Abstract
Flap endonuclease (Fen1) is required for DNA replication and repair, and defects in the gene encoding Fen1 cause increased accumulation of mutations and genome rearrangements. Because mutations in some genes involved in these processes cause cancer predisposition, we investigated the possibility that Fen1 may function in tumorigenesis of the gastrointestinal tract. Using gene knockout approaches, we introduced a null mutation into murine Fen1. Mice homozygous for the Fen1 mutation were not obtained, suggesting absence of Fen1 expression leads to embryonic lethality. Most Fen1 heterozygous animals appear normal. However, when combined with a mutation in the adenomatous polyposis coli (Apc) gene, double heterozygous animals have increased numbers of adenocarcinomas and decreased survival. The tumors from these mice show microsatellite instability. Because one copy of the Fen1 gene remained intact in tumors, Fen1 haploinsufficiency appears to lead to rapid progression of cancer.
There are several forms of human colorectal cancer, the most frequent being the sporadic form. Germ line mutations in the Apc gene are responsible for the rare cancer predisposition syndrome, familial adenomatous polyposis (FAP) (1–7), although additional genetic changes are required for tumor formation. Mutations in Apc and other genes involved in tumor progression are a prerequisite for a majority of sporadic colorectal tumors. Hereditary nonpolyposis colorectal cancer (HNPCC) is another colorectal cancer predisposition syndrome. Germ line mutations in the DNA mismatch repair (MMR) genes Msh2 and Mlh1 are the major cause of HNPCC (refs. 8–12; http://www.nfdht.nl/database/mdbchoice.htm). Most HNPCC cases can be accounted for by missense, nonsense, frameshift, and splice mutations in these two genes or deletion mutations, primarily in Msh2 (9, 13–16). A small proportion of HNPCC cases appear to be caused by germ line mutations in two other MMR genes, Msh6 and Pms2, and mutations in Msh6 are also found in familial non-HNPCC cases (17–21). Tumors from HNPCC patients often exhibit microsatellite instability (MSI) (22–26). MSI results from expansion or contraction of mono- or multinucleotide repeats due to failure to repair insertion/deletion mismatches in DNA. Although a large number of HNPCC kindreds can be accounted for by mutations in Msh2, Mlh1, and other MMR genes, there are reports of suspected HNPCC cases that do not have mutations in any of these genes but have tumors with MSI, raising the possibility that mutations in other genes involved in MMR might be implicated in these cases (27–30).
A proportion of sporadic tumors in the intestinal tract as well as of tumors from other sites exhibit MSI (16, 23, 24, 31), suggesting that sporadic defects in MMR genes play a role in either the initiation or progression of a number of tumor types. Indeed, mutations in Msh2 and Mlh1 or silencing of Mlh1 were detected in some but not all of these tumor types (8–12). This observation also suggests that other DNA repair genes could be involved in these cases. One such gene is Flap Endonuclease 1 (Fen1).
FEN1 functions in the processing of the 5′ ends of Okazaki fragments in lagging strand synthesis and long patch-base excision type repair (32–37). FEN1 protein binds to proliferating nuclear cell antigen (38–39) and potentially competes with p21, xeroderma pigmentosum gene product, 5′ methyl cytosine methyl transferase, and other proteins for a specific binding motif, implying roles for FEN1 in DNA replication, repair, epigenetic inheritance, and cell cycle control. Null mutations in this gene (also referred to as rad27) in Saccharomyces cerevisiae cause temperature-sensitive viability, increased sensitivity to UV light, mutagen sensitivity, genomic instability, plasmid loss, and destabilization of telomeric repeats and are nonviable in combination with mutations in genes involved in homologous recombination (40–46). rad27 mutants exhibit a complex mutator phenotype. They have increased frequencies of accumulating frame-shift mutations and because of this, it has been suggested that rad27 mutations might cause a partial MMR defect (41–42, 47). rad27 mutants accumulate insertion mutations that result from duplication of sequences flanked by repeated sequences as well as deletion mutations that result from deletion of sequences flanked by repeated sequences (42), and these are thought to arise from improper processing of Okazaki fragments (42, 48). Finally, rad27 mutants accumulate extensive genome rearrangements that have been suggested to result from errors during DNA replication (43). The phenotypes caused by rad27 mutations make Fen1 a potential candidate cancer susceptibility gene.
To examine the role of FEN1 in tumor initiation and progression, we generated mice that carry a null mutation in the Fen1 gene. We found that homozygosity of the Fen1 mutation leads to early embryonic lethality. Mice that are heterozygous for the mutation are viable and show a mild tumor predisposition phenotype. A proportion of the mice (17%) develop non-Hodgkin's lymphoma of the B cell type, and some mice show premature thymus involution. To assess the role of Fen1 in GI tumor progression, we generated mice that are double heterozygotes for mutations in the Apc and Fen1 genes. Mice that are heterozygous for the Apc mutation (Apc1638N) have a tumor predisposition phenotype. They develop colonic polyps and adenomas of the small intestine that do not show MSI, and they have a median survival of 13 mo. When Apc1638N and Fen1null are combined, the median survival of the resulting double heterozygous mice is reduced to 9 mo, the tumors are more advanced, and all tumors show MSI. The wild-type (WT) copy of the Fen1 allele remains intact in these tumors. These results suggest that haploinsufficiency of Fen1 is an important contributor to gastrointestinal (GI) tumor progression in our mouse models.
Materials and Methods
Fen1 Gene Targeting.
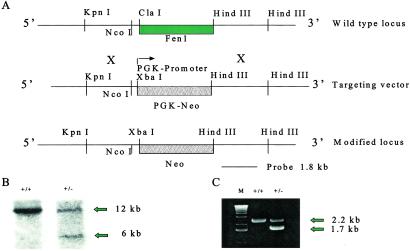
A 9-kb fragment of mouse genomic DNA was isolated from BAC 325J22 (RP-22 BAC library) and cloned into pUC19. The fragment contained the Fen1 coding region, as shown by DNA sequencing. A 1.2-kb KpnI fragment located on the 5′ end of the flanking region was deleted to facilitate identification of target vector insertion in embryonic stem (ES) cells. ClaI and HindIII sites were found to encompass the coding region and 826 bp of 3′ flanking sequence. The ClaI site was filled in and converted to an XbaI site. The Fen1 coding region was then deleted by digestion with XbaI and HindIII and replaced with the neomycin gene under control of the phosphoglycerol kinase promoter. The 826 bp of 3′ flanking region previously deleted was reinserted at the HindIII site by using a PCR product containing HindIII sites at each end. Proper orientation and sequence was confirmed. The targeting vector (Fig. 1A) was linearized with KpnI for transfecting ES cells. G418-resistant clones were screened by PCR by using primers within the targeting vector and 3′ flanking region. Of 165 ES cell clones screened, 8 contained the correctly targeted mutant allele (Fen1null). Chimeric mice were generated by standard techniques with C57/BL6 blastocysts and germ line transmission monitored by using coat color markers. Chimeric males were bred to C57/BL6 females and offspring genotyped by PCR (Fig. 1C) or Southern hybridization (Fig. 1B). Offspring were intercrossed as required.
Figure 1.
Strategy for the production of Fen1null mutant mice. (A) Gene-targeting strategy. (B) Southern blot of liver DNA from Fen1 WT (12-kb fragment) and Fen1null mutant mouse (6-kb fragment). DNA was digested with HindIII and hybridized with an 1,800-bp probe from the 3′ flanking region of the Fen1 gene. (C) PCR analysis of tail DNA from Fen1 WT (WT, 2.2-kb fragment) and Fen1null mutant mouse (1.7-kb fragment).
Generation of Fen1null/Apc1638N Animals.
Mice heterozygous for the Fen1null allele were mated with Apc1638N animals that spontaneously develop intestinal and colonic polyps and colon cancer. The Apc1638N allele was in the C57/BL6 background. The Fen1null mice were of a mixed genetic background estimated to be 60% C57/BL6, 37.5% 129/SV, and 2.5% SJL/J. All animals were maintained in an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility under barrier conditions.
Analysis of Tumors.
After mice were killed, the GI tract was removed, opened longitudinally, and fixed in 10% neutral-buffered formalin. The gross specimens were examined under a dissecting microscope for tumors, and the number and location of tumors were recorded. Representative tumors were chosen for histological and molecular analysis. A histological diagnosis was made on all tissue sections after hematoxylin and eosin staining. Early invasive adenocarcinomas were those that had invaded into the submucosa, and invasive adenocarcinomas were those that had invaded into the muscularis and beyond. Microadenomas were counted in five serial sections of flat mucosa adjacent to tumors. Statistical analyses were performed by using the Fisher exact probability and χ2 tests for analysis of tumor incidence and the Mann–Whitney test and binomial exact calculation for tumor multiplicity.
MSI Analysis.
DNA was extracted from tumor tissue and used for PCR amplification (49) under limiting dilution conditions. Approximately 25–30 reactions were performed on each tumor DNA sample after dilution to 10−5. The D7Mit91 dinucleotide repeat locus (left: TCTTGCTTGCATACACTCACG and right: GAGACAAACCGCAGTCTCCT) was amplified by using end-labeled left primer. Amplified products were separated on a denaturing polyacrylamide gel and autoradiographed for analysis. Instability was judged by determining the proportion of PCR reactions that contained a microsatellite allele that differed from the WT allele.
Sequencing of Remaining Fen1 Allele in Tumors.
Primer pairs were constructed that spanned the Fen1 gene and the four mouse/human homology blocks implicated in transcriptional control (50). PCR products were sequenced.
Analysis of Apc Truncation Mutations.
Codons 677-1674 of the mouse Apc gene were analyzed for protein truncating mutations by PCR and in vitro transcription and translation (IVTT) (51–52). PCR amplification of the WT Apc allele was performed in two stages to eliminate coamplification of the inactivated Apc1638N allele. Ten nanograms of genomic DNA was amplified in 10-μl reactions containing Pfu (Pyrococcus furiosus) DNA polymerase reaction buffer [20 mM Tris⋅HCl (pH 8.8)/2 mM MgSO4/10 mM KCl/10 mM (NH4)2SO4/0.1% Triton X-100/0.1 mg/ml of nuclease-free BSA] and 0.05 units/μl of Pfu turbo (Stratagene). Cycling conditions were one cycle of 94°C for 5 min, followed by 20 cycles of 94°C for 1 min, 60°C for 1 min, and 72°C for 5 min, with one final extension cycle at 72°C for 5 min. This procedure resulted in amplification products of ≈3.2 kb. Two overlapping segments of the Apc gene covering codons 677-1223 and 1100–1674 were subsequently amplified from aliquots of the first reactions using two pairs of PCR primers specific for IVTT. For the amplification of 677-1223 fragment, forward primer 5′-CGGGATCCTAATACGACTCACTATAGGGAGACCACCATGGATGCATGTGGAACTTTGTGG and reverse primer: 5′-CGAACAGCTAGCATTAGATGGAGGTACAGC were used. For amplification of the 1100–1674 fragment, forward primer: 5′-GCGGATCCTAATACGACTCACTATAGGGAGACCACCATGGGTATGATGATGT- ATAGGTCAAGGGGAACCAGT and reverse primer: 5′-CAACTTGCTAGCTCTGACCCCATCTCCAG were used. PCR was performed in 20-μl reactions containing 1-μl aliquots of the first-stage reactions. Cycling conditions for both segments were as above, except that 25 cycles of PCR were performed and the annealing temperature was 57°C. One microliter of the resulting PCR products was directly used as template in 6-μl IVTT reactions (TNT T7 Quick for PCR DNA, Promega) containing 2.5 μCi of [35S]-methionine (Amersham Pharmacia). The reactions were incubated at 30°C for 1 h. Aliquots of the IVTT reactions were then analyzed by 12% SDS/PAGE and fluorography. For further characterization of tumor-specific mutations, the PCR products were cloned into a vector, individual clones screened by IVTT to identify mutations, and their DNA sequence was determined.
Results
Homozygous Fen1null Animals Are Not Viable.
We generated mouse ES cells in which one copy of the Fen1 gene was replaced by a null allele in which the entire gene was completely deleted (Fig. 1A). Correct gene targeting events were identified by Southern hybridization (Fig. 1B) and PCR (Fig. 1C). We obtained eight gene-targeted cell lines. Four, designated 126, 111, 102, and 146, were used for further experiments. ES cells were injected into mouse blastocysts to generate chimeric mice. Chimeric mice from cell lines 126 and 111 transmitted the mutant allele (Fen1null) through their germ line. The resulting Fen1null heterozygotes were viable and fertile. They were intercrossed and their offspring genotyped by PCR. We examined 219 mice from 34 matings. Of these, 71 were wild type and 148 were Fen1null heterozygotes. Fen1null homozygotes were not detected. These results suggest that expression of Fen1 is necessary for normal embryogenesis, and lack of both functional copies of the Fen1 gene leads to embryonic lethality.
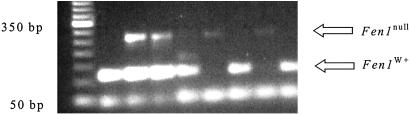
To determine whether the lack of gene expression leads to early lethality, we examined 37 blastocysts from two Fen1null intercrosses. Although we detected four blastocysts that were homozygous (Fen1 +/+: Fen1 −/+: Fen1 −/− = 23:10:4), such blastocysts were severely underrepresented (X2 = 26.9, P < 0.05) (Fig. 2.). We attempted to establish cell lines from 60 blastocysts obtained from several intercrosses; we were able to establish 14 (23%). Six of these were wild type and eight were heterozygotes, whereas none were homozygous for the Fen1null allele. These results suggest that Fen1 expression may be necessary for continued cell viability.
Figure 2.
Genotyping of embryonic day 3.5 blastocysts by PCR. Blastocysts were placed into distilled H2O, boiled for 10 min, and used directly as substrate for PCR reactions. A 260-bp fragment was amplified from the Neomycin gene in the Fen1null locus, and a 120-bp fragment amplified from the Fen1 WT locus.
Properties of Fen1null Heterozygotes.
Mice that are heterozygous for the Fen1null mutation are viable, fertile, and have a life span that is indistinguishable from their WT littermates. Among two small groups of mice from Fen1null heterozygotes, some mice became moribund. In one group of seven mice, there were a total of three heterozygotes and four WT animals; two of the three heterozygotes were found moribund at 11 mo of age, and one was found moribund at 5 mo of age. All three of these mice had thymuses that were four to five times smaller than those of their four WT littermates or healthy animals of comparable age. Histological examination revealed severe atrophy of the thymus, both in the medulla and cortex, with an overall reduction in the density and uniformity of the lymphocyte population. The overall observations favor an interpretation of premature thymus involution by a mechanism of accelerated loss of individual lymphocytes. A second cohort contained 36 mice, of which 12 were Fen1null heterozygotes. When they were examined at an average age of 12.3 mo, two of the heterozygotes had non-Hodgkins lymphoma of the B cell type, and one also had a Paneth cell hyperplasia of the small intestine.
Effect of Fen1 Haploinsufficiency in Apc1638NMice.
We then generated mice heterozygous for a mutation in the Apc gene (Apc1638N) (53) and heterozygous for Fen1null. Kaplan–Meier survival curves for the different groups of mice are shown in Fig. 3. Fen1null/+ mice have survival curves that are indistinguishable from their WT littermates. Apc1638N mice have a median survival of 13 mo. The addition of the Fen1null allele into these mice further reduces their median survival to 9 mo (P < 0.07).
Figure 3.
Kaplan–Meier survival plot of Fen1 +/+ Apc +/+; Fen1 +/− Apc +/+; Fen1 +/+ Apc +/−; Fen1 +/− Apc +/− mice. Time of death or when mice became moribund was recorded. The colors for the different genotypes and the number of mice are as follows: green, (106) Fen1 +/+ Apc +/+; red, (161) Fen1 +/− Apc +/+; dark blue, (33) Fen1 +/+ Apc +/−; light blue, (25) Fen1 +/− Apc +/−.
Mice with different genetic compositions were killed and examined at approximately 1 yr of age. The tumor incidence and types of tumors observed in the different types of mice are summarized in Table 1. The WT and Fen1null heterozygous mice had no tumors in the GI tract. Both Apc1638N and the double heterozygotes had a substantial tumor burden. Tumor multiplicity was slightly higher in Fen1null/Apc1638N animals than in Apc1638N mice (Table 2). The range of tumor multiplicity was considerably greater for Fen1null/Apc1638N mice than for Apc1638N mice alone. The range of tumors per animal in Apc1638N mice was 4–13 and in Fen1null/Apc1638N double heterozygotes, the range was 3–20. A definitive difference between the two strains of mice was obtained from the histological typing of tumors (Table 3; Fig. 5). The Fen1null/Apc1638N double heterozygous mice had a 9-fold greater incidence of malignant tumors (early invasive and invasive adenocarcinomas) compared with Apc1638N heterozygotes (P < 0.0053). Apc1638N animals had more benign tumors (tubular adenomas) (3.13 ± 1.89) than Fen1null/Apc1638N double heterozygotes (2.22 ± 1.48, P < 0.05). An increase in the number of adenomas in the large intestine was also observed in double heterozygous mice (0.56 ± 1.01) compared with Apc1638N (0.25 ± 0.71, P < 0.05, Table 2).
Table 1.
Tumor incidence of Fen1 mice
| Genotype (n) | Sex (M/F) | Age, mo | No., % of mice with tumors
|
||
|---|---|---|---|---|---|
| Overall | GI | Extra-GI | |||
| WT (7) | 1:6 | 12.6 ± 1.1 | 0 (0%) | 0 (0%) | 0 (0%) |
| Fen1null (12) | 1:2 | 12.3 ± 2.5 | 2 (17%) | 0 (0%) | 2 (17%) |
| Fen1null/Apc1638N (9) | 1:0.5 | 11.1 ± 1.7 | 9 (100%) | 9 (100%)* | 0 (0%) |
| Apc1638N (8) | 1:0.6 | 13.0 ± 1.9 | 8 (100%) | 8 (100%) | 0 (0%) |
n, number of mice studied. Statistical results from comparison with Fisher exact probability test: compared to Fen1null:
, P < 0.001. No significant difference found between Fen1null/Apc1638N double heterozygous and Apc1638N mice. Mean ± SD.
Table 2.
Multiplicity of GI tumors of Fen1null mice
| Overall | Stomach | Small intestine | Large intestine | |
|---|---|---|---|---|
| WT | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Fen1null | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Fen1null/Apc1638N | 8.44 ± 7.13* | 0.56 ± 1.13† | 7.32 ± 6.20* | 0.56 ± 1.01† |
| Apc1638N | 7.13 ± 2.85 | 1.88 ± 2.10 | 5.00 ± 1.85 | 0.25 ± 0.71 |
Statistical results were from the comparison with binomial exact calculation: compared to Fen1null:
, P < 0.001; †, P < 0.02. No significant difference was found between Fen1null/Apc1638N double heterozygous and Apc1638N mice with Mann-Whitney test. Mean ± SD.
Table 3.
Histologic types of tumors in Fen1null mice
| Apc1638N | Fen1null/Apc1638N | |
|---|---|---|
| Total no. of tumors found | 57 | 76 |
| No. (%) of tumors with histological examination | 36 (63%) | 48 (63%) |
| Histologic types of tumors: | ||
| Total | 36 (100%) | 48 (100%) |
| Malignant | 1 (2.8%) | 12 (25%) |
| Early invasive carcinoma | 0 (0%) | 2 (4.2%) |
| Adenocarcinoma | 1 (2.8%) | 10 (20.8%) |
| Benign | ||
| Villous-tubular adenoma | 4 (11.1%) | 7 (14.6%) |
| Tubular adenoma | 25 (69.4%) | 23 (47.9%) |
| Microadenoma | 6 (16.7%) | 6 (12.5%) |
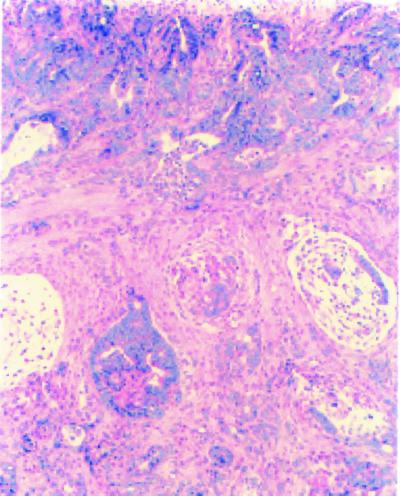
Figure 5.
Invasive adenocarcinoma of the small intestine of a Fen1nullApc1638N of a double mutant mouse. Tumor tissue was composed of neoplastic glands with irregular shape and size infiltrating the muscularis. Mucinous pools formed. Ulceration was shown on the surface of tumor. Desmoplastic response was observed near neoplastic glands in the invasive area. Hematoxylin/eosin ×100.
Tumors from Fen1null/Apc1638N Double Heterozygous Animals Have MSI.
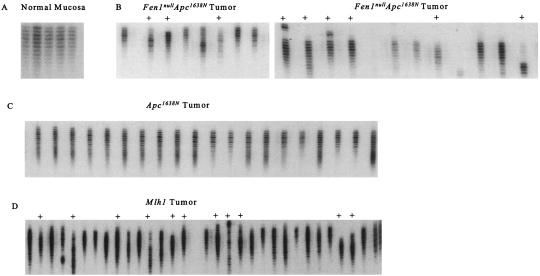
We determined whether the tumors from the double heterozygotes exhibited MSI by using limiting dilution PCR. DNA from the normal intestinal mucosa of two healthy C57/BL6 mice (0 of 60 PCR reactions positive), and three tumors from Apc1638N mice (7 of 91 PCR reactions positive) showed no instability (Fig. 4 A and C). DNA from Mlh1 tumors (42 of 62 PCR reactions positive) and all four tumors from Fen1null/Apc1638N heterozygotes (93 of 123 PCR reactions positive) had extensive MSI (Fig. 4 B and D). Normal liver tissue from an Apc1638N mouse that had adenomas showed no MSI (0 of 31 PCR reactions positive; data not shown). Normal liver tissue from a Fen1null Apc1638N mouse that had adenocarcinomas did not show MSI (0 of 30 PCR reactions).
Figure 4.
MSI in Fen1nullApc1638N tumor DNA. Primers for the D7Mit91 locus were used to amplify a 262-bp fragment under limiting dilution conditions (10−5). (A) Five PCR amplifications (normal intestine) show no MSI. (B) Twenty-one PCR amplifications from two Fen1null Apc1638N tumor DNAs show MSI as indicated. (C) Twenty PCR amplifications from the same Apc1638N tumor DNA show no MSI. (D) Thirty-one PCR amplifications from an Mlh1 tumor show MSI.
The Remaining Fen1 Allele in Tumors Is Intact.
We examined the status of the WT Fen1 allele in DNA from tumors derived from Apc1638N allele and Fen1null heterozygous mice. DNA from 15 Fen1null Apc1638N tumors and from 5 Apc1638N tumors was examined. The entire coding region of the Fen1 gene and an additional 820 bp in the 5′ untranslated region that contains four homology blocks conserved between mouse and human Fen1 genes were analyzed by DNA sequencing. In all cases, the Fen1 gene was intact and had no detectable mutations (data not shown).
Apc Mutations in Tumor DNA.
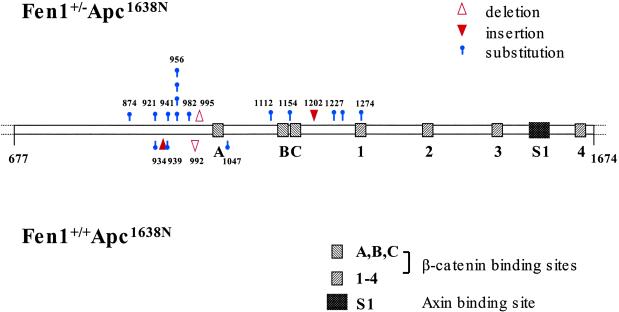
We then examined the fate of the WT Apc allele in tumors from the two groups of mice. A total of 41 tumors were analyzed from Fen1null/Apc1638N mice, and the results were compared with those obtained from the analysis of 15 tumors from Apc1638N mice (51). IVTT analysis indicated that 15 (37%) of the tumors from the double heterozygotes had Apc truncation mutations in the region encoding codons 876-1274, and the sequence alterations are shown in Table 4. In general, the Apc truncation mutations detected in both groups of tumors were point mutations. Insertion/deletion mutations were identified in 2 of 15 tumors from double heterozygous mice (Fig. 6). We also detected two insertion/deletions in tumors from Apc1638N animals.
Table 4.
Sequence of Apc mutations in Apc1638N and Fen1null/Apc1638N tumors
| Codon | Mutation | Consequence | WT sequence* | Apc1638N | Fen1null/Apc1638N |
|---|---|---|---|---|---|
| 874 | C-T | Arg-Stop | TCA AAA CGA GGT CTG | – | 1 |
| 921 | C-T | Arg-Stop | GCG GCA CGA AGA AGC | 1 | 1 |
| 934 | ins† TACA | Frameshift | AAC ACA TAC AAC TTC | 1 | – |
| 939 | G-T | Glu-Stop | AAG TCG GAA AAT TCA | 1§ | – |
| 941 | C-A | Ser-Stop | GAA AAT TCA AAT AGG | – | 1 |
| 956 | C-T | Arg-Stop | TAT AAA CGA TCT TCA | – | 4 |
| 982 | G-T | Glu-Stop | TCA GTT GAA TCC TAT | – | 1 |
| 992 | del‡ 8 bp + A | Frameshift | AAA TTTTGCAGTTAT GGT | 1§ | – |
| 995 | del‡ 10 bp | Frameshift | AGT TATGGTCAGTAT CCA | – | 1 |
| 1047 | G-A | Trp-Stop | GAA AGG TGG GCA AGA | 1§ | – |
| 1112 | C-T | Arg-Stop | ACA AAT CGA ATG GGT | – | 1 |
| 1154 | G-T | Glu-Stop | GAA GAA GAA GAA GAG | – | 1 |
| 1202 | ins† ATCA | Frameshift | AAT TCA TCA GCA CAA | – | 1 |
| 1227 | C-T | Gln-Stop | AAA AGG CAG AAT CAG | – | 1 |
| 1242 | C-T | Gln-Stop | CAG ACT CAA AAA GGC | – | 1 |
| 1274 | C-A | Ser-Stop | TGC AGT TCA TTA TCA | – | 1 |
| Total | 5 | 15 |
The WT sequence surrounding each mutation is shown and the site of mutation is shown in bold.
ins, insertion.
del, deletion.
Data from Kuraguchi et al. (51).
Figure 6.
Distribution of Apc mutations found in Fen1null Apc1638N tumor DNA and in tumor DNA from Apc1638N mice. Diagram of Apc between codons 677 and 1674 showing positions and characteristics of mutations detected in Fen1nullApc1638N (Upper) and Apc1638N (Lower) intestinal tumors. ▵, deletion; ▾, insertion;  , substitution. Each symbol represents an independent mutation. The three 15-aa (A–C) and four 20-aa (1–4) β-catenin-binding repeats and one SAMP (Ser, Ala, Met, Pro sequence) repeat (S1) in this segment of Apc are indicated.
, substitution. Each symbol represents an independent mutation. The three 15-aa (A–C) and four 20-aa (1–4) β-catenin-binding repeats and one SAMP (Ser, Ala, Met, Pro sequence) repeat (S1) in this segment of Apc are indicated.
Discussion
We generated mice that have a loss of function mutation in the Fen1 gene and examined the phenotype caused by this mutation. Mice lacking two copies of the Fen1 gene die early in embryogenesis, indicating that the Fen1 gene product is essential for development. The relative paucity of homozygous blastocysts, together with our failure to establish Fen1null homozygous ES cell lines, suggests that Fen1 expression might be necessary for cell survival or proliferation. The role of FEN1 in DNA replication and repair might explain its importance in cell survival. The Fen1 heterozygotes have a normal life span but do exhibit some phenotypes. Several of these mice showed features of premature thymus involution that may be the result of early T cell depletion. The proposed function of Fen1 in nonhomologous end joining (54) and in V(D)J recombination might explain the observed result. A proportion of Fen1 heterozygotes also had B cell lymphomas. The precise mechanism by which Fen1 hemizygosity leads to B cell lymphomas needs further investigation.
We have obtained strong evidence for a role of Fen1 in tumor progression. When the Fen1null mutation was combined with the Apc1638N mutation, the double heterozygous mice exhibited significant differences from those that are heterozygous for the Apc1638N mutation alone. The double heterozygotes had a lower median survival. Although the average tumor burden in both groups of mice is similar, there appears to be a significant increase in the rate of progression of tumors in the double mutant mice. The latter had nine times as many adenocarcinomas as those carrying the Apc1638N mutation alone. These results suggest that reduction of Fen1 does not alter the tumor initiation process but has a profound effect on tumor progression. None of the tumors we examined showed mutation or loss of the WT Fen1 allele. This observation is consistent with the view that complete absence of Fen1 function might lead to impaired proliferation. A clue to the mechanism by which Fen1 heterozygosity results in rapid tumor progression came from the observation that the tumors from the double heterozygotes exhibit MSI at the same level as seen in tumors from Mlh1 mutant mice; this suggests that a reduction in Fen1 expression results in impairment of DNA repair.
That Fen1 heterozygotes show some tumor susceptibility and that reduction in copy number is sufficient to result in advanced tumors in the Apc1638N background suggest that under some conditions, haploinsufficiency of the Fen1 gene causes a mutator phenotype in mammalian cells. A total of 41 tumors were analyzed from Fen1null/Apc1638N mice. Fifteen (37%) of these had Apc truncation mutations. Several of these mutations were novel. They include one 4-bp insertion and one 10-bp deletion. Because most of the truncation mutations detected in these tumors were similar to alterations previously identified in the remaining Apc allele in tumors of Apc1638N mice, and because Fen1 heterozygous mice do not have appreciable tumor burden, we suggest that Fen1 does not play a significant role in GI tumor initiation. The more advanced nature of tumors in the double heterozygotes suggests a greater role for Fen1 in tumor progression.
The maintenance of genomic stability is of prime importance to the cell, and it is not surprising that genes involved in replication and repair contribute to the process. We observed MSI in Fen1null/Apc1638N haploinsufficient tumors that was consistent with the increased accumulation of frameshift mutations observed in S. cerevisiae rad27 mutants. That the Fen1 WT allele is intact in these tumors suggests that the quantity of the product plays an important role in tumor progression. Eukaryotic replication initiates at 2 × 104 to 1 × 105 sites in the human haploid genome. The formation of competent replication complexes on the leading and lagging strands of DNA must depend on the concentration and availability of the individual components that make up the complexes. Haploinsufficiency of a component may not make a difference to a cell undergoing normal replication; however, if the cell cycle is perturbed by mutations in oncogenes or tumor suppressor genes (e.g., Apc), additional levels of at least some gene products might be necessary to accommodate the change in rates of cell division. If one or more critical product necessary for replication and repair is not there in sufficient quantity, the result may be detrimental to genomic stability. Replication or repair factors are involved in the maintenance of genomic stability, and results described here imply that a quantitative measure of the expression of some of these gene products may be useful as a prognostic indicator, particularly genes controlling cell cycle concomitantly with genes involved in replication and repair.
Acknowledgments
This work was supported by grants to R.K. (CA84301-04 and ES11040) and to R.D.K. (GM50006 and ES11040) from the National Institutes of Health, and by a fellowship to M.K. from the American Association for Cancer Research–Cancer Research Foundation of America.
Abbreviations
- FAP
familial adenomatous polyposis
- HNPCC
hereditary nonpolyposis colorectal cancer
- MMR
mismatch repair
- MSI
microsatellite instability
- GI
gastrointestinal
- ES
embryonic stem
- IVTT
in vitro transcription and translation
- WT
wild type
References
- 1.Yang K, Edelmann W, Fan K, Lau K, Kolli V, Fodde R, Khan P M, Kucherlapati R, Lipkin M. J Exp Zool. 1997;277:245–254. [PubMed] [Google Scholar]
- 2.Bussey H J R. Familial Polyposis Coli. Baltimore: Johns Hopkins Univ. Press; 1975. [Google Scholar]
- 3.Bodmer W F, Bailey C J, Bodmer J, Bussey H J R, Ellis A, Gorman P, Lucibello F C, Murday V A, Rider S H, Scambler P, et al. Nature (London) 1987;328:614–616. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- 4.Leppert M, Dobbs M, Scambler P, O'Connell P, Nakamura Y, Stauffer D, Woodward S, Burt R, Hughes J, Gardner E, et al. Science. 1987;238:1411–1413. doi: 10.1126/science.3479843. [DOI] [PubMed] [Google Scholar]
- 5.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spiro L, Robertson M, et al. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 6.Nishisho I, Nakamura Y, Miyoshi Y, Miki Y, Ando H, Horii A, Koyama K, Utsunomiya J, Baba S, Hedge P, et al. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 7.Kinzler K W, Nilbert M C, Su L, Vogelstein B, Bryan T M, Levy D B, Smith K J, Preisinger A C, Hedge P, McKechnie D, et al. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 8.Kuismanen S A, Holmberg M T, Salovaara R, de la Chapelle A, Peltomaki P. Am J Pathol. 2000;156:1773–1779. doi: 10.1016/S0002-9440(10)65048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peltomaki P, Vasen H F. Gastroenterology. 1997;113:1146–1158. doi: 10.1053/gast.1997.v113.pm9322509. [DOI] [PubMed] [Google Scholar]
- 10.Peltomaki P. Hum Mol Genet. 2001a;10:735–740. doi: 10.1093/hmg/10.7.735. [DOI] [PubMed] [Google Scholar]
- 11.Peltomaki P. Mutat Res. 2001b;488:77–85. doi: 10.1016/s1383-5742(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler J M, Loukola A, Aaltonen L A, Mortensen N J, Bodmer W F. J Med Genet. 2000;37:588–592. doi: 10.1136/jmg.37.8.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan H, Papadopoulos N, Marra G, Perrera C, Jiricny J, Boland C R, Lynch H T, Chadwick R B, de la Chapelle A, Berg K, et al. Nature (London) 2000;403:723–724. doi: 10.1038/35001659. [DOI] [PubMed] [Google Scholar]
- 14.Wijnen J, van der Klift H, Vasen H, Khan P M, Menko F, Tops C, Meijers Heijboer H, Lindhout D, Moller P, Fodde R. Nat Genet. 1998;20:326–328. doi: 10.1038/3795. [DOI] [PubMed] [Google Scholar]
- 15.Charbonnier F, Olschwang S, Wang Q, Boisson C, Martin C, Buisine M P, Puisieux A, Frebourg T. Cancer Res. 2002;62:848–853. [PubMed] [Google Scholar]
- 16.Thibodeau S N, French A J, Roche P C, Cunningham J M, Tester D J, Lindor N M, Moslein G, Baker S M, Liskay R M, Burgart L J, et al. Cancer Res. 1996;56:4836–4840. [PubMed] [Google Scholar]
- 17.Liu T, Yan H, Kuismanen S, Percesepe A, Bisgaard M L, Pedroni M, Benatti P, Kinzler K W, Vogelstein B, Ponz de Leon M, et al. Cancer Res. 2001;61:7798–7802. [PubMed] [Google Scholar]
- 18.Kolodner R D, Tytell J D, Schmeits J, Kane M F, Das Gupta R, Weger J, Wahlberg S, Fox E A, Peel D J, Ziogas A, et al. Cancer Res. 1999;59:5068–5074. [PubMed] [Google Scholar]
- 19.Akiyama Y, Sato H, Yamada T, Nagasaki H, Tsuchiya A, Abe R, Yuasa Y. Cancer Res. 1997;57:3920–3923. [PubMed] [Google Scholar]
- 20.Miyaki M, Konishi M, Tanaka K, Kituchi-Yanoshita R, Muraoka M, Igari T, Koike M, Chiba M, Mori T. Nat Genet. 1997;17:271–272. doi: 10.1038/ng1197-271. [DOI] [PubMed] [Google Scholar]
- 21.Wijnen J, de Leeuw W, Vasen H, van der Klift H, Moller P, Stormorken A, Meijers-Heijboer H, Lindhout D, Menko F, Vossen S, et al. Nat Genet. 1999;23:142–144. doi: 10.1038/13773. [DOI] [PubMed] [Google Scholar]
- 22.Aaltonen L A, Peltomaki P, Mecklin J P, Jarvinen H, Jass J R, Green J S, Lynch H T, Watson P, Tallqvist G, Juhola M, et al. Cancer Res. 1994;54:1645–1648. [PubMed] [Google Scholar]
- 23.Ionov Y, Peinado M A, Malkhosyan S, Shibata D, Perucho M. Nature (London) 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 24.Dietmaier W, Wallinger S, Bocker T, Kullmann F, Fishel R, Ruschoff J. Cancer Res. 1997;57:4749–4756. [PubMed] [Google Scholar]
- 25.Boland C, Thibodeau S, Hamilton S R, Sidransky D, Eshleman J, Burt R, Meltzer S, Rodriguez-Bigas M, Fodde R, Ranzani N, Srivastava S. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 26.Terdiman J P, Gum J R, Jr, Conrad P G, Miller G A, Weinberg V, Crawley S C, Levin T R, Reeves C, Schmitt A, Hepburn M, et al. Gastroenterology. 2001;120:21–30. doi: 10.1053/gast.2001.20874. [DOI] [PubMed] [Google Scholar]
- 27.Park J G, Vasen H F, Park K J, Peltomaki P, Ponz de Leon M, Rodriguez-Bigas M A, Lubinski J, Beck N E, Bisgaard M L, Miyaki M, et al. Dis Colon Rectum. 1999;42:710–715. doi: 10.1007/BF02236922. [DOI] [PubMed] [Google Scholar]
- 28.Wijnen J, Khan P M, Vasen H, van der Klift H, Mulder A, van Leeuwen-Cornelisse I, Bakker B, Losekoot M, Moller P, Fodde R. Am J Hum Genet. 1997;61:329–335. doi: 10.1086/514847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Berends M J, Mensink R G, Kempinga C, Sijmons R H, van Der Zee A G, Hollema H, Kleibeuker J H, Buys C H, Hofstra R M. Am J Hum Genet. 1999;65:1291–1298. doi: 10.1086/302612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farrington S M, Lin-Goerke J, Ling J, Wang Y, Burczak J D, Robbins D J, Dunlop M G. Am J Hum Genet. 1998;63:749–759. doi: 10.1086/301996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eshleman J R, Markowitz S D. Curr Opin Oncol. 1995;7:83–89. [PubMed] [Google Scholar]
- 32.Harrington J J, Lieber M R. EMBO J. 1994;13:1235–1246. doi: 10.1002/j.1460-2075.1994.tb06373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrington J J, Lieber M R. J Biol Chem. 1995;270:4503–4508. doi: 10.1074/jbc.270.9.4503. [DOI] [PubMed] [Google Scholar]
- 34.Lindahl T. Eur J Biochem. 1971;18:407–414. doi: 10.1111/j.1432-1033.1971.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 35.Guggenheimer R A, Nagata K, Kenny M, Hurwitz J. J Biol Chem. 1984;259:7815–7825. [PubMed] [Google Scholar]
- 36.Siegal G, Turchi J J, Myers T W, Bamabara R A. Proc Natl Acad Sci USA. 1992;89:9377–9381. doi: 10.1073/pnas.89.20.9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim K, Biade S, Matsumoto Y. J Biol Chem. 1998;273:8842–8848. doi: 10.1074/jbc.273.15.8842. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Li J, Harrington J, Lieber M R, Burgers P. J Biol Chem. 1995;270:22109–22112. doi: 10.1074/jbc.270.38.22109. [DOI] [PubMed] [Google Scholar]
- 39.Wu X, Li J, Hsieh C L, Burgers P, Lieber M R. Nucleic Acids Res. 1996;24:2036–2043. doi: 10.1093/nar/24.11.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reagan M S, Pittenberger C, Siede W, Friedberg E C. J Bacteriol. 1995;177:364–371. doi: 10.1128/jb.177.2.364-371.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson R E, Kovvali G, Prakash L, Prakash S. Sci USA. 1995;269:238–240. doi: 10.1126/science.7618086. [DOI] [PubMed] [Google Scholar]
- 42.Tishkoff D X, Filosi N, Gaida M, Kolodner R D. Cell. 1997;88:253–263. doi: 10.1016/s0092-8674(00)81846-2. [DOI] [PubMed] [Google Scholar]
- 43.Myung K, Chen C, Kolodner R D. Nature (London) 2001;411:1073–1076. doi: 10.1038/35082608. [DOI] [PubMed] [Google Scholar]
- 44.Kolodner R D, Marsischky G T. Curr Opin Genet Dev. 1999;9:89–96. doi: 10.1016/s0959-437x(99)80013-6. [DOI] [PubMed] [Google Scholar]
- 45.Moreau S, Morgan E A, Symington L S. Genetics. 2001;159:1423–1433. doi: 10.1093/genetics/159.4.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vallen E A, Cross F R. Mol Cell Biol. 1995;15:4291–4302. doi: 10.1128/mcb.15.8.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kokoska R J, Stefanovic L, Tran H T, Resnick M A, Gordenin D A, Petes T D. Mol Cell Biol. 1998;18:2779–2788. doi: 10.1128/mcb.18.5.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin Y H, Obert R, Burgers P M, Kunkel T A, Resnick M A, Gordenin D A. Proc Natl Acad Sci USA. 2001;98:5122–5127. doi: 10.1073/pnas.091095198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edelmann W, Umar A, Yang K, Heyer J, Kucherlapati M, Lia M, Kneitz B, Avdievich E, Fan K, Wong E, et al. Cancer Res. 2000;60:803–807. [PubMed] [Google Scholar]
- 50.Karanjawala Z, Shi X, Hsieh C-L, Lieber M. Microbial Comp Genom. 2000;5:173–177. doi: 10.1089/omi.1.2000.5.173. [DOI] [PubMed] [Google Scholar]
- 51.Kuraguchi M, Edelmann W, Yang K, Lipkin M, Kucherlapati R, Brown A M C. Oncogene. 2000;19:5755–5763. doi: 10.1038/sj.onc.1203962. [DOI] [PubMed] [Google Scholar]
- 52.Kuraguchi M, Yang K, Wong E, Avdievich E, Fan K, Kolodner R D, Lipkin M, Brown A M C, Kucherlapati R, Edelmann W. Cancer Res. 2001;61:7934–7942. [PubMed] [Google Scholar]
- 53.Fodde R, Edelmann W, Yang K, van Leeuwen C, Carlson C, Renault B, Breukel C, Alt F, Lipkin M, Khan P M, Kucherlapati R. Proc Natl Acad Sci USA. 1994;91:8969–8973. doi: 10.1073/pnas.91.19.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu X, Wilson T E, Lieber M R. Proc Natl Acad Sci USA. 1999;96:1303–1308. doi: 10.1073/pnas.96.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]