to 28 for commercial purposes. Forest clearance for agriculture, environmental destruction for human habitation, dam construction (66), and any other major human encroachments on nature all potentially invited increased contacts between unrecognized, indigenous virus and humans (as in a Kyasanur Forest disease outbreak in India) or between vectors and vertebrates.
In other examples of the accelerated contacts between indigenous viruses and exogenous hosts or vice versa, all due to human activities, the breeds of horses introduced by the European settlers to Southern Africa were susceptible to African horse sickness virus, resulting in many serious epizootics beginning in the 18th century. The introduction of sheep to Scotland was retrospectively determined to be the beginning of tick-borne louping ill disease (97). Likewise, extensive distribution of other ectoparasitic tick-borne viruses, in particular Crimean-Congo hemorrhagic fever virus, has been strongly believed to be the result of animal trade. Other examples of imported animals playing a role in transmission include urban cycle of SLE mediated by the imported, peridomestic house sparrow, eastern and western equine encephalitis outbreaks in imported emus in North America, and Rift Valley fever virus transmission in Africa (13, 86, 123, 328). The importation of cryptically infected zebras was the cause of an outbreak of African horse sickness in horses in Spain (17).
Vector-Vertebrate Interactions
In a mathematical model on the maintenance of Ross River virus, it was concluded that overwintering of the virus in adult freshwater mosquitoes would require a large host population; while overwintering in infected eggs of saltwater mosquitoes would be effective when filial infection rates are high. Furthermore, it was predicted that when marsupial host is replaced by a host with higher birth rate and shorter infectious period, the virus would survive longer under all mathematical models (94).
As for the maintenance mechanism of TBEV, Randolph et al. (252) advanced a theory that synchronization of aggregation for cofeeding of noninfected larvae and virus-infected nymphs (of Ixodes ricinus) was crucial to facilitate infection of larvae from nymphs cofeeding on the same vertebrate host. On the other hand, Dermacentor reticulatus (a competent vector of TBEV under laboratory conditions) could not serve as natural vector because the life cycle pattern of the tick did not facilitate synchronized cofeeding between larvae and nymphs (252). On the other hand, in a more recent field investigation in a TBEV-endemic focus in central Europe, the results did not support the above theory and instead pointed out the importance of mass cofeeding of larvae alone both in the spring and in autumn (63).
Other field studies of TBEV transmission in Europe also revealed how vertebrate hosts were selected. Among many virus-susceptible vertebrates, high levels of viremia developed in bank voles, but only 28% of nymphs were infected due to a strong immune response by the hosts. Pine voles were highly susceptible, developing high virus titers during viremia and also in organs. However, nearly 50% of the voles died before the ticks could finish engorgement. This, in turn, resulted in only about 7 to 10% transmission to ticks. In contrast, field mice (Apodemus spp.) developed comparatively much lower (or even undetectable) titers of viremia, and yet 68% of the nymphs feeding on those field mice became infected (171, 236).
Viral Dispersal
Patterns of viral dispersal vary considerably among arboviruses, some viruses remaining in enzootic foci for years, while others are dispersed extensively. One of the interesting examples of rapid dispersal is demonstrated in the primatophilic chikungunya virus, which has caused dengue-like disease outbreaks in rural communities in Africa and more often in urban areas in Asia in the past. Although the virus is transmitted in urban environments, unlike dengue, chikungunya outbreaks are characterized by shorter duration of outbreak and puzzlingly quick disappearance and reappearance in other remote locations, as demonstrated in a recent report from Indonesia (173). Because of the sharing of the same vector species and human as hosts between DENV and chikungunya virus, an intriguing question raised is if the difference in the pattern of urban transmission between these two viruses reflects the different evolutionary stages of viral adaptation to the urban vector as optimal reservoir, difference in size of human population (threshold) required, or something else. Other viruses or viral subpopulations (or genotypes) also disperse rapidly. For example, it took WNV only 4 years after its introduction to eastern North America to complete transcontinental spread. On the other hand, the Kunjin subtype of the dispersing lineage I of WNV has been confined to Australia and parts of Indonesia.
One unsolved issue on viral dispersal relates to the possibility of repeated, nearly annual introductions of viruses from warmer regions to northern regions of temperate climate. This possibility for eastern equine encephalitis virus in North America could not be supported, since a phylogenetic study revealed that the genotypes in North America dispersed southwards rather than in the opposite direction (31). Regarding St. Louis encephalitis virus in North America, local persistence in southern regions of the United States rather than annual introduction has become a shared view among some professionals. On the other hand, in northern Australia, a genotyping study provided a supporting evidence of repeated southward invasions of JEV from Papua New Guinea (143).
Regarding arboviral dispersal in much larger scale, it has been noted that antigenically similar or phylogenetically related groups of arboviruses are distributed to multiple continents. Mattingly (202) speculated on two possibilities for the mosquito-borne flaviviruses: convergent evolution to account for the similarity among the viruses found in the New World and the New World and dispersal of JEV complex viruses from the Old World to North America. Similarly, Sabin (274) wondered if JEV found its way across the islands linking Asia and North America and then evolving to St. Louis encephalitis virus in the New World. In a more recent molecular phylogenetic analysis, repeated one-way dispersal of the flaviviruses from the Old World to the New World was proposed (98).
Before the advent of molecular tools for investigation, continental drift was also one of the speculations proposed to explain the global distribution of the California Serogroup bunyaviruses, with an exceptional absence in Australia (43). On the other hand, the branching order of the phylogenetic tree of alphaviruses (family Togaviridae) revealed an interesting pattern. When followed from the root to the terminal branches of the tree, extant alphaviruses from the Old World and those from the New World were clustered in multiple branches. This suggested the possibility of multiple two-way crossings of this virus lineage between the two worlds (247).
PECULIARITIES OF BIOLOGICAL TRANSMISSION
Few DNA Arboviruses
Beyond the facts that mutation rates are generally far higher and genome sizes shorter in RNA viruses than in DNA viruses, little is known why, with the only exception of African swine fever virus, all arboviruses are RNA viruses. If ticks (but not insect vectors) were suitable hosts for DNA viruses to become arboviruses, why is African swine fever virus the only DNA arbovirus found in ticks? Similarly, the speculation that insects are not suitable as vectors for the evolution of DNA arboviruses is also questionable.
In fact, besides African swine fever virus, there are actually many vector-borne DNA viruses. They are the vector-borne geminiviruses, single-stranded DNA plant viruses. The mode of transmission of those plant viruses is very similar to the biological transmission of arboviruses. These plant viruses, like arboviruses, must penetrate the body cavity (hemocoel) of the vector (whiteflies, leafhoppers, and treehoppers) before being discharged from the salivary gland to infect plants. However, geminiviruses do not replicate in the vectors. And yet, incubation in vectors is obligatory for transmission. This mode of transmission (termed circulative, nonpropagative mode) of geminiviruses thus appears to be in the intermediate mode between mechanical transmission and biological transmission. Some insect poxviruses (genus Entomopoxvirus) share with mammalian poxviruses similar cell entry and uncoating mechanisms as well as biochemical strategy for replication, the major difference being that the former viruses are defective in late gene expression in vertebrate cells, while the latter are defective in proteolytic processing of late viral proteins in insect cells (180, 181).
Polydnaviruses are obligate, symbiotic, double-stranded DNA viruses that infect insects. Those viruses replicate only in the reproductive organ of the endoparasitic wasps and are vertically transmitted. The genome is either integrated into the chromosome of the wasp or exists as multiple, circular, extrachromosomal DNAs. The viruses are also horizontally transmitted to the wasp's real hosts (i.e., caterpillars), when the wasps lay eggs. Thus, the role of wasps in horizontal transmission is carrier, remotely resembling vector. The major difference from biological transmission is that those DNA viruses do not replicate in the caterpillars; and their function is to suppress defensive mechanisms of the caterpillars, thereby ensuring normal growth of immature endoparasitic wasps (343).
Multiphylum Pathogens
Recent surge of zoonotic viral infections due to host shift has raised an interest in the mechanism involved. A survey of emerging infectious diseases clearly demonstrated that of the 26 viruses known to infect both birds and mammals all were RNA viruses, despite statistical projection that predicts DNA viruses to represent as much as 24.6% of emerging viruses. In addition, the pathogens infecting more than one order of animals were found generally more likely to emerge as zoonotic agents than those infecting only one order (54, 349).
Although small in number, microbes that replicate in multiple phyla or even in multiple kingdoms of hosts exist. For example, spiroplasmas are the mollicutes that infect plants, arthropods (including mosquitoes and ticks), and vertebrates; and some of them are vertically transmitted in insects (27). Although it is not an arbovirus, Flock house virus (a member of the family Nodaviridae) was isolated from a beetle (order Coleoptera). This virus is unique in that it multiplies also in plants and yeast; furthermore, it orally infects mosquitoes (64, 282).
Shared mechanism for viral adsorption to host cell.
It was proposed that the broad host range of some arboviruses is due to the utilization of two kinds of receptors at least one of which is highly prevalent in most hosts and another more host-specific (304). Data supporting the aforementioned theory were demonstrated in differential adsorption of G1 and G2 glycoproteins of La Crosse encephalitis virus to mammalian and mosquito cells, respectively (190). However, it was disputed that G1 glycoprotein alone was involved in infection of both vertebrate and invertebrate cells (105). The available data nonetheless strongly suggest that each virus most likely utilizes multiple receptors of variable binding properties, although probably not all are equally important and/or specific. Furthermore, some of the receptors are utilized by multiple viruses. As described earlier in the section on vertebrate virus receptors, integrin and laminin receptors are most likely the receptors for many viruses.
Among many glycosaminoglycans that are probably involved, the importance of heparan sulfate needs to be interpreted carefully, since the enhanced adsorption observed for the arboviruses (DENV, Sindbis virus, TBEV, and Venezuelan equine encephalitis virus) were found in laboratory-adapted viruses with repeated cell passages. On the other hand, wild strains without in vitro cell passage bound poorly to heparan sulfate (154). Various groups of nonarboviral pathogens also utilize heparan sulfate, Thus, clearly it is not a receptor uniquely exploited by arboviruses alone (329). Integrin is also likely involved in virus binding. Still, generalization for all arboviruses has met with difficulty, because RGD-mediated viral binding to integrin was found to play a minor role, at least for YFV (326); and other flaviviruses (such as DENV) do not have the RGD motif.
Shared viral replication mechanisms.
For a virus to replicate in both arthropod and vertebrate cells, sharing of conserved cellular proteins for all stages of viral replication in two phyla of susceptible hosts is strongly suspected. Because multiple viral genes and cellular proteins are considered to regulate viral replication either alone or in combination, elucidation of such shared cellular factors has been complicated. In an in vitro experiment of eastern equine encephalitis virus passage in alternating host cells (vertebrate and mosquito), it was concluded that host alternation selected virus populations well adapted for replication in both types of hosts (58). At least for WNV, the conserved cellular proteins that bind specifically to 3′ terminal end of the viral genome were found to be important as host range determinants. p52 protein, for example, is an elongation factor 1α and is involved in carrying the charged positive strand RNA to ribosomes (35, 284).
Extrinsic Incubation Period
Arboviruses are unique in that, in addition to the intrinsic incubation period in vertebrate, the incubation period in vector has a considerable significance in the transmission cycle. In practice, an extrinsic incubation period has been determined for convenience under laboratory conditions typically without a specific reference to minimum period. Two problems observed were the discrepancy of the data obtained under natural and laboratory conditions and difference in life cycle among different groups of vectors.
For example, although the mean longevity of adult Aedes aegypti mosquitoes under natural conditions in the tropics has been determined to be only about 8.5 days (283), under laboratory conditions, YFV-infected and DENV-infected females of this mosquito could transmit virus as long as 101 days and 75 days, respectively (286, 301).
Second, its application to ticks was found to be problematic (238) because ticks, unlike mosquitoes, feed at both immature and adult stages (and hence a high frequency of transstadial transmission), they molt between stages, which often reduces virus titer and alters viral replication dynamics, and they have a much longer life span compared to adult mosquitoes. Furthermore, determination of extrinsic incubation period in ticks individually is even more complicated because infective ticks transmit virus efficiently only by feeding in congregation of many individuals rather than feeding singly on a vertebrate host (208, 345). In addition, under natural conditions, some infected ticks survive without feeding on vertebrate hosts for more than a few years; thus, extrinsic incubation period for these ticks under natural conditions heavily depends on the timing of the availability of vertebrate hosts. This prolonged questing period of ticks is considered by some to be a functional equivalent of a very long extrinsic incubation period (251). This complication is not limited to ticks. As mentioned earlier, some avian cimicid bugs, the vectors of a few alphaviruses, remain infected for nearly a year or longer and can survive without vertebrate hosts for up to 2 years.
Reservoirs
The current concept of reservoirs is old and derived from the anthropocentric view of the origins of the pathogens of human diseases in animals. In contrast to the vertebrate viruses, for arboviruses, at least theoretically, both vectors and vertebrate hosts could be reservoirs (202, 267, 344). However, one of the common problems of the topics related to reservoirs in many fields of infectious diseases has been lack of consensus over the definition of a reservoir itself (11, 115, 297, 307). In the World Health Organization characterization of arboviruses (351), although the roles of hosts were classified into maintenance and amplification, the two kinds of hosts were not clearly defined, and the examples of the reservoirs cited actually referred to the rodent hosts of vertebrate viruses but not of arboviruses.
Vector as reservoir.
The probability of a reservoir role of vectors is substantial, given three sources of data. First, there exist multiple records of isolation of such viruses as western equine encephalitis virus, La Crosse encephalitis virus, JEV, St. Louis encephalitis virus, and WNV from overwintering mosquitoes in temperate and subtropical regions where complete cessation of vector activity is clearly marked (15, 22, 40, 177, 230, 334). Also, Fort Morgan virus was isolated from avian bugs in winter in North America and Crimean-Congo hemorrhagic fever virus from overwintering ticks in temperate Central Asia (128). Second, there are an increasing number of records of vertical transmission in vectors in the field. Third, long-term viral persistence through transstadial transmission in ticks has been documented many times.
In regions with a seasonal dry period, which may last as long as 3 to 6 months depending on location, desiccation-resistant eggs provide a means of viral survival. For example, eggs of the mosquito vectors of YFV, such as Aedes and Haemagogus spp., survive desiccation in tree holes and hatch with the return of rain. In fact, as much as 2.9% of nulliparous females of Haemagogus janthinomys were found to be infected with YFV in the Amazon (219). For St. Louis encephalitis virus, persistence in southern regions of the United States, besides vertical transmission, gonotrophic dissociation (blood feeding in late summer or early fall by female mosquitoes destined to diapause) (110), and continuous horizontal transmission by reproductively active Culex mosquitoes were speculated on as possible mechanisms (258). Collectively, these data strongly favor the possibility of vectors serving as reservoirs as well.
Vertebrate as reservoir.
Because most arboviruses have been considered essentially zoonotic infectious agents transmitted by vectors, existence of chronically infected vertebrate hosts (in particular wildlife) has been assumed even though they were not definitively identified. Thus, inclusion of vertebrate reservoir has been an established tradition in depicting arboviral transmission scheme (336). The three commonly used data for identifying vertebrate reservoirs for arboviruses have been (i) virus isolation from suspected animals, (ii) relatively high antibody prevalence in the animals captured in the field, and (iii) demonstration of viremia (of higher virus titer and duration) in the suspected animals typically obtained under laboratory conditions.
However, the combination of these three sources of data alone is still insufficient to identify them as vertebrate reservoirs, because the crucial evidence is lacking for long-term infection in the field that satisfies the requirements of true reservoir. The reservoirs of vertebrate viruses, once infected, would remain infected for the remainder of life; or the length of viral infection is measured in those hosts at least in many months but more often in many years. In contrast, for arboviruses, the recorded lengths of long-term infection were too short to fall into the category of persistent infection (165).
In the temperate and subtropical regions where cessation of biting activity of vectors is clearly marked, the strongest evidence is the combination of two sets of data. The first is detection of persistently infected vertebrate hosts during the period of total absence of biting activity of vectors. The second is demonstration of viremia when vector activities resume with the return of favorable weather.
Unfortunately, the definitive identity of those vertebrates has largely remained elusive despite many years of field investigation. Furthermore, by the definition, to satisfy the requirement as reservoirs of arboviruses, infected vertebrates must be either constantly viremic during maintenance period or somehow become viremic again by activation after a latent period, when vector's biting activity resumes. No reliable example of the first possibility has been found, with the sole exception of bluetongue virus in cattle. The second possibility is highly unlikely because it requires a precise synchronization of reactivated viremia and vector biting activity. In fact, such reports documenting recurrent viremia have been found very infrequently (165). Furthermore, an attempt to demonstrate relapse of St. Louis encephalitis virus viremia with an immunosuppressant failed (260).
Accordingly, the significance or the validity of rare virus isolation from overwintering vertebrates, such as western equine encephalitis virus from snakes in North America (91), persistence of bluetongue virus in cattle for nearly 5 years (192), and unusual detection of JEV genome (but not infectious virus) in blood cells from pigs obtained in winter in Far East Asia (353), was seriously questioned. The skepticism arose because subsequent field or laboratory investigations either failed to reproduce the same results or to isolate virus in the spring from the animals that were PCR-positive in winter or because vectors feeding on those infected vertebrates in winter failed to get infected (254, 258). Other examples of prolonged infections obtained under laboratory conditions, such as WNV shedding for 8 months by the golden hamster (312), require a careful interpretation, because the animals used were not natural hosts for the viruses tested.
However, the possibility of identifying true vertebrate reservoirs of arboviruses still exists. Even for such zoonotic viruses as Ebola viruses to which the concept of reservoirs in wildlife is best applied, thus far the search for the reservoir has not been fruitful after many years of arduous research (243). Furthermore, capturing wildlife in sufficient quantity, unlike capturing flying vectors with a trap, is more labor-intense and difficult. These problems notwithstanding, the recent WNV isolation from a red-tail hawk in winter in North America (88) rekindled the interest in vertebrate reservoir. Furthermore, based on an in vitro experiment, persistence of bluetongue virus in specialized T cells was proposed to account for its possible overwintering mechanism (309).
Unusual reports of viral genome integration in vertebrate host chromosome, such as that of Sindbis virus (360) and isolation of TBEV from a human patient 17 years after infection (reviewed in reference 166), usually have been received with skepticism. However, the recent confirmation of chromosomal integration of an ancestral flavivirus genome in mosquitoes collected in nature (59) renewed interest with respect to viral persistence. In fact, it was shown that conversion of vial RNA to cDNA was not restricted to retroviruses and was found possible in certain breeds of rodents that have a unique reverse transcriptase (152).
Thus, theoretically, it is still possible to eventually identify vertebrate reservoirs for some arboviruses.
As one of the solutions for the vertebrate reservoir issue, vertebrate hosts involved in viral maintenance are simply called enzootic hosts without reference to their potential role as reservoirs, if direct application of this term originally conceived for vertebrate viruses ultimately proves to be difficult. Then, arboviruses would be considered unique zoonotic viruses whose arthropod vectors play a role of primary natural reservoirs as well; while vertebrates serve in enzootic or epizootic transmission primarily for viral amplification with a possibility of playing an occasional role in viral maintenance for shorter durations. Another solution is to use the terms (reservoir and vector) with a direction (297). Thus, reservoir is the host that carries virus passively; while vector is defined as the host that carries virus with direction.
Whichever is the more optimal solution, DENV must be considered different. DENV-infected humans are amplifying hosts and/or dead-end hosts in urban environments. Furthermore, if they move around during the viremic stage, they even serve as vectors (by the original definition) (112). Regardless, the concept of vertebrate reservoir cannot be applied to DENV, because humans are not reservoirs, contrary to the perception by the others (217, 266). As for the notion of subhuman primates as reservoirs of YFV, it was considered a perpetuated myth (92).
Comparison with Other RNA Viruses
Animal RNA viruses currently not considered arboviruses.
Animal viruses that are not arboviruses nonetheless demonstrate interesting relationships with arthropods and vertebrates (167). Lymphocytic choriomeningitis virus (an arenavirus of rodents) was found to be transmitted transstadially in ticks or to guinea pigs by mosquito under laboratory conditions (57, 210). Quaranfil virus, which is currently unclassified but proposed to be an arenavirus (358), replicates in and is transmitted by ticks; furthermore, it has been isolated from febrile humans, pigeons, cattle egrets, and other animals. In the members of the genus Hantavirus (of the family Bunyaviridae) directly transmitted by persistently infected rodents, biological transmission by ectoparasitic acarines under laboratory conditions has recently been observed in both Asia and North America (114, 362). In the family Nodaviridae, Nodamuravirus, which was originally isolated from Culex mosquitoes in the field, is transmitted by mosquitoes, at least under laboratory conditions, and replicates in both insects and vertebrates, causing paralysis or mortality. Another nodavirus, Flock house virus, originally isolated from a beetle, multiplies in mosquito, mammalian cells, plants, and yeasts (64).
Among the vertebrate viruses of the genus Vesiculovirus (family Rhabdoviridae), a few unclassified viruses (Kotonkan virus, Obodhiang virus, and Rochambeau virus) have been isolated from mosquitoes in the field. Mokola virus of the genus Lyssavirus is a human pathogen that replicates in mosquitoes in vitro and in vivo and can be transmitted vertically in mosquitoes at least under laboratory conditions (2). This virus was thus speculated to have derived from an insect virus (2, 285).
Vector-borne plant RNA viruses.
Because many plant viruses are also transmitted biologically by vectors, comparison of the modes of transmission between arboviruses and plant viruses may yield useful information. Of the two major circulative modes of plant virus transmission (nonpropagative and propagative) that require an obligatory latent period in vector, the latter is closest to biological transmission, since viruses must replicate in the vectors to be transmitted. Although there exists no example of the former mode (nonpropagative, circulative transmission) in arboviruses, this mode is also interesting from the evolutionary point of view, because it resembles the intermediate mode between mechanical and biological transmissions. According to speculation, some of the single-strand DNA plant viruses of the genus Geminivirus are apparently moving to become propagative in vectors, based on cytopathological evidence in vectors (99).
Advantages.
The intriguing question regarding the advantages of biological transmission of arboviruses is similar to the unresolved, perennial, and controversial question about the evolutionary advantages of the complicated life cycles of helminthic parasites, with some developmental stages in aquatic hosts and others in terrestrial hosts. One of the advantages common to all disease transmissions that entail pathogen maintenance in reservoirs (such as arboviral transmission) is that basic reproductive number remains above unity even when susceptible vertebrate host populations are low (8). Also, this number is strongly speculated to be higher for TBEV by the cofeeding of ticks on the same vertebrate hosts (171, 252).
The other frequently speculated advantage for arboviruses has been improvement of viral survival. This can be achieved by arboviruses moving out of vertebrate populations with increased herd immunity levels and finding susceptible hosts elsewhere through exploitation of the mobility of vectors. Another benefit provided by the mobility of vectors is the theoretical advantage for virulent pathogens to withstand high mortality in vertebrate hosts because they can be transported to vertebrate populations on the fringes of their transmissible range (151). In contrast, the viruses that developed a strategy to overcome the defensive mechanism of vertebrate hosts did not need the vector's assistance for survival. For example, no-vector group of flaviviruses, which are considered ancestral to the vector-borne flaviviruses (Fig. 1), either persist in hosts without inducing neutralizing antibody (such as Rio Bravo virus) or persist despite induction of neutralizing antibody (such as Modoc virus) for long periods (>6 months) (165).
For the arboviruses that could have derived from arthropod viruses, the significance of viral replication in vertebrates may be interpreted as a necessary and supplementary amplification mechanism because those virus populations cannot be maintained within vectors alone, however efficient vertical transmission may be (82). A mathematical model for the transmission of TBEV by Ixodes ticks also confirmed that the tick-borne virus could not be maintained for a long period if the virus depended solely on transstadial and transovarial transmissions in ticks (158). And yet, according to other thought, the function of the vector in biological transmission is to serve as a selective sieve to avoid viral competition for replication (such as interference) when more than one virus infects the same host (250). The purification theory proposed by Igarashi (137) concerned elimination in arthropods of defective interfering particles generated in vertebrates. According to another thought without involvement of defective interfering particles, a viral propagative cycle involving two phyla of hosts was considered advantageous because it contributes to the genetic stability of arboviruses through purification (125, 337).
GENETIC ANALYSIS OF RELATIONSHIPS BETWEEN ARBOVIRUS LINEAGE AND HOST
Evaluating the correlation between viral genome or taxonomic affiliation and phenotypic traits (such as disease syndrome or host range) has been one of the interests in arbovirology. However, lack of sequence data has often precluded such an analysis for many arboviruses. Nevertheless, at least for the mosquito-borne flaviviruses, strong correlations between neurotropic JEV complex viruses and their Culex vectors and between viscerotropic viruses (such as DENV and YFV) and Aedes vectors were recognized very early in the history of arbovirus research (274). The result of a recent phylogenetic study (89) partially confirmed the earlier observations.
Regarding the history of host shift, thus far, the best evidence has been obtained in flaviviruses. As shown in Fig. 1, the host range of flaviviruses apparently shifted multiple times. Figure 1 was prepared by a neighbor-joining inference program (MEGA2; version 2.1) (161). Distances were calculated using Poisson correction. The numbers at nodes indicate bootstrap supports (%) calculated by sampling of 500 replicates. This basic tree topology was found to be essentially identical to the tree produced by a Bayesian inference method based on 1 million replicates (MrBayes; version 3.0b4) (132), except that the branch topology of the three viruses (Tamana bat virus, cell-fusing agent virus, and Kamiti River virus) (hereafter called the three distant flaviviruses) was trifurcated, indicating that the exact branching order among the three viruses could not be determined by that method (Kuno and Chang, unpublished).
In Fig. 1, from the three distant flaviviruses (cell-fusing agent virus, Kamiti River virus, and Tamana bat virus) at the root of the tree evolved the no-vector group; and from the no-vector group evolved two vector-borne groups, tick-borne and mosquito-borne in that order. Thus, our results support the earlier speculations that the flaviviral association with ticks was more primitive than with mosquitoes (279) and that the vector-borne mode of transmission was an acquired trait. Regarding the distant viruses, both cell-fusing agent virus and Kamiti River virus are insect viruses because they cannot replicate either in tick cells or in vertebrate cells (278, 303) (Kuno, unpublished). On the other hand, Tamana bat virus (248) is a no-vector vertebrate virus which cannot replicate in mosquito or tick cells. Thus, none of them are arboviruses.
At least Fig. 1 demonstrates that vector-borne groups evolved from a group of vertebrate viruses (no-vector group). In turn, the no-vector group evolved either from insect viruses (cell-fusing agent virus and Kamiti River virus) or from a no-vector vertebrate virus (Tamana bat virus). As far as speciation is concerned, evidently the mosquito-borne group was the most successful because the numbers of extant species in the no-vector, tick-borne, and mosquito-borne groups, including viruses not shown in Fig. 1, are 11, 12, and 41, respectively (169).
Is it possible for a virus lineage to shift host range dramatically many times? Hurlbut and Thomas (136) theorized that insectivorous vertebrates became infected by ingesting arthropods infected with arthropod viruses, implying that this was one of the possible mechanisms to explain major host range shift from insect to vertebrate. Interestingly, Tamana bat virus and several members of the no-vector group are viruses of bats.
The evolutionary process must be analyzed in both progressive and regressive directions. In the mosquito-borne flaviviruses, a puzzling question of possible regression was raised because three viruses, Entebbe bat virus, Sokuluk virus (not shown in Fig. 1), and Yokose virus, that have been isolated only from bats but never from vectors nonetheless clustered in the mosquito-borne group (Fig. 1) (169). Although having no known vector thus far, these viruses, unlike the members of the no-vector group of vertebrate flaviviruses, replicate well in mosquito cells in vitro (327).
Two possibilities were proposed to account for these discrepant data. First, the absence of vectors is simply due to incomplete field investigation. Second, it is a consequence of regression of vector association after becoming mosquito-borne viruses. According to Mattingly (202), the loss of vector association of Entebbe bat virus could be interpreted to represent the more advanced level in the evolution of host-parasite relationship illustrated in the stepwise evolutionary theory of Baker (16). Alternatively, we previously interpreted the in vitro replication of those viruses in mosquito cells as vestigial evidence of the former mosquito-borne status (169).
Regarding host shift in other arboviruses, both YFV and DENV infect subhuman primates in sylvatic environments and humans in urban environments. And yet examination of the phylogenetic trees of YFV and DENV revealed further differences in host range history. In the YFV tree, sylvatic strains (isolated from monkeys) and urban virus strains (isolated from humans) cluster in the same branch, indicating continuous gene flow (178, 227). On the other hand, in the DENV tree, sylvatic virus strains are clearly segregated from urban strains because they belong to different branches (331). This contrast in two viruses thus agrees quite well with the epidemiologic observations. In YFV, no subpopulation that is completely adapted to urban environments and genetically segregated from the sylvatic strains has ever evolved; thus, each urban epidemic is caused by the incursion of a sylvatic virus population (or populations) into urban areas (218). On the other hand, for DENV, once the virus became established in urban environments, the genetic linkage with sylvatic viral populations was completely severed; and the viral populations in urban areas have been independently perpetuated between domesticated mosquito and human. Interestingly, both Aedes aegypti and Aedes albopictus, the vectors of urban dengue, were found to be susceptible to infection by the DENV-2 strains isolated in urban outbreaks but not by the strains isolated in sylvatic environments (217).
The history of host adaptation of bunyaviruses is also intriguing, because each of the five genera has a unique host range: genus Bunyavirus mostly in mosquitoes with a small number in other vectors, such as midges; genus Phlebovirus strictly in sand flies; genus Nairovirus only in ticks; genus Hantavirus as no-vector vertebrate viruses; and genus Tospovirus transmitted to plants by insect vectors (thrips). Unfortunately, because neither a monophyletic relationship nor a satisfactory phylogenetic relationship among the five genera has ever been firmly established, at this moment, it is difficult to discuss the history of host range selection in bunyaviruses. However, in the tick-borne viruses of the genus Nairovirus, perfect segregation of the viruses into two branches, soft tick-borne and hard tick-borne, was clearly shown, which strongly suggested to the investigators evidence of coevolution (more appropriately cospeciation) between virus and vector (127).
PAST THEORIES ON BIOLOGICAL TRANSMISSION OF ARBOVIRUSES
Because reconstruction of the evolutionary history of biological transmission is difficult in the absence of fossil records, a variety of early theories proposed were invariably based a priori on the virulence dogma. Huff (133), based on his malaria studies, favored the idea that parasitism originated in invertebrates and was later transferred to vertebrates when invertebrates developed hematophagy. The evolutionary direction of arthropod-parasite-human relationships by Baker (16) was based on his anthropocentric belief that direct transmission of parasites with increased virulence to humans in the absence of vectors (which played the intermediate role) was most advanced.
Based on more abundant data on arthropod-animal virus relationships available by the early 1950s, Andrewes (9) proposed a theory of vector origin of arboviruses and conceived the existence of multiple virus lineages by recognizing the complexity of virus-host transmission mechanisms. Most importantly for this review, Andrewes emphasized the importance of studying the evolutionary significance of seemingly insignificant or odd virus-host relationships that usually receive little attention, which he termed blind-alley infections (9). Also, Mattingly (202), based on vector-vertebrate relations, postulated that most of mosquito-borne arboviruses originated in culicine mosquitoes and that their introduction to mammals was the secondary development. He further considered narrow vector host range to be the result of progressive loss of host adaptational potential, by holding the viruses with a wide host range to be more primitive and the viruses without a known vector (i.e., no-vector flaviviruses) to be in the advanced stage (202). As for biological transmission, Blok and Gibbs (23) are of the opinion that the arthropod transmission of flaviviruses was the acquired trait of directly transmitted vertebrate viruses. On the other hand, according to Schlesinger (279), flaviviruses were thought to have evolved from a common precursor virus infecting arthropods and moved in two directions regarding host association, gaining the ability to replicate in vectors in one direction and becoming the no-vector group flaviviruses in the other.
CONCLUDING REMARKS
Given the enormous number of blood-sucking arthropod species and the diverse hosts available in the numerous and varied environments they occupy, it is not surprising that each of many arbovirus lineages evolved at a different time independently in association with a unique sets of available competent hosts, using vector-dependent replication, survival, and virus transportation between vertebrate hosts as the shared biologic traits. Accordingly, just like the strongly speculated, convergent evolution of hematophagy in many different groups of arthropods (which emerged at different geologic periods), biological transmission most likely evolved by convergence in many viruses.
The very initial event leading to the establishment of biological transmission has always been the encounter among the three essential components, virus, vector, and vertebrate. Although infinite number of new encounters with new partners occur daily worldwide, nearly all are purely accidental or abortive, allowing only extremely small number of the contacts that meet all required conditions in time and space to proceed to establish biological transmission. Still, among the very small number of encounters that somehow succeed, sooner or later most will become extinct when the required conditions are disrupted irrevocably. Accordingly, the extant arboviruses represent an infinitely small proportion of the successful encounters that have survived for many years. Even in the well-established endemic (or enzootic) areas, dynamic changes in virus population, including repeated extinction and reintroduction (or displacement) are expected to occur constantly, in particular among the viruses transmitted by flying vectors. This was demonstrated in the genotype change over years in the St. Louis encephalitis virus populations in the Central Valley of California (160) and DENV in the Pacific and Asia (10, 314). However, it is cautioned that extinction of a virus species, as opposed to the extinction of subpopulations or genotypes, should not be determined in short terms because of long intervals of inactivity of some arboviruses. Such examples include nearly 30 years of quiescence for Murray Valley encephalitis virus in Australia between epidemics in the early 1920s and reemergence in 1951 and O'nyong nyong virus in Africa between 1959 and 1996.
Throughout this review, the difficulty of generalization of the observed phenomena by vector group, vertebrate host, or by virus group was repeatedly commented. The enormous variation in combination of the partners in biological transmission among arboviruses is one of the reasons for the difficulty. However, the difficulty also partly derives from the fact that many concepts, including the definition of arbovirus, were traditionally established based primarily on the observations of mosquito-transmitted viruses. The recent revelations that the mechanisms of transmission and viral survival for tick-borne viruses are quite different despite sharing of biological transmission (251) presented an urgent need to make a comprehensive reappraisal of this unique mode of viral transmission.
Inasmuch as evolution is an on-going biologic process, biological transmission also must be evolving constantly either progressively or regressively. Most of the virus-vector-vertebrate relationships that do not entirely satisfy the requirements of biological transmission have been too often dismissed as either accidental contacts without a biological significance or observations of unknown importance. However, as pointed out in blind-alley infections by Andrewes (9), some of those observations whose full significance is not entirely clear might indeed represent the putative transient stages in either progressive or regressive direction of biological transmission.
As demonstrated clearly throughout the sections of this review, like in typical research initially set to answer certain questions, more new questions were raised than the number of questions answered. Many fundamental questions on biological transmission still remain unanswered. Nevertheless, with the volume of new information accumulating sharply, periodic review exercise, such as this, would be useful for further reducing the puzzles and unresolved issues and for refining more plausible thoughts on the evolution of this unique mode of animal virus transmission. In that regard, the rapidly spreading problems of such arboviruses as Crimean-Congo hemorrhagic fever virus, DENV, JEV, TBEV, and WNV that are currently in progress as well as continuing problems with Venezuelan equine encephalitis virus and YFV provide excellent opportunities for scientists to test the hypotheses discussed in this review as well as new proposals.
Acknowledgments
Comments by the anonymous referees were invaluable for improving the text. Translation of Russian articles by M. Kosoy of CDC (Fort Collins, CO) and permission by Pedro Fernando da Costa Vasconcelos of the Instituto Evandro Chagas, Belém, Brazil, to reproduce the tables with minor modifications are very much appreciated.
REFERENCES
- 1.Ahmed, M., M. O. McKenzie, S. Puckett, M. Hojnacki, L. Poliquin, and D. S. Lyles. 2003. Ability of the matrix protein of vesicular stomatitis virus to suppress beta interferon gene expression is genetically correlated with the inhibition of host RNA and protein synthesis. J. Virol. 77:4646-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aitken, T. H. G., R. W. Kowalski, B. J. Beaty, S. M. Buckley, J. D. Wright, R. E. Shope, and B. R. Miller. 1984. Arthropod studies with rabies-related Mokola virus. Am. J. Trop. Med. Hyg. 33:945-952. [DOI] [PubMed] [Google Scholar]
- 3.Alekseev, A. N., and S. P. Chunikhin. 1990. Exchange of tick-borne encephalitis virus between Ixodidae simultaneously feeding on animals with subthreshold levels of viremia. Med. Parazitol. Bolezni. 2:48-50. (In Russian.) [PubMed] [Google Scholar]
- 4.Alekseev, A. N., S. P. Chunikhin, M. Y. Khkhkyan, and L. F. Stefutkina, L. F. 1991. Possible role of Ixodoidea salivary gland substrates as an adjuvant enhancing the arbovirus transmission. Med. Parazitol. Parazitol. Bolezni 1:28-31. (In Russian.) [PubMed] [Google Scholar]
- 5.Alonso, W. J., T. D. Wyatt, D. W. Kelly. 2003. Are vectors able to learn about their hosts? A case study with Aedes aegypti mosquitoes. Mem. Inst. Oswaldo Cruz 98:665-672. [DOI] [PubMed] [Google Scholar]
- 6.Anderson, G. W., J. A. Rosebrock, A. J. Johnson, G. B. Jennings, and C. J. Peters. 1991. Infection of inbred rat strains with Rift Valley fever virus: development of a congenic resistant strains and observations on age-dependence of resistance. Am. J. Trop. Med. Hyg. 44:475-480. [DOI] [PubMed] [Google Scholar]
- 7.Anderson, I., L. Bladh, M. Mousavi-Jazi, K.-E. Magnusson, A. Lundkvist, O. Haller, and A. Mizrazimi. 2004. Hum. MxA protein inhibits the replication of Crimean-Congo hemorrhagic fever virus. J. Virol. 78:4323-4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson, R. M., and R. M. May. 1992. Infectious diseases of humans: dynamics and control. Oxford University Press, Oxford, United Kingdom.
- 9.Andrewes, C. H. 1957. Factors in virus evolution. Adv. Virus Res. 4:1-24. [DOI] [PubMed] [Google Scholar]
- 10.A-Nuegoonpipat, A., A. Berlioz-Arthaud, V. Chow, T. Endy, K. Lowry, L. Q. Mai, T. U. Ninh, A. Pyke, M. Reid, J.-M. Reynes, S.-T. S. Yun, H. M. Thu, S.-S. Wong, E. C. Holmes, and J. Aaskov. 2004. Sustained transmission of dengue virus type 1 in the Pacific due to repeated introductions of different Asian strains. Virology 329:505-512. [DOI] [PubMed] [Google Scholar]
- 11.Ashford, R. W. 2003. What is a reservoir not a reservoir? Emerg. Infect. Dis. 9:1495-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Austin, R. J., T. L. Whiting, R. A. Anderson, and M. A. Drebot. 2004. An outbreak of West Nile virus-associated disease in domestic geese (Anser domesticus) upon initial introduction to a geographic region, with evidence of bird to bird transmission. Can. Vet. J. 45:117-123. [PMC free article] [PubMed] [Google Scholar]
- 13.Ayers, J. R., T. L. Lester, and A. B. Angulo. 1994. An epizootic attributable to western equine encephalitis virus infection in emus in Texas. J. Am. Vet. Med. Assoc. 205:600-601. [PubMed] [Google Scholar]
- 14.Baer, G. M., and D. F. Woodall. 1966. Bat salivary gland virus carrier state in a naturally infected Mexican freetail bat. Am. J. Trop. Med. Hyg. 15:769-771. [DOI] [PubMed] [Google Scholar]
- 15.Bailey, C. L., B. F. Eldridge, D. E. Hayes, D. M. Watts, R. F. Tammariello, and J. M. Dalrymple. 1978. Isolation of St. Louis encephalitis virus from overwintering Culex pipiens mosquitoes. Science 199:1346-1349. [DOI] [PubMed] [Google Scholar]
- 16.Baker, A. C. 1943. The typical epidemic series. Am. J. Trop. Med. 23:559-566. [Google Scholar]
- 17.Barnard, B. J. H., R. Bengis, D. Keet, and E. H. Dekker. 1994. Epidemiology of African horse sickness: duration of viremia in zebra (Equus burchelli). Onderstepoort J. Vet. Res. 61:391-393. [PubMed] [Google Scholar]
- 18.Beaty, B. J., M. Holterman, W. Tabachnick, E. J. Rozhon, and D. H. L. Bishop. 1981. Molecular basis of bunyavirus transmission by mosquitoes: role of the middle-sized RNA segment. Science 211:1433-1435. [DOI] [PubMed] [Google Scholar]
- 19.Bennett, S. N., E. C. Holmes, M. Chirivella, D. M. Rodríguez, M. Beltran, A. V. Vorndam, D. J. Gubler, and W. O. McMillan. 2003. Selection-driven evolution of emergent dengue virus. Mol. Biol. Evol. 20:1650-1658. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi, T. I., G. Aviles, T. P. Monath, and M. S. Sabattini. 1993. Western equine encephalomyelitis: virulence markers and their epidemiologic significance. Am. J. Trop. Med. Hyg. 49:322-328. [DOI] [PubMed] [Google Scholar]
- 21.Bielefeldt-Ohmann, H., M. Meyer, D. R. Fitzpatrick, and J. S. Mackenzie. 2001. Dengue virus binding to human leukocyte cell lines: receptor usage differs between cell types and virus strains. Virus Res. 73:81-89. [DOI] [PubMed] [Google Scholar]
- 22.Blackmore, J. S., and J. F. Winn. 1956. A winter isolation of western equine encephalitis virus from hibernating Culex tarsalis Coquillett. Proc. Soc. Exp. Biol. Med. 91:146-148. [DOI] [PubMed] [Google Scholar]
- 23.Blok, J., and A. J. Gibbs. 1995. Molecular systematics of the flaviviruses and their relatives, p. 270-289. In A. J. Gibbs, C. H. Calisher, and F. García-Arenal (ed.), Molecular basis of virus evolution. Cambridge University Press, Cambridge, United Kingdom.
- 24.Boehme, K. W., and T. Compton. 2004. Innate sensing of viruses by Toll-like receptors. J. Virol. 78:7867-7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boehme, K. W., J. C. Williams, R. E. Johnson, and H. W. Heidner. 2000. Linkage of an alphavirus host range restriction to the carbohydrate-processing phenotypes of the host cells. J. Gen. Virol. 81:161-170. [DOI] [PubMed] [Google Scholar]
- 26.Bouloy, M., C. Janzen, P. Vialat, H. Khan, J. Pavlovic, M. Huerre, and O. Haller. 2001. Genetic evidence for an interferon-antagonistic function of Rift Valley fever virus nonstructural protein NSs. J. Virol. 75:1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bové, J. M. 1997. Spiroplasmas: infectious agents of plants, arthropods and vertebrates. Wien. Klin. Wochenschr. 109:604-612. [PubMed] [Google Scholar]
- 28.Bowen, G. S. 1977. Prolonged western equine encephalitis viremia in the Texas tortoise (Gopherus berlandieri). Am. J. Trop. Med. Hyg. 26:171-175. [DOI] [PubMed] [Google Scholar]
- 29.Bowers, D. F., C. G. Coleman, and D. T. Brown. 2003. Sindbis virus-associated pathology in Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 40:698-705. [DOI] [PubMed] [Google Scholar]
- 30.Bowman, A. S., L. B. Coons, G. R. Needham, and J. R. Sauer. 1997. Tick saliva: recent advances and implications for vector competence. Med. Vet. Entomol. 11:277-285. [DOI] [PubMed] [Google Scholar]
- 31.Brault, A. C., A. M. Powers, C. L. V. Chávez, R. N. López, M. F. Chacón, L. F. L. Gutiérrez, W.-L. Kang, R. B. Tesh, R. E. Shope, and S. C. Weaver. 1999. Genetic and antigenic diversity among eastern equine encephalitis viruses from North, Central, and South America. Am. J. Trop. Med. Hyg. 61:579-586. [DOI] [PubMed] [Google Scholar]
- 32.Bridgen, A., D. A. Dalrymple, F. Weber, and R. M. Elliott. 2004. Inhibition of Dugbe nairovirus replication by human MxA protein. Virus Res. 99:47-50. [DOI] [PubMed] [Google Scholar]
- 33.Brinton, M. A. 1981. Genetically controlled resistance to flaviviruses and lactate-dehydrogenase-elevating virus-induced disease. Curr. Top. Microbiol. Immunol. 92:1-14. [DOI] [PubMed] [Google Scholar]
- 34.Brinton, M. A. 1997. Host susceptibility to viral disease, p. 303-328. In N. Nathanson, R. Ahmed, F. Gonzalez-Scarano, D. E. Griffin, K. V. Holmes, F. A. Murphy, and H. L. Robinson (ed.), Viral pathogenesis. Lippincott-Raven, Philadelphia, Pa.
- 35.Brinton, M. A. 2001. Host factors involved in West Nile virus replication. Ann. N. Y. Acad. Sci. 951:207-219. [DOI] [PubMed] [Google Scholar]
- 36.Brossard, M., and S. K. Wikel. 1997. Immunology of infections between ticks and hosts. Med. Vet. Entomol. 11:270-276. [DOI] [PubMed] [Google Scholar]
- 37.Bryant, J., H. Wang, C. Cabezas, D. Watts, K. Russell, and A. Barrett. 2003. Enzootic transmission of yellow fever virus in Peru. Emerg. Infect. Dis. 9:926-933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buck, C., G. P. Paulino, D. J. Medina, G. D. Hsiung, T. W. Campbell, and M. T. Welsh. 1993. Isolation of St. Louis encephalitis virus from a killer whale. Clin. Diagn. Virol. 1:109-112. [DOI] [PubMed] [Google Scholar]
- 39.Budiansky, S. 2002. Creatures of our own making. Science 298:80-86. [DOI] [PubMed] [Google Scholar]
- 40.Bugbee, L. M., and L. R. Forte. 2004. The discovery of West Nile virus in overwintering Culex pipiens (Diptera: Culicidae) mosquitoes in Lehigh County, Pennsylvania. J. Am. Mosq. Control Assoc. 20:326-327. [PubMed] [Google Scholar]
- 41.Burt, F. J., A. A. Grobbelaar, P. A. Leman, F. S. Anthony, G. V. F. Gibson, and R. Swanepoel. 2002. Phylogenetic relationships of Southern African West Nile virus isolates. Emerg. Infect. Dis. 8:820-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Byrnes, A. P., and D. E. Griffin. 1998. Binding of Sindbis virus to cell surface heparin sulfate. J. Gen. Virol. 72:7349-7359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calisher, C. H. 1980. Antigenic relationships of the arboviruses, p. 117-152. In New aspects in ecology of arboviruses. Slovak Academy of Sciences, Bratislava, Slovakia.
- 44.Carn, V. M. 1996. The role of dipterous insects in the mechanical transmission of animal viruses. Br. Vet. J. 152:377-393. [DOI] [PubMed] [Google Scholar]
- 45.Casadevall, A., and L. A. Pirofski. 2001. Host-pattern interactions: the attributes of virulence. J. Infect. Dis. 184:337-344. [DOI] [PubMed] [Google Scholar]
- 46.Chadee, D. D., and R. Martinez. 2000. Landing periodicity of Aedes aegypti with implications for dengue transmission in Trinidad, West Indies. J. Vector Ecol. 25:158-163. [PubMed] [Google Scholar]
- 47.Chamberlain, R. W., and W. D. Sudia. 1961. Mechanism of transmission of viruses by mosquitoes. Annu. Rev. Entomol. 6:371-390. [DOI] [PubMed] [Google Scholar]
- 48.Chen, Y., T. Maguire, R. E. Hileman, J. R. Fromm, J. D. Esko, R. J. Linhardt, and R. M. Marks. 1997. Dengue virus infectivity depends on envelope protein binding to target cell heparin sulfate. Nat. Med. 3:866-871. [DOI] [PubMed] [Google Scholar]
- 49.Cheng, T. C. 1969. The biology of animal parasites. W. B. Saunders, Co., Philadelphia, Pa.
- 50.Chevillon, C., R. Eritja, N. Pasteur, and M. Raymond. 1995. Communalism, adaptation, and gene flow: mosquitoes of the Culex pipiens complex in different habitats. Genet. Res. 66:147-157. [DOI] [PubMed] [Google Scholar]
- 51.Chihota, C. M., L. F. Rennie, R, P. Kitching, and P. S. Mellor. 2001. Mechanical transmission of lumpy skin disease virus by Aedes aegypti (Diptera: Culicidae). Epidemiol. Infect. 126:317-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Childs, J. E., and C. J. Peters. 1993. Ecology and epidemiology of arenaviruses and their hosts, p. 331-384. In M. S. Salvato (ed.), The Arenaviridae. Plenum Press, New York, N.Y.
- 53.Chu, J. J., and M. L. Ng. 2004. Interaction of West Nile virus with alpha v beta 3 integrin mediates virus entry into cells. J. Biol. Chem. 279:54533-54541. [DOI] [PubMed] [Google Scholar]
- 54.Cleaveland, S., M. K. Laurenson, and L. H. Taylor. 2001. Diseases of human and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Phil. Trans. R. Soc. London B. 356:991-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clements, A. N. 1992. The biology of mosquitoes. Development, nutrition, and reproduction. Vol. 1. Chapman and Hall, London, England.
- 56.Clifford, C. M. 1979. Tick-borne viruses of seabirds, p. 83-100. In E. Kurstak (ed.), Arctic and tropical arboviruses. Academic Press, New York, N.Y.
- 57.Coggeshall, L. T. 1939. The transmission of lymphocytic choriomeningitis by mosquitoes. Science 89:515-516. [DOI] [PubMed] [Google Scholar]
- 58.Cooper, L. A., and T. W. Scott. 2001. Differential evolution of eastern equine encephalitis virus populations in response to host cell types. Genetics 157:1403-1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Crochu, S., S. Cook, H. Attoui, R. N. Charrel, R. de Chesse, M. Belhouchet, J.-J. Lamasson, P. de Micco, and X. de Lamballerie. 2004. Sequences of flavivirus-related RNA viruses persist in DNA form integrated in the genome of Aedes spp. mosquitoes. J. Gen. Virol. 85:1971-1980. [DOI] [PubMed] [Google Scholar]
- 60.Crosskey, R. W. 1988. Old tools and new taxonomic problems in blood-sucking insects, p. 1-18. In M. W. Service (ed.), Biosystematics of haematophagous insects. Clarendon Press, Oxford, United Kingdom.
- 61.Cupp, E. W., C. J. Mare, M. S. Cupp, and F. B. Ramberg. 1992. Biological transmission of vesicular stomatitis virus (New Jersey) by Simulium vittatum (Diptera: Simuliidae). J. Med. Entomol. 29:137-140. [DOI] [PubMed] [Google Scholar]
- 62.Daniel, M., V. Danielova, B. Kriz, A. Jirsa, and J. Nožicka. 2003. Shift of the tick Ixodes ricinus and tick-borne encephalitis to higher altitudes in Central Europe. Eur. J. Clin. Microbiol. 22:327-328. [DOI] [PubMed] [Google Scholar]
- 63.Danielova, V., J. Holubová, M. Rejcoch, and M. Daniel. 2002. Potential significance of transovarial transmission in the circulation of tick-borne encephalitis virus. Folia Parasitol. 49:323-325. [PubMed] [Google Scholar]
- 64.Dasgupta, R., L. L. Cheng, and L. C. Bartholomy. 2003. Flock house virus replicates and expresses green fluorescent protein in mosquitoes. J. Gen. Virol. 84:1789-1797. [DOI] [PubMed] [Google Scholar]
- 65.Davis, J. W., and J. L. Hardy. 1974. Characterization of persistent Modoc viral infections in Syrian hamsters. Infect. Immunol. 10:328-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dégallier, N., A. P. A. Travassos da Rosa, P. F. C. Vasconcelos, J.-P. Herve, G. C. Sá Filho, J. F. S. Travassos da Rosa, E. S. Travassos da Rosa, and S. G. Rodriguez. 1992. Modifications of arbovirus transmission in relation to construction of dams in Brazilian Amazonia. Cie. Cultura 44:124-135. [Google Scholar]
- 67.Dégallier, N., A. P. A. Travassos da Rosa, P. F. C. Vasconcelos, G. C. Sá Filho, E. S. Travassos da Rosa, S. G. Rodrigues, and J. F. S. Travassos da Rosa. 1998. Evolutionary aspects of the ecology of arboviruses in Bazilian Amazonia, South America, p. 42-60. In A. P. A. Travassos da Rosa, P. F. C. Vasconcelos, and J. F. S. Travassos da Rosa (ed.), An overview of arbovirology in Brazil and neighboring countries. Instituto Evandro Chagas, Belem, Brazil.
- 68.Diallo, M., Y. Ba, A. A. Sall, O. M. Diop, J. A. Ndione, M. Mondo, L. Girault, and C. Mathiot. 2003. Amplification of the sylvatic cycle of dengue virus type 2, Senegal, 1999-2000: entomologic findings and epidemiologic considerations. Emerg. Infect. Dis. 9:362-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Diamond, M. 2003. Evasion of innate and adaptive immunity by flaviviruses. Immunol. Cell. Biol. 81:196-206. [DOI] [PubMed] [Google Scholar]
- 70.Diarrassouba, S., and J. Dossou-Yovo. 1997. Rythme d'activité atypique chez Aedes aegypti en zone de savane sub-soudonienne de Côte d'Ivore. Bull. Soc. Pathol. Exot. 90:361-363. [PubMed] [Google Scholar]
- 71.Dixon, L. K., C. C. Abrams, G. Bowick, L. C. Goatley, P. C. Kay-Jackson, D. Chapman, E. Liverani, R. Nix, R. Silk, and F.-Q. Zhang. 2004. African swine fever virus proteins involved in evading host defence systems. Vet. Immunol. Immunopathol. 100:117-134. [DOI] [PubMed] [Google Scholar]
- 72.Downes, J. A. 1970. The ecology of blood-sucking Diptera: an evolutionary perspective, p. 232-258. In A. M. Fallis (ed.), Ecology and physiology of parasites. University of Toronto Press, Toronto, Canada.
- 73.Eaton, B. T. 1975. Defective interfering particles of Semliki Forest virus generated in BHK cells do not interfere with viral RNA sysnthesis in Aedes albopictus cells. Virology 68:534-538. [DOI] [PubMed] [Google Scholar]
- 74.Edman, J. D., and A. Spielman. 1988. Blood-feeding by vectors: physiology, ecology, behavior, and vertebrate defense, p. 153-189. In T. P. Monath (ed.), The arboviruses: epidemiology and ecology. CRC Press, Boca Raton, Fla.
- 75.Edwards, J. F., S. Higgs, and B. J. Beaty. 1998. Mosquito feeding-induced enhancement of Cache Valley virus (Bunyaviridae) infection in mice. J. Med. Entomol. 35:269-265. [DOI] [PubMed] [Google Scholar]
- 76.Eisler, R. 2003. Health risks of gold miners: a synoptic review. Env. Geochem. Health 25:325-345. [DOI] [PubMed] [Google Scholar]
- 77.Endris, R. G., W. R. Hess, and J. M. Caiado. 1992. African swine fever virus infection in the Iberian soft tick, Ornithodoros (Pavlovskyella) macrocanus (Acari: Argasidae). J. Med. Entomol. 29:874-878. [DOI] [PubMed] [Google Scholar]
- 78.Faran, M. E., M. J. Turell, W. S. Romoser, R. G. Routier, P. H. Gibbs, and C. L. Bailey. 1987. Reduced survival of adult Culex pipiens infected with Rift Valley fever virus. Am. J. Trop. Med. Hyg. 37:403-409. [DOI] [PubMed] [Google Scholar]
- 79.Fenner, F. 1982. Transmission cycles and broad patterns of observed epidemiological behavior in humans and other animal populations, p. 103-119. In R. M. Anderson, and R. M. May (ed.), Population biology of infectious diseases. Springer-Verlag, Berlin, Germany.
- 80.Ferguson, N., R. Anderson, and S. Gupta. 1999. The effect of antibody-dependent enhancement on the transmission dynamics and persistence of multiple-strain pathogens. Proc. Natl. Acad. Sci. USA 96:790-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Findley, G. M., and F. O. MacCallum. 1939. The transmission of yellow fever virus to monkeys by mouth. J. Pathol. Bacteriol. 49:53-60. [Google Scholar]
- 82.Fine, P. E. M. 1975. Vectors and vertical transmission: an epidemiologic perspective. Ann. N. Y. Acad. Sci. 266:173-194. [DOI] [PubMed] [Google Scholar]
- 83.Fonseca, D. M., N. Keyghobadi, C. A. Malcolm, C. Mehmet, F. Schaffner, M. Mogi, R. C. Fleischer, and R. C. Wilkerson. 2004. Emerging vectors in the Culex pipiens complex. Science 303:1535-1538. [DOI] [PubMed] [Google Scholar]
- 84.Frese, M., M. Weeber, F. Weber, V. Speth, and O. Haller. 1997. Mx1 sensitivity: Batken virus is an orthomyxovirus closely related to Dhori virus. J. Gen. Virol. 78:2453-2458. [DOI] [PubMed] [Google Scholar]
- 85.Frolov, I. 2004. Persistent infection and suppression of host response by alphaviruses. Arch. Virol. Suppl. 18:139-147. [DOI] [PubMed] [Google Scholar]
- 86.Gad, A. M., F. M. Feinsod, I. H. Allam, M. Eisa, A. N. Hassan, B. A. Soliman, S. el Said, and A. J. Saah. 1986. A possible route for the introduction of Rift Valley fever virus into Egypt during 1977. J. Trop. Med. Hyg. 89:233-236. [PubMed] [Google Scholar]
- 87.Gaidamovich, S. Y. 1995. Tick-borne flavivirus infections, p. 203-221. In J. S. Porterfield (ed.), Exotic viral infections. Chapman and Hall, London, United Kingdom.
- 88.Garmendia, A. E., H. J. Van Krainingen, R. A. French, J. F. Anderson, T. G. Andreadis, A. Kumar, and B. West. 2000. Recovery and identification of West Nile virus from a hawk in winter. J. Clin. Microbiol. 38:3110-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gaunt, M., A. A. Sall, X. de Lamballerie, A. K. I. Falconar, T. I. Dzhiranian, and E. A. Gould. 2001. Phylogenetic relationships of flaviviruses correlate with their epidemiology, disease association and biogeography. J. Gen. Virol. 82:1867-1876. [DOI] [PubMed] [Google Scholar]
- 90.Gaydos, J. K., W. R. Davidson, F. Elvinger, D. G. Mead, E. W. Howerth, and D. E. Stalknecht. 2002. Innate resistance to epizootic hemorrhagic diseases in white-tailed deer. J. Wildl. Dis. 38:713-719. [DOI] [PubMed] [Google Scholar]
- 91.Gebhardt, L. P., and D. W. Hill. 1960. Overwintering of western equine encephalitis virus. Proc. Soc. Exp. Biol. Med. 104:695-698. [DOI] [PubMed] [Google Scholar]
- 92.Gillett, J. D. 1994. The monkey myth. Lancet 343:126. [DOI] [PubMed] [Google Scholar]
- 93.Gillies, M.T. 1964. Selection for host preference in Anopheles gambiae. Nature 203:852-854. [DOI] [PubMed]
- 94.Glass, K. 2005. Ecological mechanisms that promote arbovirus survival: a mathematical model of Ross River virus transmission. Trans. R. Soc. Trop. Med. Hyg. 99:252-260. [DOI] [PubMed] [Google Scholar]
- 95.González-Salazar, D., J. G. Estrada-Franco, A.-S. Carrara, J. F. Aronson, and S. C. Weaver. 2003. Equine amplification and virulence of subtype IE Venezuelan equine encephalitis viruses isolated during the 1993 and 1996 Mexican epizootics. Emerg. Infect. Dis. 9:161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gordon, S. W., K. J. Linthicum, J. R. Moulton. 1993. Transmission of Crimean-Congo hemorrhagic fever virus in two species of Hyalomma ticks from infected adults to cofeeding immature forms. Am. J. Trop. Med. Hyg. 48:576-580. [DOI] [PubMed] [Google Scholar]
- 97.Gould, E. A., X. de Lamballerie, P. M. A. Zanotto, and E. C. Holmes. 2001. Evolution, epidemiology and dispersal of flaviviruses revealed by molecular phylogenies. Adv. Virus Res. 57:71-103. [DOI] [PubMed] [Google Scholar]
- 98.Gould, E. A., S. R. Moss, and S. L. Turner. 2004. Evolution and dispersal of encephalitic flaviviruses. Arch. Virol. Suppl. 18:65-84. [DOI] [PubMed] [Google Scholar]
- 99.Gray, S. M., and N. Banerjee. 1999. Mechanisms of arthropod transmission of plant and animal viruses. Microbiol. Mol. Biol. Rev. 63:128-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grimstad, P. R., Q. E. Ross, and G. B. Craig. 1980. Aedes triseriatus (Diptera: Culicidae) and La Crosse virus II. Modification of mosquito feeding behavior by virus infection. J. Med. Entomol. 17:1-7. [DOI] [PubMed] [Google Scholar]
- 101.Gubler, D. J. 1988. Dengue, p. 223-260. In T. P. Monath (ed.), The arboviruses: epidemiology and ecology. CRC Press, Boca Raton, Fla.
- 102.Gubler, D. J., R. Novak, and C. J. Mitchell. 1982. Arthropod vector competence: epidemiological, genetic, and biological considerations, p. 343-378. In W. W. M. Steiner, W. J. Tabachnick, K. S. Rai, and S. Narang (ed.), Recent developments in the genetics of insect disease vectors. Stipes, Champaign, Ill.
- 103.Guo, J.-T., J. Hayashi, and C. Seeger. 2005. West Nile virus inhibits the signal transduction pathway of alpha interferon. J. Virol. 79:1343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Guthrie, A. J., P. G. Howell, I. A. Gardneer, R. E. Swanepoel, J. P. Nurton, C. K. Harper, A. Pardini, D. Groenewald, C. W. Visage, J. F. Hedges, U. B. Balasuriya, A. J. Cornel, and N. J. MacLachlan. 2003. West Nile virus infection of throughbred horses in South Africa (2000-2001). Equine Vet. J. 35:601-605. [DOI] [PubMed] [Google Scholar]
- 105.Hacker, J. K., L. E. Volkman, and J. L. Hardy. 1995. Requirements for the G1 protein of California encephalitis virus infection in vitro and in vivo. Virology 206:945-953. [DOI] [PubMed] [Google Scholar]
- 106.Hagmaier, K., S. Jennings, J. Buse, F. Weber, and G. Kochs. 2003. Novel gene product of Thogotovirus segment 6 codes for an interferon antagonist. J. Virol. 77:2747-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hajnická, V., N. Fuchsberger, M. Slovák, P. Kocakova, and M. Labuda. 1998. Tick salivary gland extracts promote virus growth in vitro. Parasitology 116:533-538. [DOI] [PubMed] [Google Scholar]
- 108.Haller, O., M. Frese, D. Rost, P. A. Nuttall, and G. Kochs. 1995. Tick-borne Thogotovirus infection in mice is inhibited by the orthmyxovirus resistance gene product Mx1. J. Virol. 69:2596-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hanada, K., Y. Suzuki, and T. Gojobori. 2004. A large variation in the rates of synonymous substitutions for RNA viruses and its relationship to a diversity of viral infection and transmission mode. Mol. Biol. Evol. 21:1074-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hardy, J. L., F. J. Houk, L. D. Kramer, and W. C. Reeves. 1983. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu. Rev. Entomol. 28:229-262. [DOI] [PubMed] [Google Scholar]
- 111.Harrington, L. C., J. D. Edman, and T. W. Scott. 2001. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J. Med. Entomol. 38:411-422. [DOI] [PubMed] [Google Scholar]
- 112.Harrington, L. C., T. W. Scott, K. Lerdthusnee, R. C. Coleman, A. Costero, G. G. Clark, J. J. Jones, S. Kitthawee, P. Kittayapong, R. Sithiprasasna, and J. D. Edman. 2005. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am. J. Trop. Med. Hyg. 72:209-220. [PubMed] [Google Scholar]
- 113.Harrison, R. L., and B. C. Bonning. 2003. Comparative analysis of the genomes of Rachiplusia ou and Autographa californica multiple nucleopolyhedrosis virus. J. Gen. Virol. 84:1827-1842. [DOI] [PubMed] [Google Scholar]
- 114.Hauck, M. A., H. Qin, and H. R. Robert. 2001. Hantavirus transmission: potential role of ectoparasites. Vector-Borne Infect. Dis. 1:75-79. [DOI] [PubMed] [Google Scholar]
- 115.Haydon, D. T., S. Cleaveland, L. H. Taylor, and M. K. Laurenson. 2002. Identifying reservoirs of infection: a conceptual and practical challenge. Emerg. Infect. Dis. 8:1468-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Herrera-Basto, E., D. R. Prevots, M. L. Zárate, J. L. Silva, and J. Sepulveda-Amor. 1992. First reported outbreak of classical dengue fever in Guerrero State, Mexico, June 1988. Am. J. Trop. Med. Hyg. 46:649-653. [DOI] [PubMed] [Google Scholar]
- 117.Hess, W. 1988. African swine fever, p. 19-37. In T. P. Monath (ed.), The arboviruses: epidemiology and ecology. CRC Press, Boca Raton, Fla.
- 118.Hess, W. T., R. G. Endris, A. Lousa, and J. M. Caiado. 1989. Clearance of African swine fever virus from infected tick (Acari) colonies. J. Med. Entomol. 26:314-317. [DOI] [PubMed] [Google Scholar]
- 119.Higgs, S., B. S. Schneider, D. L. Vanlandingham, K. A. Klingler, and E. A. Gould. 2005. Nonviremic transmission of West Nile virus. Proc. Natl. Acad. Sci. USA 124:8871-8874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hilleman, M. R. 2004. Strategies and mechanisms for host and pathogen survival in acute and persistent viral infections. Proc. Natl. Acad. Sci. USA 101:14560-14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hoch, A. L., T. P. Gargan, and C. L. Bailey. 1985. Mechanical transmission of Rift Valley fever virus by hematophagous diptera. Am. J. Trop. Med. Hyg. 34:188-193. [DOI] [PubMed] [Google Scholar]
- 122.Hodes, H. L. 1946. Experimental transmission of Japanese encephalitis by mosquitoes and mosquito larvae. Johns Hopkins Bull. 79:358-360. [PubMed] [Google Scholar]
- 123.Holden, P., D. B. Francy, C. J. Mitchell, R. O. Hayes, J. S. Lazuick, and T. B. Hughes. 1973. House sparrows, Passer domesticus (L.), as hosts of arboviruses in Hale County, Texas. II. Laboratory studies with western equine encephalitis virus. Am. J. Trop. Med. Hyg. 22:254-262. [DOI] [PubMed] [Google Scholar]
- 124.Holmes, E. C. 2003. Error thresholds and the constraints to RNA virus evolution. Trends Microbiol. 11:543-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Holmes, E. C. 2003. Patterns of intra- and interhost nonsynonymous variation reveal strong purifying selection in dengue virus. J. Virol. 77:11296-11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Holmes, E. C., and A. Rambaut. 2004. Viral evolution and the emergence of SARS coronavirus. Phil. Trans. R. Soc. London 359:1059-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Honig, J. E., J. C. Osborne, and S. T. Nichol. 2004. The high genetic variation of viruses of the genus Nairovirus reflects the diversity of their predominant tick hosts. Virology 318:10-16. [DOI] [PubMed] [Google Scholar]
- 128.Hoogstraal, H. 1979. The epidemiology of tick-borne Crimean-Congo hemorrhagic fever in Asia, Europe, and Africa. J. Med. Entomol. 15:307-417. [DOI] [PubMed] [Google Scholar]
- 129.Hoogstraal, H., and A. Aeschlimann. 1982. Tick-host specificity. Mittel. Schweiz. Entomol. Ges. Bull. Soc. Entomol. Swisse 55:5-32. [Google Scholar]
- 130.Hopla, C. E., D. B. Francy, C. H. Calisher, and J. S. Lazuick. 1993. Relationship of cliff swallow, ectoparasites, and an alphavirus in west-central Oklahoma. J. Med. Entomol. 30:267-272. [DOI] [PubMed] [Google Scholar]
- 131.Hubálek, Z. 2003. Emerging human infectious diseases: anthroponoses, zoonoses, and sapronoses. Emerg. Infect. Dis. 9:403-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huelsenbeck, J. P., and F. Ronquist. 2001. MRBAYES: Bayesian inference phylogenetic trees. Bioinformatics 17:754-755. [DOI] [PubMed] [Google Scholar]
- 133.Huff, C. G. 1938. Studies on the evolution of some disease-producing organisms. Q. Rev. Biol. 13:196-206. [Google Scholar]
- 134.Hughes, A. L. 2001. Evolutionary change of predicted cytotoxic T cell epitopes of dengue virus. Infect. Genet. Evol. 1:123-130. [DOI] [PubMed] [Google Scholar]
- 135.Hung, J.-J., M.-T. Hsieh, M.-J. Young, C.-L. Kao, C.-C. King, and W. Chang. 2004. An external loop region of Domain III of dengue virus 2 envelope protein is involved in serotype-specific binding to mosquito but not mammalian cells. J. Virol. 78:378-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hurlbut, H. S., and J. I. Thomas. 1969. Further studies on the arthropod host range of arboviruses. J. Med. Entomol. 6:423-427. [DOI] [PubMed] [Google Scholar]
- 137.Igarashi, A. 1984. A hypothesis on the geographical distribution of arboviruses. Trop. Med. (Nagasaki) 26:173-179. [Google Scholar]
- 138.Inoue, Y., and H. Kato. 1963. Studies on Japanese B encephalitis virus. V. A thermoefficient mutant of Japanese B encephalitis virus. Virology 21:222-225. [DOI] [PubMed] [Google Scholar]
- 139.International Committee on Taxonomy of Viruses. 2000. Virus taxonomy, Seventh International Committee for the Taxonomy of Viruses. In M. H. V. Van Regenmortel, C. M. Fourquet, and D. H. L. Bishop (ed.), Academic Press, San Diego, Calif.
- 140.James, M. T. 1969. A study in the origin of parasitism. Bull. Entomol. Soc. Am. 15:251-253. [Google Scholar]
- 141.Jenkins, G. M., A. Rambaut, O. G. Pybus, and E. C. Holmes. 2002. Rates of molecular evolution in RNA viruses: a quantitative phylogenetic analysis. J. Mol. Evol. 54:156-165. [DOI] [PubMed] [Google Scholar]
- 142.Jindadamrongwech, S., C. Thepparit, and D. R. Smith. 2004. Identification of GRP 78 (BiP) as a liver cell expressed receptor element for dengue virus serotype 2. Arch. Virol. 149:915-927. [DOI] [PubMed] [Google Scholar]
- 143.Johansen, C. A., A. F. Van der Hurk, S. A. Ritchie, P. Zborowski, D. J. Nisbet, R. Paru, M. J. Rockarie, J. Macdonald, A. C. Drew, T. I. Khromykh, and J. S. Mackenzie. 2000. Isolation of Japanese encephalitis virus from mosquitoes (Diptera: Culicidae) collected in the western province of Papua New Guinea, 1997-1998. Am. J. Trop. Med. Hyg. 62:631-638. [DOI] [PubMed] [Google Scholar]
- 144.Johnson, R. T. 1998. Viral infections of the nervous system, 2nd ed. Lippincott-Raven, Philadelphia, Pa.
- 145.Jones, L. D., C. R. Davies, G. M. Steele, and P. A. Nuttall. 1987. A novel mode of arbovirus transmission involving a nonviremic host. Science 237:775-777. [DOI] [PubMed] [Google Scholar]
- 146.Jones, L. D., M. Gaunt, R. S. Hails, L. Laurenson, H. Reid, P. Henbest, and E. A. Gould. 1997. Transmission of louping ill virus infected and uninfected ticks co-feeding on mountain hares. Med. Vet. Entomol. 11:172-176. [DOI] [PubMed] [Google Scholar]
- 147.Jones, M., A. Davidson, L. Hibbert, P. Gruenwald, J. Schlaak, S. Ball, G. R. Foster, and M. Jacobs. 2005. Dengue virus inhibits alpha interferon signaling by reducing STAT2 expression. J. Virol. 79:5414-5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kanojia, P. C., and G. Geevarghese. 2004. First report on high-degree endophilism in Culex tritaeniorhynchus (Diptera: Culicidae) in an area endemic for Japanese encephalitis. J. Med. Entomol. 41:994-996. [DOI] [PubMed] [Google Scholar]
- 149.Katz, J., D. Alstad, G. Gustafson, and J. Evermann. 1994. Diagnostic analyses of the prolonged bluetongue virus RNA presence found in the blood of naturally infected cattle and experimentally infected sheep. J. Vet. Diagn. Investig. 6:139-142. [DOI] [PubMed] [Google Scholar]
- 150.Kharitonova, N. N., and Y. A. Leonov. 1985. Omsk hemorrhagic fever: ecology of the agent and epidemiology. Amerind Publishing Co., New Delhi, India.
- 151.Kiszewski, A. E., and A. Spielman. 1994. Virulence of vector-borne pathogens: a stochastic automata model of perpetuation. Ann. N. Y. Acad. Sci. 740:249-259. [DOI] [PubMed] [Google Scholar]
- 152.Klenerman, P., H. Hengartner, and R. M. Zinkernagel. 1997. A non-retroviral RNA virus persists in DNA form. Nature 390:298-301. [DOI] [PubMed] [Google Scholar]
- 153.Klimstra, W. B., E. M. Nangle, M. S. Smith, A. D. Yurochko, and K. D. Ryman. 2003. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J. Virol. 77:12022-12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Klimstra, W. B., K. D. Ryman, and R. E. Johnson. 1998. Adaptation of Sindbis virus to BHK cells selects for use of heparin sulfate as attachment receptor. J. Virol. 72:7357-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kochel, T. J., D. M. Watts, A. S. Gozalo, D. F. Ewing, K. R. Porter, and K. L. Russell. 2005. Cross-serotype neutralization of dengue virus in Aotus nancymae monkeys. J. Infect. Dis. 191:1000-1004. [DOI] [PubMed] [Google Scholar]
- 156.Komar, N., D. J. Dohm, M. J. Turell, and A. Spielman. 1999. Eastern equine encephalitis virus in birds: relative competence of European starlings (Sturnus vulgaris). Am. J. Trop. Med. Hyg. 60:387-391. [DOI] [PubMed] [Google Scholar]
- 157.Korenberg, E. I. 1976. Some contemporary aspects of natural focality and epidemiology of tick-borne encephalitis. Folia Parasitol. 23:357-366. [PubMed] [Google Scholar]
- 158.Korenberg, E. I., and Y. V. Kovalevskii. 1994. A model for relationships among the tick-borne encephalitis virus, its main vectors, and hosts, vol. 10: 65-92. In K. F. Harris (ed.), Advances in virus research. Springer-Verlag, New York, N.Y. [Google Scholar]
- 159.Kramer, L. D., and G. D. Ebel. 2003. Dynamics of flavivirus infection in mosquitoes. Adv. Virus Res. 60:187-232. [DOI] [PubMed] [Google Scholar]
- 160.Kramer, L. D., S. B. Presser, J. L. Hardy, and A. O. Jackson. 1997. Genotypic and phenotypic variation of selected Saint Louis enceaphalitis viral strains in California. Am. J. Trop. Med. Hyg. 57:222-229. [DOI] [PubMed] [Google Scholar]
- 161.Kumar, S. K., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: Molecular evolutionary genetic analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 162.Kuno, G. 1995. Review of the factors modulating dengue transmission. Epidemiol. Rev. 17:321-335. [DOI] [PubMed] [Google Scholar]
- 163.Kuno, G. 1997. Factors influencing the transmission of dengue viruses, p. 61-88. In D. J. Gubler and G. Kuno (ed.), Dengue and dengue hemorrhagic fever. CAB International, Wallingford, United Kingdom.
- 164.Kuno, G. 2001. Transmission of arboviruses without involvement of arthropod vectors. Acta Virol. 45:139-150. [PubMed] [Google Scholar]
- 165.Kuno, G. 2001. Persistence of arboviruses and antiviral antibodies in vertebrate hosts: its occurrence and impacts. Rev. Med. Virol. 11:165-190. [DOI] [PubMed] [Google Scholar]
- 166.Kuno, G. 2003. Serodiagnosis of flaviviral infections and vaccinations in humans. Adv. Virus Res. 61:3-65. [DOI] [PubMed] [Google Scholar]
- 167.Kuno, G. 2004. A survey of the relationships among the viruses not considered arboviruses, vertebrates, and arthropods. Acta Virol. 48:135-143. [PubMed] [Google Scholar]
- 168.Kuno, G. 2005. Letter to the editor: Dengue transmission without involvement of mosquito vector. Clin. Infect. Dis. 40:774-775. [DOI] [PubMed] [Google Scholar]
- 169.Kuno, G., G.-J.J. Chang, K. R. Tsuchiya, N. Karabatsos, and C. B. Cropp. 1998. Phylogeny of the genus Flavivirus. J. Virol. 72:73-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Labuda, M., L. D. Jones, T. Williams, and P. A. Nuttall. 1993. Enhancement of tick-borne encephalitis virus transmission by tick salivary gland extracts. Med. Vet. Entomol. 7:193-196. [DOI] [PubMed] [Google Scholar]
- 171.Labuda, M., and S. E. Randolph. 1999. Survial strategy of tick-borne encephalitis virus: cellular basis and environmental determinants. Zentbl. Bakteriol. 289:513-524. [DOI] [PubMed] [Google Scholar]
- 172.Lanciotti, R. S., J. T. Roehrig, V. Deubel, J. Smith, M. Parker, K. Steele, B. Crise, K. E. Volpe, M. B. Crabtree, J. H. Scherret, R. A. Hall, J. S. Mackenzie, C. B. Cropp, B. Panigrahy, E. Ostlund, B. Schmitt, M. Malkinson, C. Banet, J. Weissman, N. Komar, H. M. Savage, W. Stone, T. McNamara, and D. J. Gubler. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333-2337. [DOI] [PubMed] [Google Scholar]
- 173.Laras, K., N. C. Sukri, R. P. Larasati, M. J. Bangs, R. Kosim, Djauzi, T. Wandra, J. Master, H. Kosasih, S. Hartati, C. Beckett, E. R. Sedyaningsih, H. J. Beecham, and A. L. Corwin. 2005. Tracking the reemergence of epidemic chikungunya virus in Indonesia. Trans. R. Soc. Trop. Med. Hyg. 99:128-141. [DOI] [PubMed] [Google Scholar]
- 174.Lawrie, C. H., N. Y. Uzcátegui, N. Y., E. A. Gould, and P. A. Nuttall. 2004. Ixodid and argasid tick species and West Nile virus. Emerg. Infect. Dis. 10:653-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lee, C.-H., and R. H. Schloemer. 1981. Mosquito cells infected with Banzi virus secrete an antiviral activity which is of viral origin. Virology 110:402-410. [DOI] [PubMed] [Google Scholar]
- 176.Lee, E., and M. Lobigs. 2000. Substitutions at the putative receptor-binding site of an encephalitic flavivirus alter virulence and host cell tropism and reveal a role for glycosaminoglycans in entry. J. Virol. 74:8867-8875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lee, H.-W. 1971. Study on overwintering mechanisms of Japanese encephalitis virus in Korea (in Korean). J. Korean Med. Assoc. 14:871-878. [Google Scholar]
- 178.Lepiniec, L., L. Dalgarno, V. T. Q. Huong, T. P. Monath, J.-P. Digoutte, and V. Deubel. 1994. Geographic distribution and evolution of yellow fever viruses based on direct sequencing of genomic cDNA fragments. J. Gen. Virol. 75:417-423. [DOI] [PubMed] [Google Scholar]
- 179.Lewis, E. A. 1946. Nairobi sheep disease: the survival of the virus in the tick, Rhipicephalus appendiculatus. Parasitology 37:55-59. [DOI] [PubMed] [Google Scholar]
- 180.Li, Y., R. L. Hall, and R. W. Moyer. 1997. Transient, non-lethal expression of genes in vertebrate cells by recombinant entomopoxviruses. J. Virol. 71:9557-9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li, Y., S. Yuan, and R. W. Moyer. 1998. The non-permissive infection of insect (gypsy moth) LD-652 cells by vaccinia virus. Virology 248:74-82. [DOI] [PubMed] [Google Scholar]
- 182.Lin, R.-J., C.-L. Liao, E. Lin, and Y.-L. Lin. 2004. Blocking of the alpha interferon-induced Jak-STAT signaling pathway by Japanese encephalitis virus infection. J. Virol. 78:9285-9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lin, S.-R., Hsieh, S.-C., Y.-Y. Yueh, T.-H. Lin, D.-Y. Chao, W.-J. Chen, C.-C. King, and W.-K. Wang. 2004. Study of sequence variation of dengue type 3 virus in naturally infected mosquitoes and human hosts: implications for transmission and evolution. J. Virol. 78:12717-12721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Linn, M. L., J. Gardner, D. Warrilow, G. A. Darnell, C. R. McMahon, I. Field, A. D. Hyatt, R. W. Slade, and A. Suhrbier. 2001. Arbovirus of marine mammals: a new alphavirus isolated from the elephant seal louse Lepidophthirus macrorhini. J. Virol. 75:4103-4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lipsitch, M., and E. R. Myxon. 1997. Virulence and transmissibility of pathogen: what is the relationship? Trends Microbiol. 5:31-37. [DOI] [PubMed] [Google Scholar]
- 186.Littaua, R., I. Kurane, and F. A. Ennis. 1990. Human IgG Fc receptor II mediates antibody-dependent enhancement of dengue virus infection. J. Immunol. 144:3183-3186. [PubMed] [Google Scholar]
- 187.Liu, W.-J., H. B. Chen, X. J. Wang, H. Huang, and A. A. Khromykh. 2004. Analysis of adaptive mutations in Kunjin virus replicon RNA reveals a novel role for the flavivirus nonstructural protein NS2A in inhibition of beta interferon promoter-driven transcription. J. Virol. 78:12225-12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lok, S. M., M. L. Ng, and J. Aaskov. 2001. Amino acid and phenotypic changes in dengue 2 virus associated with escape from neutralization by IgM antibody. J. Med. Virol. 65:315-323. [DOI] [PubMed] [Google Scholar]
- 189.Ludwig, G. V., P. P. Calle, J. A. Mangiafico, B. L. Raphael, D. K. Danner, J. A. Hile, T. L. Clippinger, J. F. Smith, R. A. Cook, and T. McNamara. 2002. An outbreak of West Nile virus in a New York City captured wildlife population. Am. J. Trop. Med. Hyg. 67:67-75. [DOI] [PubMed] [Google Scholar]
- 190.Ludwig, G. V., B. A. Israel, B. M. Christensen, T. M. Yuill, and K. T. Schultz. 1991. Role of La Crosse virus glycoproteins in attachment of virus to host cells. Virology 181:564-571. [DOI] [PubMed] [Google Scholar]
- 191.Ludwig, G. V., J. P. Kondig, and J. F. Smith. 1996. A putative receptor for Venezuelan equine encephalitis virus from mosquito cells. J. Virol. 70:5592-5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Luedke, A. J., R. H. Jones, and T. E. Walton. 1977. Overwintering mechanism for bluetongue virus: biological recovery of latent virus from a bovine by bites of Culicoides variipennis. Am. J. Trop. Med. Hyg. 26:313-325. [DOI] [PubMed] [Google Scholar]
- 193.Luo, C.-H., and L.-B. Zheng. 2000. Independent evolution of Toll and related genes in insects and mammals. Immunogenetics 51:92-98. [DOI] [PubMed] [Google Scholar]
- 194.Mackenzie, J., C. A. Johansen, S. A. Ritchie, A. van den Hurk, and R. A. Hall. 2002. Japanese encephalitis as an emerging virus: the emergence and spread of Japanese encephalitis virus in Australasia. Curr. Top. Microbiol. Immunol. 267:49-73. [DOI] [PubMed] [Google Scholar]
- 195.MacLachlan, N. J., B. Barratt-Boyes, A. W. Brewer, and J. L. Stott. 1991. Bluetongue virus infection of cattle, p. 725-736. In T. E. Walton and B. I. Osburn (ed.), Bluetongue, African horse sickness and related orbiviruses. CRC Press, Boca Raton, Fla.
- 196.Mady, B. J., I. Kurane, D. V. Erbe, M. W. Fanger, and F. A. Ennis. 1993. Neuraminidase augments Fc gamma receptor II-mediated antibody-dependent enhancement of dengue virus infection. J. Gen. Virol. 74:839-844. [DOI] [PubMed] [Google Scholar]
- 197.Mahon, R., and A. Gibbs. 1982. Arbovirus infected hens attract more mosquitoes, p. 502-505. In J. S. Mackenzie (ed.), Viral diseases in Southeast Asia and the Western Pacific. Academic Press, Sydney, Australia.
- 198.Mahy, B. W. J. 2001. A dictionary of virology, 3rd ed. Academic Press, San Diego, Calif.
- 199.Mandl, C. W., S. L. Allison, H. Holzmann, T. Meixner, and F. X. Heinz. 2000. Attenuation of tick-borne encephalitis virus by structure-based site-specific mutagenesis of a putative flavivirus receptor binding site. J. Virol. 74:9601-9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Mans, B. J., A. I. Louw, and A. W. Neitz. 2002. Evolution of hematophagy in ticks: common origins for blood coagulation and platelet aggregation inhibitors from soft ticks of the genus Ornithodoros. Mol. Biol. Evol. 19:1695-1705. [DOI] [PubMed] [Google Scholar]
- 201.Mashimo, T., M. Lucas, D. Simon-Chazottes, M.-P. Frenkie, X. Montagutelli, P.-E. Ceccaldi, V. Deubel, J. L. Guénet, and P. Desprès. 2002. A nonsense mutation in the gene encoding 2′-5′-oligoadenylate synthetase/L1 isoform is associated with West Nile virus susceptibility in laboratory mice. Proc. Natl. Acad. Sci. USA 99:11311-11316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mattingly, P. F. 1960. II. Ecological aspects of the evolution of mosquito-borne virus diseases. Trans. R. Soc. Trop. Med. Hyg. 54:97-112. [Google Scholar]
- 203.McLean, R. G. 1991. Arboviruses of wild birds and mammals. Bull. Soc. Vector Ecol. 16:3-16. [Google Scholar]
- 204.Mead, D. G., E. W. Howerth, M. D. Murphy, E. W. Gray, R. Noblet, and D. E. Stallknecht. 2004. Black fly involvement in the epidemic transmission of vesicular stomatitis New Jersey virus (Rhabdoviridae: Vesiculovirus). Vector-Borne Zoonotic Dis. 4:351-359. [DOI] [PubMed] [Google Scholar]
- 205.Mead, D. G., F. B. Ramberg, D. G. Besselsen, and C. J. Maré. 2000. Transmission of vesicular stomatitis virus from infected to non-infected blackflies co-feeding on nonviremic deer mice. Science 287:485-487. [DOI] [PubMed] [Google Scholar]
- 206.Mellor, P. S., J. Boorman, and M. Baylis. 2000. Culicoides biting midges: their role as arbovirus vectors. Annu. Rev. Entomol. 45:307-340. [DOI] [PubMed] [Google Scholar]
- 207.Miller, B. R., K. O. Kloter, B. J. Beaty, and L. A. Magnarelli. 1983. Nonreplication of Jamestown Canyon and Keystone (California group) viruses in tabanids (Diptera: Tabanidae). J. Med. Entomol. 20:214-215. [DOI] [PubMed] [Google Scholar]
- 208.Miller, B. R., R. Loomis, A. Dejean, and H. Hoogstraal. 1985. Experimental studies on the replication and dissemination of Qalyub virus (Bunyaviridae: Nairovirus) in the putative tick vector, Ornithodoros (Pavlovskyella) erraticus. Am. J. Trop. Med. Hyg. 34:180-187. [DOI] [PubMed] [Google Scholar]
- 209.Miller, D. L., M. J. Manuel, C. Baldwin, G. Burtle, D. Ingram, M. E. Hines, and K. S. Frazier. 2003. West Nile virus in farmed alligators. Emerg. Infect. Dis. 9:794-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Milzer, A. 1942. Studies on the transmission of lymphocytic choriomeningitis virus by arthropods. J. Infect. Dis. 70:152-172. [Google Scholar]
- 211.Mims, C. A., M. F. Day, and I. D. Marshall. 1966. Cytopathic effects of Semliki Forest viruses in the mosquito Aedes aegypti. Am. J. Trop. Med. Hyg. 15:775-784. [DOI] [PubMed] [Google Scholar]
- 212.Mitchell, C. J. 1983. Mosquito vector competence and arboviruses, p. 63-92. In K. F. Harris (ed.), Current topics in vector research. Praeger, New York, N.Y.
- 213.Mizutani, T., M. Kobayashi, Y. Eshita, K. Shirata, T. Kimura, Y. Ako, H. Miyoshi, T. Takasaki, I. Kurane, H. Kariwa, T. Umemura, I. Takashima. 2003. Involvement of the JNK-like protein of the Aedes albopictus mosquito cell line, C6/36, in phagocytosis, endocytosis and infection of the West Nile virus. Insect Mol. Biol. 12:491-499. [DOI] [PubMed] [Google Scholar]
- 214.Monath, T. P. 1988. The arboviruses: epidemiology and ecology. In T. P. Monath (ed.), The arboviruses: epidemiology and ecology. CRC Press, Boca Raton, Fla.
- 215.Monath, T. P. 1988. Yellow fever, p. 139-231. In T. P. Monath (ed.), The arboviruses: epidemiology and ecology. CRC Press, Boca Raton, Fla.
- 216.Monath, T. P. 1994. Yellow fever and dengue—the interactions of virus, vector and host in the re-emergence of epidemic disease. Semin. Virol. 5:133-145. [Google Scholar]
- 217.Moncayo, A. C., Z. Fernandez, D. Ortiz, et al. 2004. Dengue emergence and adaptation to peridomestic mosquitoes. Emerg. Infect. Dis. 10:1790-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mondet, B. 2001. Considerations sur l'epidemiologie de la fiebre jaune au Brésil. Bull. Soc. Pathol. Exot. 94:260-267. [PubMed] [Google Scholar]
- 219.Mondet, B., P. F. C. Vasconcelos, A. P. A. Travassos da Rosa, E. S. Travassos da Rosa, S. G. Rodrigues, J. F. S. Travassos da Rosa, and D. J. Bicout. 2002. Isolation of yellow fever virus from nulliparous Haemagogus (Haemagogus) janthinomys in eastern Amazonia. Vector-Borne Zoonotic Dis. 2:47-50. [DOI] [PubMed] [Google Scholar]
- 220.Morgan, I. R., H. A. Westbury, amd J. Campbell. 1985. Viral infections of little blue penguins (Eudyptula minor) along southern coast of Australia. J. Wildl. Dis. 21:193-198. [DOI] [PubMed] [Google Scholar]
- 221.Moshkin, M., L. Gerlinskaya, O. Morozova, V. Bakhvalova, and V. Evsikov. 2002. Behaviour chemosignals and endocrine functions in male mice infected with tick-borne encephalitis virus. Psychoneuroendocrinology. 27:603-608. [DOI] [PubMed] [Google Scholar]
- 222.Mourya, D. T., M. D. Ranadive, P. V. Gokhale, V. S. Barde, V. S. Padbidri, and K. Banerjee. 1998. Putative chikungunya virus-specific receptor proteins on the midgut brush border membrane of Aedes aegypti mosquito. Indian J. Med. Res. 107:10-14. [PubMed] [Google Scholar]
- 223.Muñoz, M. L., A. Cisneros, J. Cruz, P. Das, R. Tovar, and A. Ortega. 1998. Putative dengue virus receptors from mosquito cells. FEMS Microbiol. Lett. 168:251-258. [DOI] [PubMed] [Google Scholar]
- 224.Muñoz-Jordán, J. L., M. Laurent-Rolle, J. Ashour, L. Martínez-Sobrido, M. Ashok, W. I. Lipkin, and A. García-Sastre. 2005. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J. Virol. 79:8004-8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Muñoz-Jordán, J. L., G. G. Sánchez-Burgos, M. Laurent-Rolle, and A. García-Sastre. 2003. Inhibition of interferon signaling by dengue virus. Proc. Natl. Acad. Sci. USA 100:14333-14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Murray, C. H. 1885. Young trout destroyed by mosquitoes. Bull. U.S. Fish Comm. 5:243. [Google Scholar]
- 227.Mutebei, P., H. W. Wang, L. Li, J. E. Bryant, and A. D. T. Barrett. 2001. Phylogenetic and evolutionary relationships among yellow fever virus isolates in Africa. J. Virol. 75:6999-7008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Mwandawiro, C., M. Boots, N. Tuno, W. Suwungkerd, and Y. Tsuda. 2000. Heterogeneity in the host preference of Japanese encephalitis vectors in Chiang Mai, northern Thailand. Trans. R. Soc. Trop. Med. Hyg. 94:238-242. [DOI] [PubMed] [Google Scholar]
- 229.Myles, K. M., D. J. Pierro, and K. E. Olson. 2003. Deletions in the putative cell receptor-binding domain of Sindbis virus strain MRE16E2 glycoprotein reduce midgut infectivity in Aedes aegypti. J. Virol. 77:8872-8881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Nasci, R. S., H. M. Savage, D. J. White, J. R. Miller, B. C. Cropp, M. S. Godsey, A. J. Kerst, P. Bennett, K. Gottfield, and R. S. Lanciotti. 2001. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg. Infect. Dis. 7:742-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Naumov, R. L., and V. P. Gutova. 1984. Experimental study of the participation of gamasid mites and fleas in circulating the tick-borne encephalitis virus. Parazitologia 18:106-115. (In Russian.) [PubMed] [Google Scholar]
- 232.Newton, S. E., and L. Dalgarno. 1983. Antiviral activity released from Aedes albopictus cells persistently infected with Semliki Forest virus. J. Virol. 47:652-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nichol, S. T., J. E. Rowe, and W. M. Fitch. 1993. Punctuated equilibrium and positive Darwinian evolution in vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 90:10424-10428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Novella, I. S., D. L. Gibertson, B. Borrego, E. Domingo, and J. J. Holland. 2005. Adaptability costs in immune escape variants of vesicular stomatitis virus. Virus Res. 107:27-34. [DOI] [PubMed] [Google Scholar]
- 235.Nunamaker, R. A., J. A. Lockwood, C. E. Stith, C. L. Campbell, S. P. Schell, B. S. Drolet, W. C. Wilson, D. M. Whilte, and G. J. Letchworth. 2003. Grasshoppers (Orthoptera: Acrididae) could serve as reservoirs and vectors of vesicular stomatitis virus. J. Med. Entomol. 40:957-963. [DOI] [PubMed] [Google Scholar]
- 236.Nuttall, P. A. 1998. Displaced tick-parasite interactions at the host interface. Parasitology 116(Suppl.):S65-S72. [DOI] [PubMed] [Google Scholar]
- 237.Nuttall, P. A., L. D. Jones, M. Labuda, and W. R. Kaufman. 1994. Adaptation of arboviruses to ticks. J. Med. Entomol. 31:1-9. [DOI] [PubMed] [Google Scholar]
- 238.Nuttall, P. A., and M. Labuda. 2003. Dynamics of infection in tick vectors and at the tick-host interface. Adv. Virus Res. 60:233-272. [DOI] [PubMed] [Google Scholar]
- 239.Okudo, H., T. Toma, H. Sasaki, Y. Higa, M. Fujikawa, I. Miyagi, and T. Okazawa. 2004. A crab-hole mosquito, Ochlerotatus baisasi, feeding on mudskipper (Gobiidae: Oxudercinae) in the Ryukyu Islands, Japan. J. Am. Mosq. Control Assoc. 20:134-137. [PubMed] [Google Scholar]
- 240.Paranjape, S., B. R. Patil, and V. D. Dadam. 2003. Characterization of porcine stable kidney cell line adapted to hyperthermic temperature. In Vitro Cell Dev. Biol. 39:193-195. [DOI] [PubMed] [Google Scholar]
- 241.Parsonson, I. M., and D. A. McPhee. 1985. Bunyavirus pathogenesis. Adv. Virus Res. 30:279-316. [DOI] [PubMed] [Google Scholar]
- 242.Pelz, E. G., and J. E. Freier. 1990. Vertical transmission of St. Louis encephalitis virus to autogenously developed eggs of Aedes atropalpus mosquitoes. J. Am. Mosq. Control Assoc. 6:658-661. [PubMed] [Google Scholar]
- 243.Peterson, A. T., D. S. Carroll, J. N. Mills, and K. M. Johnson. 2004. Potential mammalian filovirus reservoirs. Emerg. Infect. Dis. 10:2073-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Platt, K. B., K. J. Linthicum, K. S. A. Myint, B. L. Innis, K. Lerdthusnee, and D. W. Vaughn. 1997. Impact of dengue virus replication on feeding behavior of Aedes aegypti. Am. J. Trop. Med. Hyg. 57:119-125. [DOI] [PubMed] [Google Scholar]
- 245.Pletnev, A. G., and R. Men. 1998. Attenuation of the Langat tick-borne flavivirus by chimerization with mosquito-borne flavivirus dengue type 4. Proc. Natl. Acad. Sci. USA 95:1746-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Poinar, G., and R. Poinar. 2005. Evidence of vector-borne disease of early Cretaceous reptiles. Vector-Borne Zoonotic Dis. 4:281-284. [DOI] [PubMed] [Google Scholar]
- 247.Powers, A. M., A. C. Brault, Y. Shirako, E. G. Strauss, W.-L. King, J. H. Strauss, and S. C. Weaver. 2001. Evolutionary relationships and systematics of the alphaviruses. J. Virol. 75:10118-10131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Price, J. L. 1978. Isolation of Rio Bravo and a hitherto undescribed agent, Tamana bat virus, from insectivorous bats in Trinidad, with serological evidence of infection in bats and man. Am. J. Trop. Med. Hyg. 27:153-161. [DOI] [PubMed] [Google Scholar]
- 249.Protopopova, Y. V., S. N. Konovalova, and V. B. Loktev. 1997. Identification of the cell surface receptor for tick-borne encephalitis virus by antiidiotypic antibodies. Voprosy Virusol. 42:264-268. (In Russian.) [PubMed] [Google Scholar]
- 250.Purcell, A. H. 1982. Evolution of the insect vector relationship, p. 121-156. In M. S. Mount, and G. H. Lay (ed.), Phytopathogenic prokaryotes. Academic Press, New York, N.Y.
- 251.Randolph, S. E. 1998. Ticks are not insects: consequences of contrasting vector biology for transmission potential. Parasitol. Today 14:186-192. [DOI] [PubMed] [Google Scholar]
- 252.Randolph, S. E., D. Miklisová, J. Lysy, D. J. Rogers, and M. Labuda. 1999. Incidence from coincidence: patterns of tick infestations on rodents facilitate transmission of tick-borne encephalitis virus. Parasitology 118:177-186. [DOI] [PubMed] [Google Scholar]
- 253.Read, A. F. 1994. The evolution of virulence. Trends Microbiol. 2:73-76. [DOI] [PubMed] [Google Scholar]
- 254.Reeves, W. C. 1990. Overwintering of arboviruses, p. 357-382. In W. C. Reeves, S. M. Asman, J. L. Hardy, M. M. Milby, and W. K. Reisen (ed.), Epidemiology and control of mosquito-borne arboviruses in California, 1943-1987. California Mosquito Control Association, Sacramento, Calif.
- 255.Reeves, W. C. 2001. Partners: serendipity in arbovirus research. J. Vector Ecol. 26:1-6. [PubMed] [Google Scholar]
- 256.Reeves, W. C., G. A. Hutson, R. E. Bellamy, amd R.P. Scrivani. 1958. Chronic latent infections of birds with western equine encephalomyelitis virus. Proc. Soc. Exp. Biol. Med. 97:733-736. [DOI] [PubMed] [Google Scholar]
- 257.Reinert, J. F. 1975. Mosquito generic and subgeneric abbreviations (Diptera: Culicidae). Mosq. Syst. 7:105-110. [Google Scholar]
- 258.Reisen, W. K. 2003. Epidemiology of St. Louis encephalitis virus. Adv. Virus Res. 61:139-183. [DOI] [PubMed] [Google Scholar]
- 259.Reisen, W. K., and P. F. L. Boreham. 1979. Host selection patterns of some Pakistan mosquitoes. Am. J. Trop. Med. Hyg. 28:408-421. [DOI] [PubMed] [Google Scholar]
- 260.Reisen, W. K., L. D. Kramer, R. E. Chiles, E. G. N. Green, and V. M. Martinez. 2001. Encephalitis virus persistence in California birds. Preliminary studies with house finches (Carpodacus mexicanus). J. Med. Entomol. 38:393-399. [DOI] [PubMed] [Google Scholar]
- 261.Reuben, R., V. Thenomozhi, P. P. Samuel, A. Gajanana, and T. R. Mani. 1992. Mosquito blood feeding patterns as a factor in the epidemiology of Japanese encephalitis in southern India. Am. J. Trop. Med. Hyg. 46:654-663. [DOI] [PubMed] [Google Scholar]
- 262.Ribeiro, J. M. C. 1995. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? Infect. Agents Dis. 4:143-152. [PubMed] [Google Scholar]
- 263.Ribeiro, J. M. C., and I. M. B. Francischetti. 2003. Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu. Rev. Entomol. 48:73-88. [DOI] [PubMed] [Google Scholar]
- 264.Ribeiro, J. M. C., P. A. Rossignol, and A. Spielman. 1985. Aedes aegypti: model for blood finding strategy and prediction of parasite manipulation. Exp. Parasitol. 60:118-132. [DOI] [PubMed] [Google Scholar]
- 265.Riedel, B., and D. T. Brown. 1979. Novel antiviral activity found in the media of Sindbis virus-persistently infected mosquito (Aedes albopictus) cell cultures. J. Virol. 29:51-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Rodhain, F. 1991. The role of monkeys in the biology of dengue and yellow fever. Comp. Immunol. Microbiol. Infect. Dis. 14:9-19. [DOI] [PubMed] [Google Scholar]
- 267.Rodhain, F. 1998. La notion de reservoir naturel en arbovirologie. Bull. Soc. Pathol. Exp. 91:279-282. [PubMed] [Google Scholar]
- 268.Rodríguez, H., and F. de la Hoz. 2005. Degue and vector behaviour in Cáqueza, Colombia, 2004. Rev. Salud Publ. (Colombia) 7:1-15. [DOI] [PubMed] [Google Scholar]
- 269.Rodríguez, L. L., W. M. Fitch, and S. T. Nichol. 1996. Ecological factors rather than temporal factors dominate the evolution of vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 93:13030-13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Rosen, L. 1987. Overwintering mechanisms of mosquito-borne arboviruses in temperate climates. Am. J. Trop. Med. Hyg. 37(Suppl. 3):69S-76S. [DOI] [PubMed] [Google Scholar]
- 271.Rossignol, P. A., J. M. C. Ribeiro, M. Jungery, M. J. Turell, A. Spielman, and C. L. Bailey. 1985. Enhanced mosquito blood-finding success on parasitemic hosts: evidence for vector-parasite mutualism. Proc. Natl. Acad. Sci. USA 82:7725-7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Rush, W. A., D. B. Francy, G. C. Smith, and C. B. Cropp. 1980. Transmission of an arbovirus by a member of the family Cimicidae. Annals Entomol. Soc. Am. 73:315-318. [Google Scholar]
- 273.Rutledge, L. C., A. A. Khan, D. L. Skidmore, and H. J. Maibach. 1975. Genetic transmission of host-seeking behavior in colonized Aedes aegypti. Mosquito News. 35:189-194. [Google Scholar]
- 274.Sabin, A. B. 1959. Survey of knowledge and problems in field of arthropod-borne virus infections. Arch. Ges. Virusforsch. 9:1-10. [DOI] [PubMed] [Google Scholar]
- 275.Salas-Benito, J. S., M. Rosa, and R. M. del Angel. 1997. Identification of two surface proteins from C6/36 cells that bind dengue type 4 virus. J. Virol. 71:7246-7252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Salvan, M., and J. Mouchet. 1994. Aedes albopictus et Aedes aegypti a l'Ile de La Reunion. Ann. Soc. Belge Med. Trop. 74:323-326. [PubMed] [Google Scholar]
- 277.Sánchez-Vargas, I., E. A. Travanty, K. M. Keene, A. W. E. Franz, B. J. Beaty, C. D. Blair, and K. E. Olson. 2004. RNA interference, arthropod-borne viruses, and mosquitoes. Virus Res. 102:65-74. [DOI] [PubMed] [Google Scholar]
- 278.Sang, R. C., A. Gichogo, J. Gachoya, M. D. Dunster, V. Ofula, A. R. Hunt, M. B. Crabtree, B. R. Miller, and L. M. Dunster. 2003. Isolation of a new flavivirus related to cell fusing agent virus (CFAV) from field-collected flood-water Aedes mosquitoes from a dambo in central Kenya. Arch. Virol. 148:1085-1093. [DOI] [PubMed] [Google Scholar]
- 279.Schlesinger, R. W. 1980. Evolutionary aspects of Togaviridae, p. 40-46. In R. W. Schlesinger (ed.), The togaviruses: biology, structure, replication. Academic Press, New York, N.Y.
- 280.Scott, T. W., and L. H. Lorenz. 1998. Reduction of Culiseta melanura fitness by eastern equine encephalitis virus. Am. J. Trop. Med. Hyg. 59:341-346. [DOI] [PubMed] [Google Scholar]
- 281.Scott, T. W., A. Naksathit, J. F. Day, P. Kittayapong, and J. D. Edman. 1997. A fitness advantage for Aedes aegypti and the viruses it transmits when females feed only on human blood. Am. J. Trop. Med. Hyg. 57:235-239. [DOI] [PubMed] [Google Scholar]
- 282.Selling, B. H., R. F. Allison, and P. Kaesberg. 1990. Genomic RNA of an insect virus directs synthesis of infectious virions in plants. Proc. Natl. Acad. Sci. USA 87:434-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Sheppard, P. M., W. M. Macdonald, R. J. Tonn, and B. Grab. 1969. The dynamics of an adult population of Aedes aegypti in relation to dengue haemorrhagic fever in Bangkok. J. Anim. Ecol. 38:661-697. [Google Scholar]
- 284.Shi, P. Y., W. Li, and M. A. Brinton. 1996. Cell proteins bind specifically to West Nile virus minus-strand 3′ stem-loop RNA. J. Virol. 70:6278-6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Shope, R. E. 1982. Rabies-related viruses. Yale J. Biol. Med. 55:271-275. [PMC free article] [PubMed] [Google Scholar]
- 286.Siler, J. F., M. W. Hall, and A. P. Hitchens. 1926. Dengue: its history, epidemiology, mechanism of transmission, etiology, clinical manifestations, immunity, and prevention. Philippine J. Sci. 29:1-304. [Google Scholar]
- 287.Silvia, O. J., L. Pantelic, J. S. Mackenzie, G. R. Shellam, J. Papadimitriou, and N. Urosevic. 2004. Virus spread, tissue inflammation and antiviral response in brains of flavivirus susceptible and resistant mice acutely infected with Murray Valley encephalitis virus. Arch. Virol. 149:447-464. [DOI] [PubMed] [Google Scholar]
- 288.Simizu, B., and N. Maeda. 1972. Virulence of a temperature-sensitive mutant of western equine encephalitis virus. Arch. Ges. Virusforsch. 38:328-337. [DOI] [PubMed] [Google Scholar]
- 289.Simpson, D. I. H., C. E. G. Smith, T. F. C. Marshall, G. S. Platt, H. J. Way, E. T. W. Bowen, W. F. Bright, J. Day, D. A. McMahon, M. N. Hill, P. J. E. Bendell, and O. H. U. Heathcote. 1976. Arbovirus infections in Sarawak: the role of the domestic pig. Trans. R. Soc. Trop. Med. Hyg. 70:66-72. [DOI] [PubMed] [Google Scholar]
- 290.Slooff, R., and E. N. Marks. 1965. Mosquitoes (Culicidae) biting a fish (Periophthalmidae). J. Med. Entomol. 2:16. [DOI] [PubMed] [Google Scholar]
- 291.Smetana, A. 1965. On the transmission of tick-borne encephalitis virus by fleas. Acta Virol. 9:375-378. [PubMed] [Google Scholar]
- 292.Smith, C. E. G. 1964. Factors influencing the bahaviour of viruses in their arthropod hosts, p. 1-31. In A. E. R. Taylor (ed.), Host-parasite relationship in invertebrate hosts. Blackwell Science Press, Oxford, United Kingdom.
- 293.Spadoni, R. D., R. L. Nelson, and W. C. Reeves. 1974. Seasonal occurrence, egg production, and blood-feeding activity of autogenous Culex tarsalis. Annals Entomol. Soc. Am. 67:895-902. [Google Scholar]
- 294.Spielman, A. 1957. The inheritance of autogeny in the Culex pipiens complex of mosquitoes. Am. J. Hyg. 65:404-425. [DOI] [PubMed] [Google Scholar]
- 295.Spielman, A. 2001. Structure and seasonality of Nearctic Culex pipiens populations. Ann. N. Y. Acad. Sci. 951:220-234. [DOI] [PubMed] [Google Scholar]
- 296.Spielman, A., T. G. Andreadis, C. S. Apperson, A. J. Cornel, J. F. Day, J. D. Edman, D. Fish, L. C. Harrington, A. E. Kiszewski, R. Lampman, G. C. Lanzaro, F. R. Matuschka, L. E. Munstermann, R. S. Nasci, D. E. Norris, R. J. Novak, P. Reiter, H. M. Savage, W. J. Tabachnick, and D. M. Wesson. 2004. Outbreak of West Nile virus in North America. Science 306:1473. [DOI] [PubMed] [Google Scholar]
- 297.Spielman, A., R. J. Pollack, A. E. Kiszewski, and S. R. Telford. 2001. Issues in public health entomology. Vector-Borne Zoonotic Dis. 1:3-19. [DOI] [PubMed] [Google Scholar]
- 298.Stamm, D. D. 1966. Relationships of birds and arboviruses. Auk. 83:84-97. [Google Scholar]
- 299.Stark, K. R., and A. A. James. 1998. Isolation and characterization of the gene encoding a novel factor Xa-directed anticoagulant from the yellow fever mosquito Aedes aegypti. J. Biol. Chem. 273:20802-20809. [DOI] [PubMed] [Google Scholar]
- 300.St. George, T. D., R. L. Doherty, J. G. Carley, C. Filippich, A. Brescia, J. Casals, D. H. Kemp, and N. Brothers. 1985. The isolation of arboviruses including a new flavivirus and a new bunyavirus from Ixodes (Ceratoxodes) uriae (Ixodoidea: Ixodidae) collected at Macquarie Island, Australia, 1975-1979. Am. J. Trop. Med. Hyg. 34:406-412. [DOI] [PubMed] [Google Scholar]
- 301.Stokes, A., J. H. Bauer, and N. P. Hudson. 1928. Experimental transmission of yellow fever to laboratory animals. Am. J. Trop. Med. 8:103-164. [Google Scholar]
- 302.Stollar, V. 1980. Defective interfering alphaviruses, p. 427-457. In R. W. Schlesinger (ed.), The togaviruses, biology, structure, replication. Academic Press, New York, N.Y.
- 303.Stollar, V., and V. L. Thomas. 1975. An agent in the Aedes aegypti cell line (Peleg) which causes fusion of Aedes albopictus cells. Virology 64:367-377. [DOI] [PubMed] [Google Scholar]
- 304.Strauss, J. H., K.-S. Wang, A. L. Schmaljohn, R. J. Kuhn, and E. G. Strauss. 1994. Host-cell receptors for Sindbis virus. Arch. Virol. Suppl. 9:473-484. [DOI] [PubMed] [Google Scholar]
- 305.Suárez, M. F., and M. J. Nelson, M. J. 1981. Registro de altitude del Aedes aegypti en Colombia. Biomédica (Bogotá) 1:225. [Google Scholar]
- 306.Sudia, W. D. 1972. Arthropod vectors of epidemic equine encephalitis. PAHO Sci. Publ. No. 243:157-161. [Google Scholar]
- 307.Süss, J. 2003. Epidemiology and ecology of TBE relevant to the production of effective vaccines. Vaccine 21:S1/19-S1/35. [DOI] [PubMed] [Google Scholar]
- 308.Tabachnick, W. J. 1991. Evolutionary genetics and arthropod-borne diseases. The yellow fever mosquito. Am. Entomol. Spring 37:14-24.
- 309.Takamatsu, H., P. S. Mellor, P. P. C. Mertens, P. A. Kirkham, J. N. Burroughs, and R. M. E. Parkhouse. 2003. A possible overwintering mechanism for bluetongue virus in the absence of the insect vector. J. Gen. Virol. 84:227-235. [DOI] [PubMed] [Google Scholar]
- 310.Tan, B.-H., E. Nason, N. Staeuber, W.-R. Jian, K. Monastryskaya, and P. R. 2001. RGD tripeptide of bluetongue virus VP7 protein is responsible for core attachment to Culicoides cells. J. Virol. 75:3937-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Tassaneetrithep, B., T. H. Burgess, A. Granelli-Piperno, C. Trampfheller, J. Finke, W. Sun, M. A. Eller, K. Pathanapanysat, S. Sarasombath, D. L. Birx, R. M. Steinman, S. Schlesinger, and M. A. Marovich. 2003. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J. Exp. Med. 197:823-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Tesh, R. B., M. Siirin, H. Guzmán, A. P. A. Travassos da Rosa, X. Wu, T. Duan, H. Lei, M. R. Nunes, and S.-Y. Xiao. 2005. Persistent West Nile virus infection in the golden hamster: studies on its mechanism and possible implications for other flavivirus infections. J. Infect. Dis. 192:287-295. [DOI] [PubMed] [Google Scholar]
- 313.Thepparit, C., and D. R. Smith. 2004. Serotype-specific entry of dengue virus into liver cells: identification of the 37-kilodalton/67-kilodalton high-affinity laminin receptor as a dengue virus serotype 1 receptors. J. Virol. 78:12647-12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Thu, H. M., K. Lowry, L. Jiang, T. Hlaing, E. C. Holmes, and J. Aaskov. 2005. Lineage extinction and replacement in dengue type 1 virus populations are due to stochastic events rather than to natural selection. Virology 336:163-172. [DOI] [PubMed] [Google Scholar]
- 315.Trpis, M., and W. Hausermann. 1978. Genetics of house-entering behaviour in East African population of Aedes aegypti (L.) (Diptera: Culicidae) and its relevance to speciation. Bull. Entomol. Res. 68:521-532. [Google Scholar]
- 316.Tsuji, N., T. Okazawa, and N. Yamamura. 1990. Autogenous and anautogenous mosquitoes: a mathematical analysis of reproductive strategies. J. Med. Entomol. 27:446-453. [DOI] [PubMed] [Google Scholar]
- 317.Tulman, E. R., and D. L. Rock. 2001. Novel virulence and host range genes of African swine fever virus. Curr. Opin. Microbiol. 4:456-461. [DOI] [PubMed] [Google Scholar]
- 318.Turell, M. J. 1988. Horizontal and vertical transmission of viruses by insect and tick vectors, p. 127-152. In T. P. Monath (ed.), The arboviruses: epidemiology and ecology. CRC Press, Boca Raton, Fla.
- 319.Turell, M. J., C. L. Bailey, and C. Rossi. 1984. Increased mosquito feeding on Rift Valley fever virus-infected lambs. Am. J. Trop. Med. Hyg. 33:1232-1238. [DOI] [PubMed] [Google Scholar]
- 320.Turell, M. J., T. P. Gargan, and C. L. Bailey. 1985. Culex pipiens (Diptera: Culicidae) morbidity and mortality associated with Rift Valley fever virus infection. J. Med. Entomol. 22:332-337. [DOI] [PubMed] [Google Scholar]
- 321.Turell, M. J., K. J. Linthicum, and J. R. Beaman. 1990. Transmission of Rift Valley fever virus by adult mosquitoes after ingestion of virus as larvae. Am. J. Trop. Med. Hyg. 43:677-690. [DOI] [PubMed] [Google Scholar]
- 322.Turell, M. J., C. N. Mores, J. S. Lee, J. J. Paragas, D. Shermanhemedova, T. P. Endy, and S. Khodjaev. 2004. Experimental transmission of Karshi and Langat (tick-borne encephalitis virus complex) viruses by Ornithodoros ticks (Acari: Argacidae). J. Med. Entomol. 41:973-997. [DOI] [PubMed] [Google Scholar]
- 323.Twiddy, S. S., E. C. Holmes, and A. Rambaut. 2003. Inferring the rate and time-scale of dengue virus evolution. Mol. Biol. Evol. 20:122-129. [DOI] [PubMed] [Google Scholar]
- 324.Twiddy, S. S., C. H. Woelk, and E. C. Holmes. 2002. Phylogenetic evidence for adaptive evolution of dengue viruses in nature. J. Gen. Virol. 83:1679-1689. [DOI] [PubMed] [Google Scholar]
- 325.Urosevic, N., and G. R. Shellam. 2002. Host genetic resistance to Japanese encephalitis group viruses. Curr. Top. Microbiol. Immunol. 267:153-170. [DOI] [PubMed] [Google Scholar]
- 326.Van der Most, R. G., J. Corver, and J. H. Strauss. 1999. Mutagenesis of the RGD motif in the yellow fever virus 17D envelope protein. Virology 265:83-95. [DOI] [PubMed] [Google Scholar]
- 327.Varelas-Wesley, I., and C. H. Calisher. 1982. Antigenic relationships of flaviviruses with undetermined arthropod-borne status. Am. J. Trop. Med. Hyg. 31:1273-1284. [DOI] [PubMed] [Google Scholar]
- 328.Veazey, R. S., C. C. Vice, D.-Y. Cho, T. N. Tully, amd S.M. Shane. 1994. Pathology of eastern equine encephalitis in emus (Dromaius novaehollandiae). Vet. Pathol. 31:109-111. [DOI] [PubMed] [Google Scholar]
- 329.Wadstrom, T., and A. Ljungh. 1999. Glycosaminoglycan-binding microbial proteins in tissue adhesion and invasion: key events in microbial pathogenicity. J. Med. Microbiol. 48:223-233. [DOI] [PubMed] [Google Scholar]
- 330.Wang, E., R. A. Bowen, G. Medina, A. M. Powers, W.-L. Kang, L. M. Chandler, R. E. Shope, and S. C. Weaver. 2001. Virulence and viremia characteristics of 1992 epizootic subtype IC Venezuelan equine encephalitis viruses and closely related enzootic subtype ID strains. Am. J. Trop. Med. Hyg. 65:64-69. [DOI] [PubMed] [Google Scholar]
- 331.Wang, E., H.-L. Ni, R.-L. Xu, A. D. T. Barrett, S. J. Watowich, D. J. Gubler, and S. C. Weaver. 2000. Evolutionary relationships of endemic/epidemic and sylvatic dengue viruses. J. Virol. 74:3227-3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Wang, K.-S., R. J. Kuhn, E. G. Strauss, S. Ou, S., and J. H. Strauss. 1992. High-affinity laminin receptor is a receptor for Sindbis virus in mammalian cells. J. Virol. 66:4992-5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Wang, T., T. Town, I. Alexopoulou, J. F. Anderson, E. Fikrig, and R. A. Flavell. 2004. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat. Med. 10:1366-1373. [DOI] [PubMed] [Google Scholar]
- 334.Watts, D. M., W. H. Thompson, T. M. Yuill, G. R. DeFoliart, G. R., and R. P. Hanson. 1974. Overwintering of La Crosse virus in Aedes triseriatus. Am. J. Trop. Med. Hyg. 23:694-700. [DOI] [PubMed] [Google Scholar]
- 335.Weaver, S. C. 1997. Vector biology in arboviral pathogenesis, p. 329-352. In N. Nathanson, R. Ahmed, F. Gonzalez-Scarano, D. E. Griffin, K. V. Holmes, F. A. Murphy, and H. L. Robinson (ed.), Viral pathogenesis. Lippincott-Raven, Philadelphia, Pa.
- 336.Weaver, S. C., and A. D. T. Barrett. 2004. Trasmission cycles, host range, evolution and emergence of arboviral disease. Nat. Rev. Microbiol. 2:789-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Weaver, S. C., A. C. Brault, W.-L. Kang, and J. J. Holland. 1999. Genetic and fitness changes accompanying adaptation of an arbovirus to vertebrate and invertebrate cells. J. Virol. 73:4316-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Weaver, S. C., C. Ferro, R. Barrera, J. Boshell, and J.-C. Navarro. 2004. Venezulelan equine encephalitis. Annu. Rev. Entomol. 49:141-174. [DOI] [PubMed] [Google Scholar]
- 339.Weaver, S. C., T. W. Scott, L. H. Lorenz, K. Lerdhusnee, amd W. S. Romoser. 1988. Togavirus-associated pathologic changes in the midgut of a natural mosquito vectors. J. Virol. 62:2083-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Weber, F., A. Bridges, J. K. Frazakerley, H. Streitenfeld, N. Kossler, R. E. Randall, and R. M. Elliott. 2002. Bunyamwera bunyavirus nonstructural protein NSs counteracts the induction of alpha/beta interferon. J. Virol. 76:7949-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Wekesa, J. W., B. Yuval, R. K. Washino, and A. M. Vázquez. 1997. Blood feeding patterns of Anopheles freeborni and Culex tarsalis (Diptera: Culicidae): effects of habitat and host abundance. Bull. Entomol. Res. 87:633-641. [Google Scholar]
- 342.Weston, J., S. Villoing, M. Bremont, J. Castric, M. Pfeffer, V. Jewhurst, M. McLoughlin, O. Rodseth, K. E. Christie, J. Koumans, and D. Todd. 2002. Comparison of two aquatic alphaviruses, salmon pancreas disease virus and sleeping disease virus, by using genome sequence analysis, monoclonal reactivity, and cross infection. J. Virol. 76:6155-6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Whitfield, J. B., and S. Asgari. 2003. Virus or not? Phylogenetics of polydnaviruses and their wasp carriers. J. Insect Physiol. 49:397-405. [DOI] [PubMed] [Google Scholar]
- 344.Whitman, L. 1951. The arthropod vectors of yellow fever, p. 233-298. In G. K. Strode (ed.), yellow fever. McGraw-Hill Book Co., Inc., New York, N.Y.
- 345.Whitman, L., and T. H. G. Aitken. 1960. Potentiality of Ornithodoros moubata Murray (Acarina; Argasidae) as a reservoir of West Nile virus. Ann. Trop. Med. Parasitol. 54:192-204. [DOI] [PubMed] [Google Scholar]
- 346.Whitman, L., and P. C. A. Antunes. 1938. Studies on Aedes aegypti infected in the larval stage with the virus of yellow fever. Proc. Soc. Exp. Biol. 37:664-666. [Google Scholar]
- 347.Williams, J. E., S. Imlarp, F. H. Top, D. C. Cavanaugh, and P. K. Russell. 1976. Kaeng Khoi virus from naturally infected bedbugs (Cimicidae) and immature free-tail bats. Bull. W.H.O. 53:365-369. [PMC free article] [PubMed] [Google Scholar]
- 348.Woelk, C. H., and E. C. Holmes. 2002. Reduced positive selection in vector-borne RNA viruses. Mol. Biol. Evol. 19:2333-2336. [DOI] [PubMed] [Google Scholar]
- 349.Woolhouse, M. E., L. H. Taylor, and D. T. Haydon. 2001. Population biology of multihost pathogens. Science 292:1109-1112. [DOI] [PubMed] [Google Scholar]
- 350.World Health Organization 1967. Arboviruses and human disease. W.H.O. Tech. Rep. Ser. No. 369. World Health Organization, Geneva, Switzerland.
- 351.World Health Organization Scientific Group. 1985. Arthropod-borne and rodent-borne viral diseases. World Health Organization Tech. Rep. Ser. No. 719. World Health Organization, Geneva, Switzerland. [PubMed]
- 352.Wu, S. C., J. R. Chiang, C. W. Lin. 2004. Novel cell adhesive glycosaminoglycan-binding proteins of Japanese encephalitis virus. Biomacromolecues 5:2160-2164. [DOI] [PubMed] [Google Scholar]
- 353.Yamanishi, S., Y. Yamanaka, T. Kameyama, K. Miki, C. Miyamoto, and I. Takshima. 1997. Detection of Japanese encephalitis virus genome in mononuclear cells from blood and spleen of swine. J. Jpn. Vet. Assoc. 50:731-734. [Google Scholar]
- 354.Yang, K. D., W.-T. Yeh, M.-Y. Yang, R.-F. Chen, and M.-F. Shaio. 2001. Antibody-dependent enhancement of heterotypic dengue infections involved in suppression of IFNγ production. J. Med. Virol. 63:150-157. [PubMed] [Google Scholar]
- 355.Young, N. A. 1972. Serologic differentiation of viruses of the Venezuelan encephalitis (VE) complex. PAHO Sci. Publ. No. 243:84-89. [Google Scholar]
- 356.Yuill, T. M. 1986. The ecology of tropical arthropod-borne viruses. Annu. Rev. Entomol. 17:189-219. [Google Scholar]
- 357.Yunker, C. E. 1975. Tick-borne viruses associated with seabirds in North America and related islands. Med. Biol. 53:302-311. [PubMed] [Google Scholar]
- 358.Zeller, H. G., N. Karabatsos, C. H. Calisher, J.-P. Digoutte, F. A. Murphy, and R. E. Shope. 1989. Electron microscopy and antigenic studies of uncharacterized viruses. I. Evidence suggesting the placement of viruses in families Arenaviridae, Paramyxoviridae, or Poxviridae. Arch. Virol. 108:191-209. [DOI] [PubMed] [Google Scholar]
- 359.Zhang, W., M. Heil, R. J. Kuhn, and T. S. Baker. 2005. Heparin binding sites on Ross River virus revealed by electron cryo-microscopy. Virology 332:511-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Zhdanov, V. M., and N. B. Azadova. 1976. Integration and transfection of an arbovirus by the mammalian cells. Mol. Biol. (Moscow) 10:1296-1302. (In Russian.) [PubMed] [Google Scholar]
- 361.Zhu, L.-H., T.-Y. Zhao, Y.-D. Dong, and B.-L. Lu. 2002. Mosquito-induced enhancement of dengue virus infection in mice. Acta Entomol. Sin. 45:588-592. (In Chinese.) [Google Scholar]
- 362.Zhuge, H.-X., Y.-C. Meng, J.-W. Wu, Z.-Y. Zhu, W.-F. Liang, and P.-P. Yao. 1998. Studies on the experimental transmission of Rattus-borne hantaviruses by Ornithonyssus bacoti. Chin. J. Parasitol. Dis. 16:445-448. (In Chinese.) [PubMed] [Google Scholar]