Abstract
Corynebacterium glutamicum is able to grow in media containing up to 12 mM arsenite and 500 mM arsenate and is one of the most arsenic-resistant microorganisms described to date. Two operons (ars1 and ars2) involved in arsenate and arsenite resistance have been identified in the complete genome sequence of Corynebacterium glutamicum. The operons ars1 and ars2 are located some distance from each other in the bacterial chromosome, but they are both composed of genes encoding a regulatory protein (arsR), an arsenite permease (arsB), and an arsenate reductase (arsC); operon ars1 contains an additional arsenate reductase gene (arsC1′) located immediately downstream from arsC1. Additional arsenite permease and arsenate reductase genes (arsB3 and arsC4) scattered on the chromosome were also identified. The involvement of ars operons in arsenic resistance in C. glutamicum was confirmed by gene disruption experiments of the three arsenite permease genes present in its genome. Wild-type and arsB3 insertional mutant C. glutamicum strains were able to grow with up to 12 mM arsenite, whereas arsB1 and arsB2 C. glutamicum insertional mutants were resistant to 4 mM and 9 mM arsenite, respectively. The double arsB1-arsB2 insertional mutant was resistant to only 0.4 mM arsenite and 10 mM arsenate. Gene amplification assays of operons ars1 and ars2 in C. glutamicum revealed that the recombinant strains containing the ars1 operon were resistant to up to 60 mM arsenite, this being one of the highest levels of bacterial resistance to arsenite so far described, whereas recombinant strains containing operon ars2 were resistant to only 20 mM arsenite. Northern blot and reverse transcription-PCR analysis confirmed the presence of transcripts for all the ars genes, the expression of arsB3 and arsC4 being constitutive, and the expression of arsR1, arsB1, arsC1, arsC1′, arsR2, arsB2, and arsC2 being inducible by arsenite.
Corynebacterium glutamicum is a biotechnologically important microorganism that is widely used for the large-scale production of amino acids such as l-glutamate and l-lysine (25, 42). Together with other members of the genera Rhodococcus, Gordonia, Nocardia, and Mycobacterium, Corynebacterium belongs to the mycolata, a broad and diverse group of mycolic-acid-containing actinomycetes. Besides a thick peptidoglycan layer, the mycolata contain large amounts of mycolic acids and other lipids in their cell walls (43). Recently, the complete genome sequence of C. glutamicum strain ATCC 13032 was determined (44) and is predicted to contain 3,002 open reading frames (32).
Arsenic is one of the most prevalent toxic metals in the environment; it is mainly of geochemical origin (rocks and minerals) in an insoluble form but also derives from anthropogenic sources (41). In soluble forms, arsenic occurs as trivalent arsenite [As(III)] and pentavalent arsenate [As(V)]. Arsenate, a phosphate structural analogue, can enter the bacterial cell via the phosphate transport system. Its toxicity is due to its interference in normal phosphorylation processes by replacing cellular phosphate. It has recently been demonstrated that arsenite enters the cells, at neutral pH, by aqua-glyceroporins (glycerol transport proteins) in bacteria, yeasts, and mammals (41) and that its toxicity lies in its ability to bind sulfhydryl groups of cysteine residues in proteins, thereby inactivating them. Arsenite is considered to be more toxic than arsenate and can be oxidized to arsenate chemically or microbiologically (20). In some gram-negative bacteria, arsenite is converted to arsenate by an arsenite oxidase, a periplasmic membrane-bound enzyme member of the dimethyl sulfoxide reductase family of molybdoenzymes (51). The toxic properties of arsenic are well known and have been exploited in the production of antimicrobial agents, such as the first specific antimicrobial drug Salvarsan 606, in addition to the commonly used wood preservative chromated copper arsenate (27).
Bacteria have developed a variety of mechanisms to avoid the toxicity of arsenic: (i) minimizing the uptake of arsenate through the system for phosphate uptake (16), (ii) by peroxidation reactions with membrane lipids (1), and (iii) using the best characterized microbial arsenic detoxification pathway involving the ars operon (55).
Bacterial operons encoding analogous arsenic resistance determinants (ars) have been found on the chromosome as well as on transmissible plasmids from gram-positive and gram-negative microorganisms. These operons generally consist of either three (arsRBC) or five (arsRDABC) genes that have been organized into a single transcriptional unit (55). The three-gene system, encoding the arsenic transcriptional repressor (arsR), arsenite permease (arsB), and arsenate reductase (arsC), was present on the chromosome of Escherichia coli, Pseudomonas aeruginosa (13), and other enterobacteria (18). The operon of three genes is also present in the Staphylococcus plasmids pI258 and pSX267 (24).
The five-gene operon (arsRDABC) encodes an arsenite-inducible repressor (arsR), a negative regulatory protein that provides the fine tuning of operon expression (arsD), an ATPase, and a membrane-located arsenite efflux pump (arsA and arsB, respectively), together with an arsenate reductase (arsC). This operon was initially discovered in the E. coli plasmids R773 and R46 (17) and then on plasmid pKW301 from Acidiphilium multivorum (57). In addition to the above-mentioned arsenic resistance operons, a broad diversity of four-gene operons have been described in different species, such as Bacillus subtilis (52), Acidithiobacillus ferrooxidans (11, 12), and a Synechocystis sp. (36). Two operons involved in arsenic resistance have recently been identified on the chromosome of the multiresistant Pseudomonas putida, although no molecular details were provided (14). In Saccharomyces, a cluster of three genes involved in arsenic resistance was identified; genes acr1, acr2, and acr3 in S. cerevisiae (7) and genes arr1, arr2, and arr3 in S.douglasii (37) seem to encode the regulator, arsenate reductase, and arsenite permease, respectively.
In view of the ubiquitous presence of arsenic in nature, we wished to determine whether the saprophytic soil bacterium C.glutamicum contained genes involved in resistance to arsenic and the possible use of C. glutamicum in the detoxification of episodic increases of arsenic in soil and water.
Here, we report the identification of genes involved in arsenic resistance in C. glutamicum, some of them forming two similar operons (called ars1 and ars2), and two accessory genes (arsB3 and arsC4) scattered throughout the chromosome. We also show that both operons (ars1 and ars2) are functional and clearly involved in arsenic resistance.
MATERIALS AND METHODS
Bacterial strains, plasmids, culture, and transformation conditions.
The bacterial strains and plasmids used in this work are listed in Table 1. E. coli strains were grown in Luria-Bertani broth or Luria-Bertani agar (26) at 37°C. Corynebacterial strains were grown at 30°C in trypticase soy broth (TSB; complex medium), TSA (TSB supplemented with 2% agar), or minimal medium for corynebacteria (MMC) (33). When necessary, antibiotics were added at the following final concentrations: kanamycin, 50 μg/ml for E. coli and 25 μg/ml for corynebacteria; ampicillin, 100 μg/ml; chloramphenicol, 50 μg/ml for E. coli and 10 μg/ml for corynebacteria; apramycin, 50 μg/ml for E. coli and 25 μg/ml for corynebacteria. Transformation of E. coli strains was carried out by the Inoue method (28), and mobilization of plasmids from E. coli S17-1 (donor strain) to coryneform recipient strains in conjugation assays was essentially accomplished as described previously (39). Transconjugants were selected on TSA medium containing 50 μg/ml of nalidixic acid and an additional antibiotic (kanamycin, chloramphenicol, or apramycin), depending on the mobilizable plasmid used for the mating. For arsenic resistance tests, strains were grown on TSA-MMC plates and single colonies were transferred into TSB medium and grown at 30°C to an optical density at 600 nm (OD600) of approximately 0.5. Ten microlitera of cell suspension was streaked on plates of TSA-MMC (supplemented with an appropriate volume of either arsenate or arsenite) and incubated for up to 2 days at 30°C. Resistance was determined by the appearance of growth and defined as the highest concentration of either arsenate or arsenite that permitted growth.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant genotype or descriptiona | Source or referenceb |
|---|---|---|
| Strains | ||
| E. coli DH5α | r− m− used for general cloning | 26 |
| E. coli S17-1 | Mobilizing donor strain, pro recA, which has an RP4 derivative integrated into the chromosome | 53 |
| E. coli W3110 | K-12 F−(rmD-rmE) | 3 |
| E. coli AW3110 | E. coli W3110 strain lacking the chromosomal ars operon (Δars::cam) | 15 |
| C. glutamicum 13869 | Wild-type strain | ATCC |
| C. glutamicum 13032 | Wild-type strain | ATCC |
| C. glutamicum RES167 | ATCC 13032 restriction-deficient derivative used as host of recombinant plasmids | 59 |
| C. glutamicum ArsB1 | RES167 derivative containing plasmid pKA1 integrated in the arsB1 gene | This work |
| C. glutamicum ArsB2 | RES167 derivative containing plasmid pKA2 integrated in the arsB2 gene | This work |
| C. glutamicum ArsB3 | RES167 derivative containing plasmid pKA3 integrated in the arsB3 gene | This work |
| C. glutamicum ArsB1-B2 | ArsB1 derivative mutant, containing plasmid pOJA2 integrated in the arsB2 gene | This work |
| C. glutamicum ArsB1-B3 | ArsB3 derivative mutant, containing plasmid pOJA1 integrated in the arsB1 gene | This work |
| C. glutamicum ArsB2-B3 | ArsB3 derivative mutant, containing plasmid pOJA2 integrated in the arsB2 gene | This work |
| Staphylococcus aureus 240 | Wild-type strain | CECT |
| Rhodococcus fascians 3001 | Wild-type strain | CECT |
| Nocardia corynebacterioides 14898 | Wild-type strain | ATCC |
| Mycobacterium smegmatis mc2 155 | Efficient plasmid transformation M. smegmatis strain | 56 |
| Bacillus subtilis 36 | Wild-type strain | CECT |
| Streptomyces lividans 1326 | Wild-type strain | JIC |
| Pseudomonas fluorescens 378 | Wild-type strain | CECT |
| Pseudomonas putida KT2440 | bdsMR | 22 |
| Plasmids | ||
| pK18mob | E. coli mobilizable plasmid containing lacZ and kan | 54 |
| pKA1 | pK18mob derivative carrying the 310-bp internal fragment of the arsB1 gene from C. glutamicum | This work |
| pKA2 | pK18mob derivative carrying the 370-bp internal fragment of the arsB2 gene from C. glutamicum | This work |
| pKA3 | pK18mob derivative carrying the 260-bp internal fragment of the arsB3 gene from C. glutamicum | This work |
| pOJ260 | E. coli mobilizable plasmid containing lacZ and apm | 6 |
| pOJA1 | pOJ260 derivative carrying the 310-bp internal fragment of the arsB1 gene from C. glutamicum | This work |
| pOJA2 | pOJ260 derivative carrying the 370-bp internal fragment of the arsB2 gene from C. glutamicum | This work |
| pECM2 | Mobilizable E. coli-Corynebacterium bifunctional plasmid containing kan and cat | 30 |
| pECAS1 | pECM2 derivative carrying the whole ars1 operon (arsRBCC) | This work |
| pECAS2 | pECM2 derivative carrying the whole ars2 operon (arsRBC) | This work |
| pJMFA24 | E. coli promoter-probe vector containing bla and the promoterless kan gene from Tn5 as reporter gene | 2 |
| pJMF-EP | pJMFA24 derivative containing the putative promoter region of arsB1 (ParsB1) | This work |
| pJMF-ER | pJMFA24 derivative containing the putative promoter region of arsC1 (ParsC1) | This work |
| pEGFP | Bifunctional E. coli-corynebacteria promoter-probe vector containing kan as selective marker and egfp2 gene under the kan promoter (Pkan) as reporter | M. Letek and L. M. Mateos, unpublished data |
| pGFP-EP | pEGFP derivative containing the putative promoter region of arsB1 (ParsB1) instead of Pkan | This work |
| pGFP-ER | pEGFP derivative containing the putative promoter region of arsC1 (ParsC1) instead of Pkan | This work |
| pEGNC | pEGFP derivative vector containing the promoterless egfp2 gene | M. Letek and L. M. Mateos, unpublished data |
kan, apm, bla, cat, and egfp2 are respectively genes for kanamycin, apramycin, ampicillin, chloramphenicol, and green fluorescent protein.
ATCC, American Type Culture Collection; CECT, Spanish Type Culture Collection; JIC, John Innes Centre, Norwich, United Kingdom.
Molecular genetic methods.
Restriction endonuclease digestion, agarose gel electrophoresis, Southern blot analysis, and molecular cloning techniques were performed by using standard procedures (34). Primers for PCR were generated by using the C. glutamicum strain ATCC 13032 genome sequence and are listed in Table S1 in the supplemental material.
Isolation of RNA from C. glutamicum strain RES167 grown in MMC [supplemented or not with 5 mM As(III)], electrophoresis, transfer to nylon membranes, and hybridization were performed as described previously (45). Probes corresponding to internal fragments of arsB1, arsB2, arsC1, arsC2, and arsC1′ were obtained by PCR amplification using specific primers (see Table S1 in the supplemental material).
For reverse transcription (RT)-PCR analysis (two-step procedure), 1 μg of RNA was used as template to generate single-strand cDNA from the desired genes using the first-strand cDNA synthesis kit for RT-PCR (Roche) and following the manufacturer's recommendations. Primers used to generate cDNA from the corresponding transcripts were designed using the specific Applied Biosystems software (Primer express software, v2.0) and can be found in Table S1 in the supplemental material. cDNAs were used as template for the second PCR amplification reaction using specific primers (see Table S1 in the supplemental material); amplified DNAs were separated by electrophoresis through 1.6% agarose gels and stained with ethidium bromide. RNA samples were tested for contamination with genomic DNA by using each RNA sample as a template for PCR.
For quantitative-PCR (Q-PCR), 1/20 of each cDNA sample (from the first RT-PCR; see above) was used as template for amplification, adding a specific pair of primers for each gene (see Table S1 in the supplemental material), a suitable volume of the master reaction mix (Applied Biosystems), and water up to 25 μl. Reactions were performed in an ABI Prism 7000 sequence detection system (Applied Biosystems), and results were processed using specific software (ABI Prism 7000 SDS software). Results are indicated relative to the cycle threshold value. In all cases, the oligonucleotide primers used in Q-PCR were optimized to amplify fragments of the same length from each gene (around 50 nucleotides) and to ensure a similar melting temperature (59°C) according to the software instructions.
Computer analyses were performed with DNASTAR; database similarity searches were performed using BLAST and FASTA public servers (National Collection of Industrial Bacteria and European Bioinformatics Institute [EBI], Hixton Hall, United Kingdom), and multiple alignments of sequences were accomplished using ClustalW (EBI). Phylogenetic analyses were performed using MEGA2 (Molecular Evolutionary Genetics Analysis) software (35) by the neighbor-joining method with 21 arsR genes, 22 arsB genes, and 29 arsC genes; these analyses are shown in the supplemental material (Fig. S1, S3, and S4).
Construction of recombinant plasmids.
To clone the whole ars1 (arsR1-B1-C1-C1′) and ars2 (arsR2-B2-C2) operons, 2.8- and 2.1-kb fragments were amplified with Pfu from the total DNA of C. glutamicum strain ATCC 13032 using primer pairs ars1/ars2 and ars37/ars38, respectively (see Table S1 in the supplemental material). Primers were designed to include at their 5′ ends sites for the restriction enzymes EcoRI and BamHI; the 2.8-kb PCR-amplified DNA band (ars1) was digested with EcoRI (Klenow fragment filled) and BamHI and ligated to the BglII- plus SmaI-digested bifunctional plasmid pECM2 (Table 1), affording plasmid pECAS1. The 2.1-kb PCR-amplified DNA fragment (ars2) was ligated to the SmaI-digested pECM2, obtaining the bifunctional plasmid pECAS2 (Table 1).
For the disruption of the arsB1, arsB2, and arsB3 genes (encoding the three arsenite permeases present in the C. glutamicum genome), internal fragments of the genes were obtained by PCR amplification of total DNA from C. glutamicum ATCC 13032 by using primers ars3/ars4 for arsB1 (310 bp), ars5/ars6 for arsB2 (370 bp), and ars7/ars8 for arsB3 (260 bp) (Fig. 1A; see Table S1 in the supplemental material). Primers were designed to include restriction sites for BamHI (upper primers) and HindIII (lower primers); plasmid pK18mob digested with BamHI and HindIII was ligated with the internal fragments of the arsenite permease genes to afford plasmids pKA1, pKA2, and pKA3 (Table 1). Similarly, the internal fragments from arsB1 and arsB2 were cloned into plasmid pOJ260 (Table 1) to obtain pOJA1 and pOJA2, respectively.
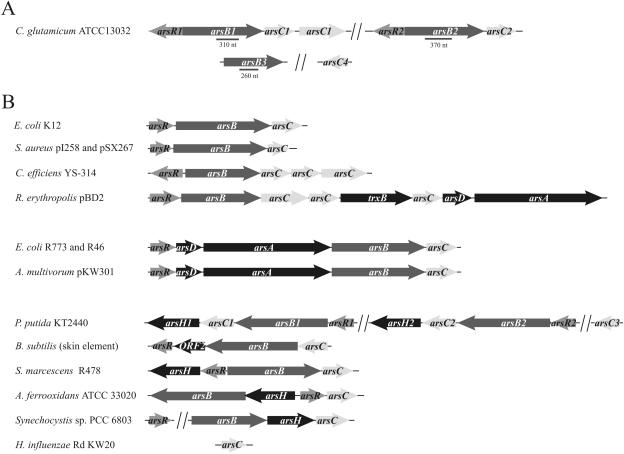
FIG. 1.
Schematic representation of genes involved in arsenic resistance in C. glutamicum ATCC 13032 (A) and in other bacteria (B). (A) Operons ars1 and ars2 are indicated. Arrows represent open reading frames. Dashed boxes indicate the probes used for the cloning of internal fragments of the desired genes obtained by PCR amplification. (B) The three-gene operon (arsRBC), encoding the transcriptional repressor (arsR), an arsenite permease (arsB), and an arsenate reductase (arsC), is present in the E. coli K-12 chromosome (accession number NC_000913), Staphylococcus plasmids pI258 (31) and pSX267 (48), C. glutamicum (NC_003450), C. efficiens (NC_004369), and R. erythropolis plasmid pBD2 (NC_005073). The five-gene operon (arsRDABC), encoding an arsenite-inducible repressor (arsR), a negative regulatory protein (arsD), an oxyanion-protruding ATPase, an arsenite efflux pump (arsA and arsB, respectively), and an arsenate reductase (arsC), is present in the E. coli plasmids R773 and R46 (10, 17) and in plasmid pKW301 from A. multivorum (57). The four-gene operon has been found in different species, such as the skin element of B. subtilis (52), A. ferrooxidans (11), plasmid R478 from S. marcescens (49), a Synechocystis sp. (36), and Pseudomonas putida (NC_002947). An isolated arsC gene is present in the genome of Haemophilus influenzae (NC_000907), and two operons involved in arsenic resistance have been identified on the chromosome of P. putida and C. glutamicum.
To identify promoters in the upstream region of arsB1 and arsC1, DNA fragments were amplified by PCR with the primers ars9/ars10 (ParsB1, 158 bp) and ars11/ars12 (ParsC1, 198 bp) (see Table S1 in the supplemental material); restriction enzyme sites for EcoRI and NdeI were included in the up and down primers, respectively. Amplified fragments were cloned into the promoter probe plasmids pJMFA24 and pEGFP (Table 1) to afford plasmids pJMF-EP/pGFP-EP (permease promoter) and pJMF-ER/pGFP-ER (reductase promoter), respectively.
RESULTS
Coryneform bacteria are highly resistant to arsenic.
Several gram-negative and gram-positive bacteria were assayed for their sensitivity/resistance to arsenic in TSA medium supplemented with sodium arsenate [AsO3−, As(V)] or sodium arsenite [AsO2−, (AsIII)]. Bacteria were classified as low resistant, moderately resistant, and highly resistant to arsenic (Table 2). As expected, the level of resistance to As(V) was 10-to 20-fold (50 to 150 mM) higher than the resistance level to As(III), except for C. glutamicum and Rhodococcus fascians, whose resistance level reached 400 to 500 mM (close to the solubility level of sodium arsenate in the media).
TABLE 2.
Resistance levels of different microorganisms to arsenite (AsIII) and arsenate (AsV)
| Microorganism | AsIII resistance (mM) | AsV resistance (mM) |
|---|---|---|
| Mycobacterium smegmatis | 1 | 25 |
| Nocardia corynebacteroides | 2 | 100 |
| Staphylococcus aureus | 2 | 50 |
| Bacillus subtilis | 2.5 | 75 |
| Streptomyces lividans | 4 | 150 |
| Escherichia coli DH5α | 5 | 100 |
| Pseudomonas fluorescens | 6 | 200 |
| Pseudomonas putida | 7 | 200 |
| Corynebacterium glutamicum 13032 | 12 | >500 |
| Corynebacterium glutamicum 13869 | 12 | >500 |
| Rhodococcus fascians | 14 | >500 |
From these experiments, it may be concluded that members of the coryneform group (C. glutamicum 13032, C. glutamicum 13869 [formerly B. lactofermentum], and R. fascians [formerly Corynebacterium fascians]) are much more resistant to arsenic than the rest of the bacteria analyzed. The genes responsible for the resistance to arsenic in C. glutamicum 13032 or C. glutamicum 13869 must be located within the bacterial chromosome because no plasmids bearing arsenic resistance genes have been described in these strains (38, 50, 58).
Several genes probably involved in arsenic resistance are present in Corynebacterium glutamicum.
Once the complete nucleotide sequence of the C. glutamicum chromosome was available (44), and assuming that proteins of similar sequences perform similar functions, the gene products possibly involved in arsenic resistance became available in the genome via homology-based analyses such as BLAST and FASTA (19). Thus, two operons, thereafter named ars1 and ars2, located distant from each other on the chromosome were found and both of them showed similar genetic structures (arsRBC). The only difference was the presence of an additional gene (arsC1′, Cgl1455 in the database) at the end of the ars1 operon (Fig. 1A). Genes homologous to arsC1′ have also been found downstream from arsC1 in Corynebacterium efficiens (accession number NC_004369) and in plasmid pBD2 from Rhodococcus erythropolis (accession number NC_005073). The organization of the ars genes in different bacteria is shown in Fig. 1B.
Based on BLAST analysis, the first genes of both clusters (ars1 and ars2) are arsR1 and arsR2, which encode putative arsenite regulatory proteins (arsR1 was not described in the database, and arsR2 was previously named Cgl 0275). ArsR1 and ArsR2 showed clear homologies to ArsR proteins from actinomycetes and Acidithiobacillus ferrooxidans (see Fig. S1 in the supplemental material). ArsR1 and ArsR2 from C. glutamicum (as in the ArsR from A. ferrooxidans) do not have the typical CXCXXC arsenite binding motif of R773 ArsR but have two joined cysteines close to the arsenite binding motif of R773 ArsR. Furthermore, ArsR1 has two additional cysteines (there is only one in ArsR2) at the N-terminal extension, like CadC (21) (see Fig. S2 in the supplemental material). Interestingly, the arsR in both operons is expressed divergently from the rest of the ars genes.
The second genes, arsB1 and arsB2, described as encoding an arsenite efflux pump, might encode enzyme arsenite permeases (formerly named Cgl 1453 and Cgl 0258 respectively). These are homologous to the arsenic protein carriers from actinomycetes, the skin element from B. subtilis (52), Saccharomyces cerevisiae Acr3p (61), and the arsenic protein carrier from a Synechocystis sp. (36) (see Fig. S3 in the supplemental material).
The rest of the genes in both clusters, arsC1, arsC1′, and arsC2 (previously named Cgl 1454, Cgl 1455, and Cgl 0259, respectively), encode predicted protein tyrosine phosphatases with sequence similarity to the thioredoxin-dependent arsenate reductases from actinomycetes and, to a lesser extent, Staphylococcus (62) and Bacillus (4) (see Fig. S4 in the supplemental material).
In addition to the above operons probably involved in arsenic resistance (operons ars1 and ars2), another putative arsenite permease gene (arsB3, Cgl 1414) and a putative arsenate reductase gene (arsC4, Cgl 1049) were present in the C. glutamicum genome (Fig. 1A). ArsB3 did not show homologies with arsenite permeases from gram-negative bacteria and seems to be different from the ArsB clade from actinomycetes, the skin element from B. subtilis, Saccharomyces cerevisiae Acr3p, and the ArsB clade from a Synechocystis sp. ArsC4 seems to be unrelated to the rest of C. glutamicum ArsCs and showed homologies with ArsC from gram-negative bacteria (see Fig. S4 in the supplemental material).
Arsenite permeases ArsB1 and ArsB2 are involved in arsenic resistance in C. glutamicum.
To confirm the involvement of the above-described genes in arsenic resistance in C. glutamicum, the integrative plasmids pKA1, pKA2, and pKA3 (Table 1) containing internal fragments of the hypothetical arsenite permease genes arsB1, arsB2, and arsB3 were introduced separately into E. coli strain S17-1 and transferred to C.glutamicum strain RES167 by conjugation. The arsenite permease gene was chosen for gene disruption because of the possible polar effect of its disruption on both ars1 and ars2 operons (Fig. 1A). Kanamycin-resistant transconjugants were obtained in all cases, suggesting that the integrative plasmids would have been incorporated into the host chromosome and that therefore the homologous chromosomal genes (and downstream arsC1, arsC1′, and arsC2 genes) might be disrupted. Plasmid integration was confirmed both by Southern hybridization and by PCR amplification (data not shown). Ten transconjugants from each conjugation experiment were analyzed for arsenite and arsenate resistance; because the behaviors of all the transconjugants obtained with a given suicide plasmid (pKA1, pKA2, or pKA3) were identical, only one transconjugant of each conjugation experiment was selected and was named C. glutamicum ArsB1, C. glutamicum ArsB2, or C. glutamicum ArsB3 (Table 1); the resistance to arsenite of these insertional mutant strains is shown in Table 3. When the above mutants were grown in TSA in the presence of arsenate, C. glutamicum ArsB2 and C. glutamicum ArsB3 were able to grow in up to 500 mM arsenate like C. glutamicum RES167 whereas C. glutamicum ArsB1 was resistant to only 200 mM.
TABLE 3.
Arsenite/arsenate resistance levels of different E. coli and C. glutamicum strains untransformed or transformed with plasmid pECAS1 (operon ars1) or pECAS2 (operon ars2)
| Strain | As(III) resistance (mM)
|
As(V) resistance (mM)
|
||||
|---|---|---|---|---|---|---|
| Untransformed | pECAS1 | pECAS2 | Untransformed | pECAS1 | pECAS2 | |
| E. coli | ||||||
| DH5α | 5 | 6 | 6 | 100 | 100 | 100 |
| W3110 | 5 | 6 | 6 | 100 | 100 | 100 |
| AW3110 | 0.2 | 6 | 6 | 2 | 20 | 20 |
| C. glutamicum | ||||||
| RES 167 | 12 | 60 | 20 | 500 | 500 | 500 |
| ArsB1 | 4 | 60 | 15 | 200 | 500 | 500 |
| ArsB2 | 9 | 60 | 20 | 500 | 500 | 500 |
| ArsB3 | 12 | 60 | 20 | 500 | 500 | 500 |
| ArsB1-B2 | 0.4 | 60 | 15 | 10 | 500 | 500 |
To construct the double arsenite permease mutant C. glutamicum ArsB1-B2, C. glutamicum ArsB1 was used as recipient in the conjugation assay, using the mobilizable plasmid pOJA2 (Table 1); mutants ArsB1-B3 and ArsB2-B3 were obtained after the transfer of plasmid pOJA1 (Table 1) and plasmid pOJA2, respectively, to the recipient strain C. glutamicum ArsB3. One transconjugant from each assay was selected and checked by Southern blot analysis. C. glutamicum mutants ArsB1-B3 and ArsB2-B3 behaved exactly like the single mutants C. glutamicum ArsB1 and C. glutamicum ArsB2, respectively. The behavior of the double mutant C. glutamicum ArsB1-B2 in relation to its resistance to arsenite is indicated in Table 3; furthermore, this mutant was also unable to grow in 10mM arsenate. The resistance level of the double mutant ArsB1-B2 was reduced 30-fold (from 12 mM to 0.4 mM) for arsenite and 50-fold (from 500 mM to 10 mM) for arsenate.
These results suggest that resistance to arsenite [and perhaps also to arsenate through the conversion of As(V) into As(III)] in C. glutamicum would mainly be due to the activity of operons ars1 and ars2, operon ars1 being more crucial than ars2.
Gene dose effect of ars1 and ars2 on resistance to arsenic in C. glutamicum and E. coli strains.
To confirm the involvement of the ars1 and ars2 operons in the resistance to arsenic, we attempted to analyze the effect of the C. glutamicum ars1 and ars2 operons in several E. coli strains (heterologous complementation) or in C. glutamicum ArsB mutants by using the multicopy bifunctional plasmids pECAS1 and pECAS2 (Table 1). Resistance levels to arsenite for transformed and untransformed E. coli and C. glutamicum strains are shown in Table 3.
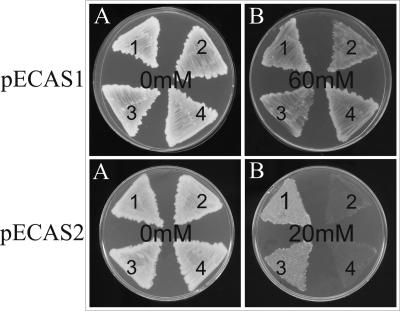
As can be observed in Fig. 2, mutations in arsB1 and arsB2 were clearly complemented by plasmid pECAS1 because transconjugants of mutants C. glutamicum ArsB1, ArsB2, and ArsB1-B2 were able to grow in up to 60 mM arsenite (Table 3; Fig. 2) and in up to 500 mM arsenate (Table 3). When plasmid pECAS2 was introduced into the C. glutamicum strains, a different behavior was observed (Table 3; Fig. 2); the control strain RES167 and mutant ArsB2 were resistant up to 20 mM arsenite, but the strains affected in the arsB1 gene (mutants ArsB1 and ArsB1-B2) were resistant only up to 15 mM arsenite. In conclusion, the presence of several copies (ca. 30 copies/cell) of the ars1 and ars2 operons not only complements the respective arsB1 and arsB2 mutations but also confers on C. glutamicum hitherto undescribed levels of resistance to arsenite.
FIG. 2.
Complementation of C. glutamicum arsB mutants by the ars1 (pECAS1) and ars2 (pECAS2) operons. C. glutamicum RES167 (1), C. glutamicum ArsB1 (2), C. glutamicum ArsB2 (3), and C. glutamicum ArsB1-B2 (4) transformed with pECAS1 or pECAS2 were inoculated in TSA containing increasing amounts of arsenite (0 mM to 60 mM), incubated at 30°C for 36 h, and photographed. Please note that some strains containing plasmid pECAS2 were unable to grow in the presence of 20 mM arsenite and that none of them were able to grow in 60 mM arsenite.
Transformation of E. coli with plasmids pECAS1 and pECAS2 indicated that the ars mutation present in E. coli AW3110 was clearly complemented by both the ars1 and ars2 operons (Table 3) whereas the resistance level of transformed E. coli DH5α and E. coli W3110 was only slightly increased (from 5 to 6 mM). Similar results were obtained when the transformed E. coli strains were analyzed for arsenate resistance; the supersensitive strain AW3110 was complemented by both ars operons, and its resistance level to arsenate increased from 2 mM to 20 mM.
Transcriptional analysis of the ars operons.
The genetic organization of the ars1 and ars2 operons suggests that genes arsB1-C1-C1′ and arsB2-C2 might be transcribed together and divergently with the transcription of arsR1 and arsR2, respectively. To analyze this possibility, C. glutamicum cells were grown in MMC to mid-exponential phase in the presence or absence of 5 mM arsenite and total RNA was isolated and used in Northern blot experiments.
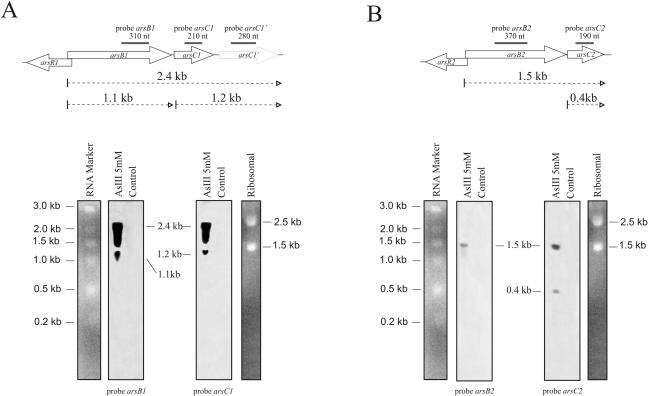
As can be observed in Fig. 3, transcription of both operons was induced in the presence of arsenite and transcription of operon ars1 was more efficient than that of operon ars2, corroborating our earlier results with the insertional mutants. The presence of transcripts of 2.4 kb and 1.1 kb when an internal fragment from arsB1 was used as probe (Fig. 3A) suggests that arsB1-C1-C1′ is transcribed mainly as a single 2.4-kb polycistronic unit and that the 1.1-kb transcript might correspond to a transcript of the gene coding for the ArsB1 permease. When an internal fragment from arsC1 was used as probe (Fig. 3A), the same 2.4-kb transcript was observed, as well as an additional 1.2-kb transcript corresponding to the dicistronic arsC1-C1′ (Fig. 3A). Similar results were obtained when the internal fragment from arsC1′ was used as probe.
FIG. 3.
Northern blot analysis of specific transcripts for arsB1, arsC1, arsC1′ arsB2, and arsC2 from C. glutamicum. Total RNA (15 μg) was isolated from C. glutamicum in the exponential phase (OD600 = 1.5) in the absence (control) or presence of 5 mM arsenite, electrophoresed, transferred to nylon filters, and hybridized with internal fragments of arsB1, arsC1, arsC1′, arsB2, and arsC2 obtained by PCR (dashed boxes) using the primers described in Table S1 in the supplemental material. Size controls corresponding to the migration of bands in the gel are indicated.
The transcriptional organization of the ars2 operon was very similar, with a transcript of 1.5 kb (dicistronic arsB2-C2) when an internal arsB2 fragment was used as probe (Fig. 3B) and transcripts of 1.5 kb (dicistronic arsB2-C2) and 0.4 kb (monocistronic arsC2) when an internal arsC2 fragment was used (Fig. 3B).
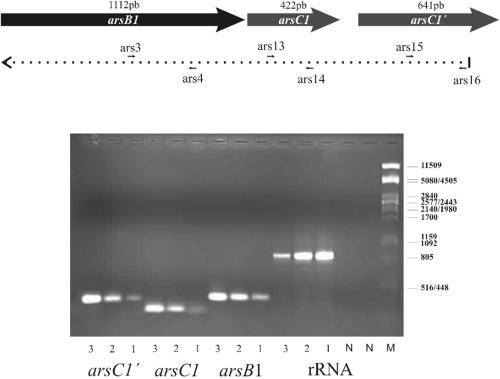
To confirm the expression of both ars operons, RT-PCR was performed using mRNA from cultures growing in the absence or presence of 5 mM arsenite. As can be observed in Fig. 4, arsB1, arsC1, and arsC1′ were induced in the presence of this compound. Similar results were obtained with the ars2 operon (data not shown).
FIG. 4.
Transcriptional analysis of arsB1-C1-C1′ by RT-PCR. Total RNA isolated from C. glutamicum in the exponential phase (OD600 = 1.5) in the absence (lanes 1) or presence (lanes 2) of 5 mM arsenite was used as the template in a reverse transcriptase reaction with primer ars16 to generate cDNA and then amplified with primers (ars3/4, ars13/14, and ars15/16) internal to each gene. A positive control using C. glutamicum chromosomal DNA instead of cDNA was included (lanes 3), as well as RT-PCR of the 16S rRNA (rRNA) using primer rrn2 for the synthesis of cDNA and primers rrn1 and rrn2 for PCR amplification (see Table S1 in the supplemental material). DNA contamination of the RNA samples was ruled out by performing PCR directly on the RNAs (N).
To quantify more precisely the level of expression of all the genes probably involved in resistance to arsenic, Q-PCR analysis was performed with all nine genes described in this work (arsR1, arsB1, arsC1, arsC1′, arsR2, arsB2, arsC2, arsB3, and arsC4) by using specific primers (see Table S1 in the supplemental material). As can be observed in Table 4, the estimated numbers of specific mRNA molecules for arsB3 and arsC4 were similar in the presence or absence of 5 mM arsenite whereas a number of molecules of specific mRNA for arsR1, arsB1, arsC1, arsC1′, arsR2, arsB2, and arsC2 were clearly induced in the presence of 5 mM arsenite. From the results of this experiment, it is possible to conclude that the expression of arsB3 and arsC4 is constitutive whereas the expression of the rest of the genes is inducible by arsenite, the induction of both regulatory genes (arsR1 and arsR2) being weaker than the rest of the ars genes.
TABLE 4.
Quantitative PCR of genes involved in arsenic resistance
| Gene | C at
|
Ratio [+As(III)/−As(III)]b | |
|---|---|---|---|
| − As(III) | + As(III) | ||
| arsR1 | 22.39 | 18.35 | 16 |
| arsB1 | 26.70 | 21.35 | 121 |
| arsC1 | 26.16 | 18.19 | 256 |
| arsC1′ | 25.92 | 18.06 | 232 |
| arsR2 | 23.12 | 19.07 | 17 |
| arsB2 | 24.84 | 19.95 | 56 |
| arsC2 | 23.12 | 16.33 | 111 |
| arsB3 | 26.40 | 26.20 | 1.1 |
| arsC4 | 24.28 | 23.68 | 1.5 |
Ct is defined as the cycle at which fluorescence is determined to be statistically significant above background, being inversely proportional to the log of the initial copy number; this value was calculated automatically by the ABI Prism 7000 SDS software.
+As(III)/−As(III) ratio indicates the relative number of specific mRNA molecules for each gene in the presence or absence of As(III). It was calculated assuming that at the threshold points, the number of amplified DNA molecules (N) in the presence or absence of arsenite would be the same as and proportional to the initial number of RNA molecules (n), as follows: N = n × 2Ct.
Promoter region analysis.
Taking into account the above results and the higher expression of the ars1 operon (Fig. 3), we checked for the presence of DNA fragments with promoter activity upstream from arsB1 or arsC1 with a view to finding new regulated promoters for use in corynebacterial expression systems.
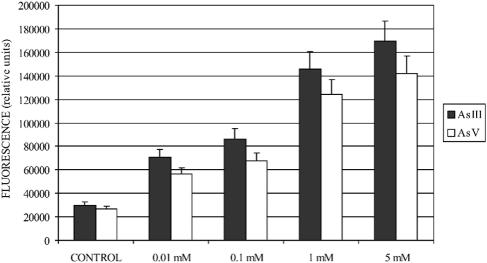
Plasmids pJMF-EP and pJMF-ER (containing the upstream regions of arsB1 and arsC1, respectively) were transformed into E. coli DH5α, and transformants were able to grow in Luria-Bertani broth containing 200 and 100 μg/ml of kanamycin, respectively. This indicated that both DNA fragments (ParsB1 and ParsC1) have promoter activity in E. coli, the permease promoter being stronger than that of reductase. Both promoter regions also showed promoter activity in C. glutamicum when plasmids pGFP-EP and pGFP-ER (Table 1) were present. Promoter activity in C. glutamicum was quantified by the level of green fluorescent protein, and as in E. coli, ParsB1 was stronger than ParsC1; the expression of ParsB1 was clearly induced in the presence of 0.01 to 5 mM arsenite/arsenate (Fig. 5), but no induction was observed in the case of ParsC1. Because in many microorganisms, the ars operon is induced by antimonite and bismuth (5, 36, 47), we studied the possible effect of different elements or compounds such as bismuth, antimonite, phosphate, phosphite, nitrates, or nitrites on the induction of ParsB1 and no induction was observed in any case.
FIG. 5.
The promoter of the arsB1 gene is induced in the presence of arsenite or arsenate. C. glutamicum [pGFP-EP] was grown in the presence of subinhibitory concentrations of arsenite (black bar) or arsenate (empty bar). C. glutamicum [pEGNC] (Table 1) was used as a negative control, and its fluorescence level was subtracted from all the values obtained. In all cases, the fluorescence level of each sample was divided by the OD600 of the sample. The values are the means of four determinations, and the standard deviation is indicated on the bar top. The fluorescence level ratio of green fluorescent protein/OD600 was measured on a Biotek Sinergy HT fluorimeter.
Interestingly, 26 nucleotides upstream from the arsB1 gene, a perfect inverted repeat (TGTCGATATT-N12-AATATCGACA) was found. Similarly, 56 nucleotides upstream from the arsB2 gene, another inverted repeat with a unique mismatch (ATGTCCGTCA-N8-TGACGcACAT) was also found. No similar sequences were found upstream from any other ars genes by using the Palindrome program (http://bioweb.pasteur.fr/seqanal/interfaces/palindrome.html).
DISCUSSION
The C. glutamicum ars system confers resistance to arsenic up to 12 mM As(III) and 500 mM As(V), and hence, C. glutamicum is one of the most resistant microorganisms described to date. This could be due to the presence of two functional ars operons (ars1 and ars2) and two accessory genes (arsB3 and arsC4) described and analyzed here. The existence of two chromosomal ars operons involved in arsenic resistance and an additional arsenate reductase gene (arsC3) scattered throughout the chromosome has also been described in P. putida (Fig. 1B) (14), although the functionality of both operons was not studied.
An unusual characteristic of the ars1 and ars2 operons of C. glutamicum is the orientation of arsR; whereas in C. glutamicum, arsR (in both ars operons) was located upstream and in the opposite orientation from arsBC, in most of the analyzed bacteria, arsR was located in the same orientation as arsBC (Fig. 1B). Another exception is A. ferrooxidans and Serratia marcescens, where the chromosomal arsenic resistance operon contains two clusters (arsCR-arsBH for A. ferrooxidans and arsBC-arsRH for S. marcescens) which are transcribed in the opposite direction (12). The possible significance of this particular gene organization remains unknown, but a divergent expression could mean an independent or differential regulation to optimize the adaptation of the microorganism to nutritional or environmental changes (9). ArsRs from either C.glutamicum, actinomycetes, or A. ferrooxidans do not have a typical arsenite binding motif (CXCXXC) present in the ArsR proteins from the E. coli plasmid R773.
Another structural difference between operons ars1 and ars2 is the presence of an extra arsenate reductase gene (arsC1′) in operon ars1. It has been claimed that a single arsC was added to the original ars operon (arsRB) when molecular oxygen appeared in the atmosphere (46); subsequent horizontal gene transfer in the evolution of arsenate reductases and sequence divergence gave the distinct arsC classes that exist today (29). However, a convergent evolution of the two mayor ArsC branches cannot be discarded (40). Three families of arsenate reductases have been described previously (40), and those encoded by genes found in the C. glutamicum ars1 and ars2 operons (and also in C. efficiens, R. erythropolis, and Streptomyces coelicolor A3 [2]) belong to the group of Bacillus enzymes where arsenate reductases use thioredoxin and are similar to protein tyrosine phosphatases but form a separate subfamily (see Fig. S4 in the supplemental material). The arsenate reductase ArsC4 from C. glutamicum, together with the ArsC4 from C. efficiens and ArsC from Corynebacterium diphtheriae, seems to be more related to the R773 ArsC purified from E. coli which requires reduced glutathione (GSH) and the small thiol transfer protein glutaredoxin (Grx) for arsenate reductase activity (23). The presence of two functional arsC genes in the ars1 operon from C. glutamicum is also intriguing, although three arsC genes are present in the unique ars operon of C. efficiens (accession number NC_004369) and R. erythropolis (accession number NC_005073) (Fig. 1B), all of them belonging to the mycolata group and being the most arsenite-resistant bacteria described to date (Table 2).
Genes encoding three hypothetical arsenite permeases were located in the genome of C. glutamicum, being the only case described up to date; permeases ArsB1 and ArsB2 are related to each other, whereas ArsB3 is phylogenetically more distant, but all the permeases belong to the same clade (see Fig. S3 in the supplemental material). The presence of multiple permeases could circumvent the absence of the oxyanion ATPase (ArsA) described in the five-gene system (Fig. 1B). The arsA gene was not found in the genome sequence of C. glutamicum, and hence, the mechanism of arsenic resistance could be driven by the ArsB permease, as has been described for several other microorganisms. In some cases, heterologous cloning of the arsA gene from E. coli resulted in increased resistance to arsenite in Staphylococcus aureus, as previously described (8).
Insertional mutation of genes encoding the three arsenite permeases found in the genome of C. glutamicum clearly indicates that arsB1 and arsB2 are involved in resistance to arsenite but that insertional mutant C. glutamicum ArsB3 behaves like the wild type. Nevertheless, Q-PCR analysis clearly indicates that arsB3 is expressed constitutively and that it is considered an accessory gene, like arsC4. The distinct level of transcription of both ars1 and ars2 operons, observed in RNA/DNA hybridization experiments, indicates that operon ars1 is transcriptionally more active than ars2. This was also confirmed when operons ars1 and ars2 were introduced into C. glutamicum (Table 3).
Operons ars1 (pECAS1) and ars2 (pECAS2) were also used to transform E. coli strains, and the resulting transformed strains were slightly more resistant to arsenite than the untransformed strains DH5α and W3110 (Table 3); operons ars1 and ars2 functionally replace the ars mutation of the supersensitive E. coli strain AW3110, which increases the resistance 30-fold (Table 3). These results confirmed the functionality of the ars1 and ars2 operons from C. glutamicum in E. coli.
The theoretical binding site for ArsR overlaps with the putative −10 and −35 promoter regions of the arsB genes, and both contain symmetric and nonidentical dyad sequences: TGTCGATATT-N12-AATATCGACA for arsB1 and ATG TCCGTCA-N8-TGACGcACAT for arsB2. Dyad sequences have been found in multiple regulatory sites involved in the binding of ArsR in E. coli (60) and Synechocystis (36). Induction of ParsB1 in the presence of As(III)/As(V) was demonstrated when plasmid pGFP-EP was used in the reporter system; however, antimonite [Sb(III)] was not able to induce pGFP-EP, although all ArsR repressors so far described do respond to Sb(III).
In conclusion, operons ars1 and ars2 are involved in arsenic resistance in C. glutamicum. Operon ars1 seems to be the main arsenic detoxification system according to different lines of experimental evidence: (i) C. glutamicum ArsB1 is less resistant to arsenite than mutant C. glutamicum ArsB2; (ii) RNA/DNA hybridization experiments reveal a higher expression of ars1 than ars2; (iii) the arsenic-supersensitive mutant C. glutamicum ArsB1-B2 transformed with the whole ars1 operon becomes resistant to 60 mM arsenite at five times the level of resistance of the wild-type strain; and (iv) C. glutamicum ArsB1-B2 transformed with operon ars2 becomes resistant to 15 mM arsenite at 1.25 times the level of resistance of C. glutamicum.
Supplementary Material
Acknowledgments
E. Ordóñez, M. Letek, and N. Valbuena were the recipients of fellowships from the Junta de Castilla y León (E.O.) and Ministerio de Educación y Ciencia (M.L. and N.V.). This work was supported by grants from the Junta de Castilla y León (LE 24/01 and LE 14/04) and the Ministerio de Ciencia y Tecnología (BIO 2002-03223).
Thanks are due to Barry Rosen (Detroit, Mich.) for providing E. coli strains W3110 and AW3110 and to N. Skinner for correcting the English version of the manuscript.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1.Abdrashitova, S. A., G. G. Abdullina, and A. N. Ilialetdinov. 1986. Role of arsenites in lipid peroxidation in Pseudomonas putida cells oxidizing arsenite. Mikrobiologiya 55:212-216. [Google Scholar]
- 2.Adham, S. A., S. Rodriguez, A. Ramos, R. I. Santamaria, and J. A. Gil. 2003. Improved vectors for transcriptional/translational signal screening in corynebacteria using the melC operon from Streptomyces glaucescens as reporter. Arch. Microbiol. 180:53-59. [DOI] [PubMed] [Google Scholar]
- 3.Bachmann, B. J. 1972. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol. Rev. 36:525-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett, M. S., Z. Guan, M. Laurberg, and X. D. Su. 2001. Bacillus subtilis arsenate reductase is structurally and functionally similar to low molecular weight protein tyrosine phosphatases. Proc. Natl. Acad. Sci. USA 98:13577-13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharjee, H., J. Li, M. Y. Ksenzenko, and B. P. Rosen. 1995. Role of cysteinyl residues in metalloactivation of the oxyanion-translocating ArsA ATPase. J. Biol. Chem. 270:11245-11250. [DOI] [PubMed] [Google Scholar]
- 6.Bierman, M., R. Logan, K. O'Brien, E. T. Seno, R. N. Rao, and B. E. Schoner. 1992. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43-49. [DOI] [PubMed] [Google Scholar]
- 7.Bobrowicz, P., R. Wysocki, G. Owsianik, A. Goffeau, and S. Ulaszewski. 1997. Isolation of three contiguous genes, ACR1, ACR2 and ACR3, involved in resistance to arsenic compounds in the yeast Saccharomyces cerevisiae. Yeast 13:819-828. [DOI] [PubMed] [Google Scholar]
- 8.Broer, S., G. Ji, A. Broer, and S. Silver. 1993. Arsenic efflux governed by the arsenic resistance determinant of Staphylococcus aureus plasmid pI258. J. Bacteriol. 175:3480-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browning, D. F., C. M. Beatty, A. J. Wolfe, J. A. Cole, and S. J. Busby. 2002. Independent regulation of the divergent Escherichia coli nrfA and acsP1 promoters by a nucleoprotein assembly at a shared regulatory region. Mol. Microbiol. 43:687-701. [DOI] [PubMed] [Google Scholar]
- 10.Bruhn, D. F., J. Li, S. Silver, F. Roberto, and B. P. Rosen. 1996. The arsenical resistance operon of IncN plasmid R46. FEMS Microbiol. Lett. 139:149-153. [DOI] [PubMed] [Google Scholar]
- 11.Butcher, B. G., S. M. Deane, and D. E. Rawlings. 2000. The chromosomal arsenic resistance genes of Thiobacillus ferrooxidans have an unusual arrangement and confer increased arsenic and antimony resistance to Escherichia coli. Appl. Environ. Microbiol. 66:1826-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butcher, B. G., and D. E. Rawlings. 2002. The divergent chromosomal ars operon of Acidithiobacillus ferrooxidans is regulated by an atypical ArsR protein. Microbiology 148:3983-3992. [DOI] [PubMed] [Google Scholar]
- 13.Cai, J., K. Salmon, and M. S. DuBow. 1998. A chromosomal ars operon homologue of Pseudomonas aeruginosa confers increased resistance to arsenic and antimony in Escherichia coli. Microbiology 144:2705-2713. [DOI] [PubMed] [Google Scholar]
- 14.Canovas, D., I. Cases, and V. de Lorenzo. 2003. Heavy metal tolerance and metal homeostasis in Pseudomonas putida as revealed by complete genome analysis. Environ. Microbiol. 5:1242-1256. [DOI] [PubMed] [Google Scholar]
- 15.Carlin, A., W. Shi, S. Dey, and B. P. Rosen. 1995. The ars operon of Escherichia coli confers arsenical and antimonial resistance. J. Bacteriol. 177:981-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cervantes, C., G. Ji, J. L. Ramirez, and S. Silver. 1994. Resistance to arsenic compounds in microorganisms. FEMS Microbiol. Rev. 15:355-367. [DOI] [PubMed] [Google Scholar]
- 17.Chen, C. M., T. K. Misra, S. Silver, and B. P. Rosen. 1986. Nucleotide sequence of the structural genes for an anion pump. The plasmid-encoded arsenical resistance operon. J. Biol. Chem. 261:15030-15038. [PubMed] [Google Scholar]
- 18.Diorio, C., J. Cai, J. Marmor, R. Shinder, and M. S. DuBow. 1995. An Escherichia coli chromosomal ars operon homolog is functional in arsenic detoxification and is conserved in gram-negative bacteria. J. Bacteriol. 177:2050-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durand, P., L. Canard, and J. P. Mornon. 1997. Visual BLAST and visual FASTA: graphic workbenches for interactive analysis of full BLAST and FASTA outputs under MICROSOFT WINDOWS 95/NT. Comput. Appl. Biosci. 13:407-413. [DOI] [PubMed] [Google Scholar]
- 20.Ehrlich, H. L. 2002. Bacterial oxidation of As(III) compounds, p. 313-329. In W. T. Frankenberger, Jr. (ed.), Environmental chemistry of arsenic. Marcel Dekker Inc., New York, N.Y.
- 21.Endo, G., and S. Silver. 1995. CadC, the transcriptional regulatory protein of the cadmium resistance system of Staphylococcus aureus plasmid pI258. J. Bacteriol. 177:4437-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franklin, F. C., M. Bagdasarian, M. M. Bagdasarian, and K. N. Timmis. 1981. Molecular and functional analysis of the TOL plasmid pWWO from Pseudomonas putida and cloning of genes for the entire regulated aromatic ring meta cleavage pathway. Proc. Natl. Acad. Sci. USA 78:7458-7462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gladysheva, T. B., K. L. Oden, and B. P. Rosen. 1994. Properties of the arsenate reductase of plasmid R773. Biochemistry 33:7288-7293. [DOI] [PubMed] [Google Scholar]
- 24.Gotz, F., J. Zabielski, L. Philipson, and M. Lindberg. 1983. DNA homology between the arsenate resistance plasmid pSX267 from Staphylococcus xylosus and the penicillinase plasmid pI258 from Staphylococcus aureus. Plasmid 9:126-137. [DOI] [PubMed] [Google Scholar]
- 25.Gourdon, P., and N. D. Lindley. 1999. Metabolic analysis of glutamate production by Corynebacterium glutamicum. Metab. Eng. 1:224-231. [DOI] [PubMed] [Google Scholar]
- 26.Hanahan, D. 1983. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166:557-580. [DOI] [PubMed] [Google Scholar]
- 27.Hingston, J. A., C. D. Collins, R. J. Murphy, and J. N. Lester. 2001. Leaching of chromated copper arsenate wood preservatives: a review. Environ. Pollut. 111:53-66. [DOI] [PubMed] [Google Scholar]
- 28.Inoue, H., H. Nojima, and H. Okayama. 1990. High efficiency transformation of Escherichia coli with plasmids. Gene 96:23-28. [DOI] [PubMed] [Google Scholar]
- 29.Jackson, C. R., and S. L. Dugas. 2003. Phylogenetic analysis of bacterial and archaeal arsC gene sequences suggests an ancient, common origin for arsenate reductase. BMC. Evol. Biol. 3:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jager, W., A. Schafer, A. Puhler, G. Labes, and W. Wohlleben. 1992. Expression of the Bacillus subtilis sacB gene leads to sucrose sensitivity in the gram-positive bacterium Corynebacterium glutamicum but not in Streptomyces lividans. J. Bacteriol. 174:5462-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji, G., and S. Silver. 1992. Regulation and expression of the arsenic resistance operon from Staphylococcus aureus plasmid pI258. J. Bacteriol. 174:3684-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kalinowski, J., B. Bathe, D. Bartels, N. Bischoff, M. Bott, A. Burkovski, N.Dusch, L. Eggeling, B. J. Eikmanns, L. Gaigalat, A. Goesmann, M. Hartmann, K. Huthmacher, R. Kramer, B. Linke, A. C. McHardy, F. Meyer, B. Mockel, W. Pfefferle, A. Puhler, D. A. Rey, C. Ruckert, O. Rupp, H. Sahm, V. F. Wendisch, I. Wiegrabe, and A. Tauch. 2003. The complete Corynebacterium glutamicum ATCC 13032 genome sequence and its impact on the production of L-aspartate-derived amino acids and vitamins. J. Biotechnol. 104:5-25. [DOI] [PubMed] [Google Scholar]
- 33.Kaneko, H., and K. Sakaguchi. 1979. Fusion of protoplasts and genetic recombination of Brevibacterium flavum. Agric. Biol. Chem. 43:1007-1013. [Google Scholar]
- 34.Kieser, T., M. J. Bibb, M. J. Buttner, B. F. Chen, and D. A. Hopwood. 2000. Practical Streptomyces genetics. The John Innes Foundation, Norwich, Conn.
- 35.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 36.Lopez-Maury, L., F. J. Florencio, and J. C. Reyes. 2003. Arsenic sensing and resistance system in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 185:5363-5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maciaszczyk, E., R. Wysocki, P. Golik, J. Lazowska, and S. Ulaszewski. 2004. Arsenical resistance genes in Saccharomyces douglasii and other yeast species undergo rapid evolution involving genomic rearrangements and duplications. FEMS Yeast Res. 4:821-832. [DOI] [PubMed] [Google Scholar]
- 38.Martin, J. F., R. I. Santamaria, H. Sandoval, G. del Real, L. M. Mateos, J. A. Gil, and A. Aguilar. 1987. Cloning systems in amino acid-producing corynebacteria. Bio/Technology 5:137-146. [Google Scholar]
- 39.Mateos, L. M., A. Schafer, J. Kalinowski, J. F. Martin, and A. Puhler. 1996. Integration of narrow-host-range vectors from Escherichia coli into the genomes of amino acid-producing corynebacteria after intergeneric conjugation. J. Bacteriol. 178:5768-5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukhopadhyay, R., and B. P. Rosen. 2002. Arsenate reductases in prokaryotes and eukaryotes. Environ. Health Perspect. 110(Suppl. 5):745-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukhopadhyay, R., B. P. Rosen, l. T. Phung, and S. Silver. 2002. Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol. Rev. 26:311-325. [DOI] [PubMed] [Google Scholar]
- 42.Nakayama, K., K. Araki, and H. Kase. 1978. Microbial production of essential amino acid with Corynebacterium glutamicum mutants. Adv. Exp. Med. Biol. 105:649-661. [DOI] [PubMed] [Google Scholar]
- 43.Ochi, K. 1995. Phylogenetic analysis of mycolic acid-containing wall-chemotype IV actinomycetes and allied taxa by partial sequencing of ribosomal protein AT-L30. Int. J. Syst. Bacteriol. 45:653-660. [DOI] [PubMed] [Google Scholar]
- 44.Ohnishi, J., S. Mitsuhashi, M. Hayashi, S. Ando, H. Yokoi, K. Ochiai, and M. Ikeda. 2002. A novel methodology employing Corynebacterium glutamicum genome information to generate a new L-lysine-producing mutant. Appl. Microbiol. Biotechnol. 58:217-223. [DOI] [PubMed] [Google Scholar]
- 45.Ramos, A., M. P. Honrubia, D. Vega, J. A. Ayala, A. Bouhss, D. Mengin-Lecreulx, and J. A. Gil. 2004. Characterization and chromosomal organization of the murD-murC-ftsQ region of Corynebacterium glutamicum ATCC 13869. Res. Microbiol. 155:174-184. [DOI] [PubMed] [Google Scholar]
- 46.Rosen, B. P. 2002. Biochemistry of arsenic detoxification. FEBS Lett. 529:86-92. [DOI] [PubMed] [Google Scholar]
- 47.Rosen, B. P., H. Bhattacharjee, and W. Shi. 1995. Mechanisms of metalloregulation of an anion-translocating ATPase. J. Bioenerg. Biomembr. 27:85-91. [DOI] [PubMed] [Google Scholar]
- 48.Rosenstein, R., K. Nikoleit, and F. Gotz. 1994. Binding of ArsR, the repressor of the Staphylococcus xylosus (pSX267) arsenic resistance operon to a sequence with dyad symmetry within the ars promoter. Mol. Gen. Genet. 242:566-572. [DOI] [PubMed] [Google Scholar]
- 49.Ryan, D., and E. Colleran. 2002. Arsenical resistance in the IncHI2 plasmids. Plasmid 47:234-240. [DOI] [PubMed] [Google Scholar]
- 50.Santamaria, R. I., J. A. Gil, J. M. Mesas, and J. F. Martin. 1984. Characterization of an endogenous plasmid and development of cloning vectors and a transformation system in Brevibacterium lactofermentum. J. Gen. Microbiol. 130:2237-2246. [Google Scholar]
- 51.Santini, J. M., and R. N. vanden Hoven. 2004. Molybdenum-containing arsenite oxidase of the chemolithoautotrophic arsenite oxidizer NT-26. J. Bacteriol. 186:1614-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato, T., and Y. Kobayashi. 1998. The ars operon in the skin element of Bacillus subtilis confers resistance to arsenate and arsenite. J. Bacteriol. 180:1655-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schafer, A., J. Kalinowski, R. Simon, A. H. Seep-Feldhaus, and A. Puhler. 1990. High-frequency conjugal plasmid transfer from gram-negative Escherichia coli to various gram-positive coryneform bacteria. J. Bacteriol. 172:1663-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schafer, A., A. Tauch, W. Jager, J. Kalinowski, G. Thierbach, and A. Puhler. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69-73. [DOI] [PubMed] [Google Scholar]
- 55.Silver, S., and L. T. Phung. 1996. Bacterial heavy metal resistance: new surprises. Annu. Rev. Microbiol. 50:753-789. [DOI] [PubMed] [Google Scholar]
- 56.Snapper, S. B., R. E. Melton, S. Mustafa, T. Kieser, and W. R. Jacobs, Jr. 1990. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol. Microbiol. 4:1911-1919. [DOI] [PubMed] [Google Scholar]
- 57.Suzuki, K., N. Wakao, T. Kimura, K. Sakka, and K. Ohmiya. 1998. Expression and regulation of the arsenic resistance operon of Acidiphilium multivorum AIU 301 plasmid pKW301 in Escherichia coli. Appl. Environ. Microbiol. 64:411-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tauch, A., A. Puhler, J. Kalinowski, and G. Thierbach. 2003. Plasmids in Corynebacterium glutamicum and their molecular classification by comparative genomics. J. Biotechnol. 104:27-40. [DOI] [PubMed] [Google Scholar]
- 59.Wehmeier, L., O. Brockmann-Gretza, A. Pisabarro, A. Tauch, A. Puhler, J. F. Martin, and J. Kalinowski. 2001. A Corynebacterium glutamicum mutant with a defined deletion within the rplK gene is impaired in (p)ppGpp accumulation upon amino acid starvation. Microbiology 147:691-700. [DOI] [PubMed] [Google Scholar]
- 60.Wu, J., and B. P. Rosen. 1993. Metalloregulated expression of the ars operon. J. Biol. Chem. 268:52-58. [PubMed] [Google Scholar]
- 61.Wysocki, R., P. Bobrowicz, and S. Ulaszewski. 1997. The Saccharomyces cerevisiae ACR3 gene encodes a putative membrane protein involved in arsenite transport. J. Biol. Chem. 272:30061-30066. [DOI] [PubMed] [Google Scholar]
- 62.Zegers, I., J. C. Martins, R. Willem, L. Wyns, and J. Messens. 2001. Arsenate reductase from S. aureus plasmid pI258 is a phosphatase drafted for redox duty. Nat. Struct. Biol. 8:843-847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.