Abstract
Inactivation of Hsp104 by guanidine is contended to be the mechanism by which guanidine cures yeast prions. We now find an Hsp104 mutation (D184N) that confers resistance to guanidine-curing of the yeast [PSI+] prion. In an independent screen we isolated an HSP104 allele altered in the same residue (D184Y) that dramatically impairs [PSI+] propagation in a temperature-dependent manner. Directed mutagenesis of HSP104 produced additional alleles that conferred varying degrees of resistance to guanidine-curing or impaired [PSI+] propagation. The mutations similarly affected propagation of the [URE3] prion. Basal and induced abundance of all mutant proteins was normal. Thermotolerance of cells expressing mutant proteins was variably resistant to guanidine, and the degree of thermotolerance did not correlate with [PSI+] stability. We thus show that guanidine cures yeast prions by inactivating Hsp104 and identify a highly conserved Hsp104 residue that is critical for yeast prion propagation. Our data suggest that Hsp104 activity can be reduced substantially without affecting [PSI+] stability, and that Hsp104 interacts differently with prion aggregates than with aggregates of thermally denatured protein.
Saccharomyces cerevisiae Hsp104 is a protein chaperone that is not required for growth at any temperature but protects cells from exposure to lethal temperature. Induced expression of Hsp104 and other heat-shock proteins by exposure to mild stress aids recovery of cells subsequently exposed to lethal levels of heat, a response termed acquired thermotolerance. Hsp104 cannot prevent aggregation of denatured proteins but acts by resolubilizing proteins that have become aggregated (1, 2). This function, which is not provided by other cytosolic proteins, is essential for acquired thermotolerance in yeast (3, 4). Hsp104 expression is induced by a variety of environmental stresses and when cells enter stationary phase or sporulate (3, 5).
A member of the conserved HSP100/Clp family of proteins, Hsp104, has two ATPase domains and forms ring-shaped hexamers in the presence of ATP (6–8). Early studies indicated that oligomerization mainly depends on the C-terminal ATPase domain [nucleotide-binding domain (NBD)2], whereas the N-terminal domain (NBD1) is responsible for most of Hsp104's ATPase activity (7, 9). More recently it was shown that in the hexamer, NBD1 and NBD2 differ in their affinity for ATP and in their ATPase activity (10). Also, ATP hydrolysis by NBD2 increases ATPase activity of NBD1, revealing communication between the two domains. The mechanism underlying the protein remodeling activity of Hsp104 likely involves ATP-driven conformational alterations of the hexamer, but the details of these changes and the interaction of Hsp104 with substrates remain to be elucidated.
Yeast prions are proteins that misfold and form self-replicating aggregates thought to be amyloid in nature (11–14). They propagate by recruiting the soluble form of the protein into the aggregates, which are transmitted between cells during cell division or fusion. Several yeast proteins can behave as prions, and all that have been tested require Hsp104 for their propagation (15–19). The requirement of Hsp104 for yeast prion propagation likely is mediated by its ability to modify protein conformation. Prion replication requires conversion of the normal form of the protein into the altered form and also the generation of new inheritable prion units (or “seeds”) that can act independently in prion propagation. Two nonexclusive mechanisms by which Hsp104 may act in prion replication are by facilitating structural changes in proteins that promote prion propagation (12, 20) or disrupting prion aggregates into smaller, more numerous particles that act as seeds (21).
The yeast [PSI+] element is a prion of Sup35p (eRF3), a component of the eukaryotic release factor that acts in translation termination (11, 22, 23). [URE3] is a prion of Ure2p, a regulator of nitrogen metabolism, and [PIN+] is a prion of Rnq1p, a nonessential protein of unknown function (11, 24). Although [URE3] and [PIN+] are insensitive to elevated levels of Hsp104, [PSI+] is lost if Hsp104 is transiently overexpressed (15, 16, 19). This disparity was seen also with artificial hybrid prion proteins and was proposed to be caused by distinct interactions of prions with Hsp104 that arise from differences in the prions' structural and dynamic properties (17).
Growing yeast in the presence of 3–5 mM guanidine was found to cause loss of [PSI+] efficiently (25). Guanidine has been shown since to cure yeast of all prions tested (11, 17, 18, 26–28) and to act by blocking replication of inheritable prion seeds, of which there are ≈60 in a typical [PSI+] cell (29, 30). After the addition of guanidine, the nonreplicating seeds are distributed randomly among dividing cells and after 4–5 cell divisions are diluted to the point at which [psi−] cells begin to appear (30). Hsp104 activity in vivo was shown to be inhibited strongly by millimolar amounts of guanidine, leading to the proposal that this inhibition is the mechanism by which guanidine cures yeast prions and implicating Hsp104 in [PSI+] prion seed regeneration (31, 32). However, it was suggested also that inhibition of Hsp104 by guanidine is not sufficient to explain its prion-curative effect (33).
To confirm Hsp104 as the target of guanidine we mutagenized HSP104 and isolated a mutant that conferred resistance to the prion-curing effects of guanidine. Remarkably, in an independent screen to identify chromosomal mutations that adversely affected [PSI+], we isolated a mutant with a different alteration of the same codon (D184). Interestingly, although this mutation considerably impaired propagation of both [PSI+] and [URE3], it had only a modest effect on the ability of Hsp104 to provide thermotolerance. Additional mutations of HSP104 at this site variously affected prion propagation and guanidine-curing, highlighting its importance in both processes.
Methods
Yeast Strains and Growth Conditions.
Strains were derived from 628-3A (MATα kar1-1 SUQ5 ade2-1 leu2Δ1 trp1Δ63 ura3-52 [PSI+]; ref. 34). Strain J104x has the coding region of HSP104 replaced by KanMX (31). Strains with J184 designations have replaced HSP104 alleles and are named for the codon at position 184 (e.g., J184D is wild type, J184Y has the hsp104D184Y allele, etc.). HSP104 gene replacements were made by transformation selecting for uracil prototrophy using alleles with a hisG-URA3-hisG cassette (35) in the 3′ noncoding region (see below). Growth on 5-fluoroorotic acid was used to select ura3 strains (36). HSP104 genes in the resulting strains were sequenced completely to verify the single codon alteration.
Yeast methods were as described (37). YPD medium contains 0.5% yeast extract, 2% peptone, and 2% dextrose. YPAD is similar but contains 1% yeast extract and 400 mg/liter adenine. Media with designations ending in/G3 contain 3 mM guanidine-hydrochloride. Solid media contain 2% agar. Unless indicated otherwise, cells were grown at 30°C.
[PSI+] causes nonsense suppression by depleting the cytosol of soluble Sup35p. The ade2-1 nonsense allele is suppressible only when [PSI+] is present (38). Nonsuppressed ade2-1 mutants are Ade− and red when grown on limiting amounts of adenine. Suppression of ade2-1 by [PSI+] allows growth without adenine and eliminates the pigmentation. The presence of [PSI+] was scored as white or pink colony color on YPD, growth without adenine at 25°C, or both. The degree of red coloration is a qualitative assay for [PSI+]-mediated nonsense suppression and can be used to identify conditions that adversely affect [PSI+] propagation (34). Curing of [PSI+] was done by growing cells in the presence of 3 mM guanidine-hydrochloride, which causes maximal curing of [PSI+] with the least growth inhibition.
The presence of [URE3] depletes the cytosol of functional Ure2p, which allows growth of ura2 mutants on ureidosuccinate in place of uracil (11, 39). Strain 819 is isogenic to 628-3A but has alleles MATa, URA3, lys2, and ura2∷KanMX. J184 strains were crossed with strain 819, and URA3 meiotic segregants with the replaced HSP104 alleles and ura2∷KanMX were selected. These strains have the additional “U” designation (JU-184D, etc.). [URE3] was transferred to these strains from strain 403-3A (40) by cytoduction (41).
Plasmids.
Plasmids pJ309 and pJ311 are pRS315 (42) with HSP104 PCR-amplified from strains 628-3A (wild type) and C39 (D184Y, see below), respectively. Plasmid pJ312 is pRS316 (42) with HSP104 from pJ309. Plasmid pJ316 was made by PCR-amplifying the hisG-URA3-hisG cassette from plasmid pNKY51 (ATCC) with primers with 5′-AatII restriction sites and ligating the product to the AatII site 190 bp 3′ to the HSP104 translation terminator in plasmid pJ309. The 6.9-kbp EcoRI–BamHI fragment from pJ316 and mutagenized derivatives was used for HSP104 gene replacements.
Mutagenesis.
Site-directed mutagenesis of HSP104 codon 184 was done on plasmid pJ316 by using the QuickChange kit from Stratagene. The primers used extended from nucleotides 532 to 567 and contained one or more base substitutions at nucleotides 550, 551, and 552 (nucleotide 1 being A of the initiator ATG).
Random mutagenesis of HSP104 was done by hydroxylamine treatment of pJ309 (43). After transforming [PSI+] strain J104x carrying plasmid pJ312 with mutagenized pJ309, transformants lacking pJ312 were obtained by growth on 5-fluoroorotic acid. These transformants were grown on YPD/G3 and replica-plated onto −ade. Plasmids from two transformants that grew on the −ade plates were recovered, and their HSP104 genes with flanking DNA were sequenced. Both had a single mutation, a G-to-A transition of HSP104 nucleotide 550, causing an aspartate-to-asparagine change at codon 184 (D184N).
Strain 628-3A was mutagenized previously with ethyl methanesulfonic acid to 60% viability (34). In the current screen of ≈20,000 colonies, 170 were isolated as appearing redder, which reflects reduced nonsense suppression. Seventeen of these colonies were verified as [PSI+]. A weakened [PSI+] phenotype segregated as a single genetic locus for clones C39, C53, B3, and B13. Clones C53 and B3 were identical to the previously described SSA1-21 (34), and HSP104 was identified on a plasmid from a genomic library that partially complemented the C39 phenotype. HSP104 subsequently was found to be linked tightly to the mutation, and subcloning confirmed that a mutant allele of HSP104 caused the effect on [PSI+]. Sequencing of the HSP104 coding region and 500 bp of flanking DNA identified a single mutation, a G-to-T transversion of nucleotide 550, causing an aspartate-to-tyrosine change of codon 184 (D184Y). The identical alteration was found for B13.
Thermotolerance.
Assays were done by using [psi−] cells as described (31) and indicated. Acquired thermotolerance primarily depends on Hsp104 and can be almost eliminated by adding guanidine to cell cultures at a final concentration of 5 mM immediately before exposure to the lethal heat shock. Because Hsp104 does not prevent protein aggregation but acts during recovery from stress, essentially identical results are obtained when cells are similarly heat-shocked without guanidine and recover on plates with 3 mM guanidine. Because prolonged growth on guanidine induces a high frequency of mitochondrial petites in our strains, the former method was used.
Results
Isolation of HSP104 Alleles That Confer Resistance to Guanidine-Curing of [PSI+] or Impair [PSI+] Propagation.
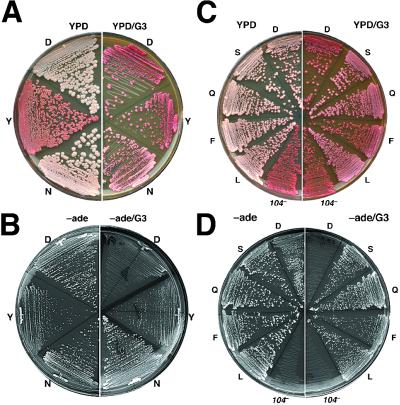
We monitor [PSI+] by its ability to suppress the ade2-1 nonsense allele (see Methods). Although [psi−] cells are red on YPD medium and Ade−, [PSI+] cells are white and Ade+. When [PSI+] is in a “weakened” state, cells are pink and display intermediate growth without adenine. Guanidine (3 mM) in growth medium blocks [PSI+] replication. When [PSI+] cells are grown on YPD that contains 3 mM guanidine (YPD/G3), a loss of [PSI+] shortly after cells begin to divide results in colonies that are red and almost entirely [psi−].
We mutagenized HSP104 expecting to identify alleles that would confer resistance to guanidine-curing of [PSI+]. Mutagenized plasmids were used to replace a plasmid with wild-type HSP104 in a [PSI+] strain lacking chromosomal HSP104. Among 5,000 transformants, two were less red on YPD/G3 plates. Most cells from these two colonies were found to be [PSI+] when transferred to medium without guanidine. The guanidine resistance conferred by both plasmids was caused by the same point mutation, an aspartate-to-asparagine change at residue 184 (D184N, see Methods).
Guanidine resistance was quantified initially by suspending three 1-mm-sized colonies in water and spreading ≈400 cells of each colony onto medium without guanidine. No wild-type (J184D) cells were [PSI+] (<0.3%), indicating that [PSI+] had been cured efficiently, but 83% of cells of J184N, which has HSP104 replaced by the hsp104D184N allele, remained [PSI+]. Thus, a point mutation in HSP104 conferred significant resistance to guanidine-curing of [PSI+].
From a separate screen of mutagenized cells that was done to identify mutations that adversely affected [PSI+], two clones were isolated (see Methods). An aspartate-to-tyrosine change at residue 184 (D184Y) of Hsp104 caused the [PSI+] propagation defect in both strains. The altered [PSI+] phenotype (Fig. 1A) segregated 2:2 among meiotic segregants of diploids heterozygous for the D184Y mutation. Also, after cytoplasmic transfer (cytoduction) of [PSI+] from strain J184Y, which has HSP104 replaced by the hsp104D184Y allele, to an isogenic wild-type strain, the recipient cells had a normal [PSI+] phenotype (data not shown). Together these results indicate that the D184Y mutation impaired propagation of a typical form of [PSI+] rather than creating a heritable conformational variant (or “strain”) of [PSI+] with reduced ability to propagate.
Figure 1.
[PSI+] phenotype of HSP104 mutant strains. Cells streaked onto YPD and YPD/G3 were grown for 2 days at 30°C and then 3 days at 25°C. The same cells streaked onto −ade and −ade/G3 were incubated at 25°C for 4 and 5 days, respectively. (A and B) J184D (D, wild type), J184Y (Y), and J184N (N) strains. (C and D) Additional HSP104 mutants designated by the single-letter code for their amino acid at position 184 in Hsp104. The isogenic hsp104 null strain J104x is shown as 104−.
Because of the antiprion effect of guanidine, growth of [PSI+] cells is inhibited severely on medium lacking adenine that contains 3 mM guanidine (−ade/G3). As expected because of the resistance of [PSI+] to guanidine-curing, J184N grew much faster than J184D on −ade/G3 (Fig. 1B). Surprisingly, although the reduced [PSI+] propagation of J184Y caused it to grow more slowly than J184D on −ade, they grew better than J184D on −ade/G3. Thus, despite its adverse effect on [PSI+], the D184Y substitution conferred resistance to guanidine inhibition of prion propagation. In agreement with this result, when J184Y colonies from YPD/G3 were assayed for curability as described above, 2% of cells (more than 6-fold over J184D) remained [PSI+]. Together these results show that amino acid residue 184 of Hsp104 is important for [PSI+] propagation and sensitivity of Hsp104 to inactivation by guanidine.
Additional HSP104 Alleles.
To test the effects of additional substitutions of codon D184 on [PSI+] phenotype and guanidine sensitivity we made mutant strains that differed only at codon 184 in HSP104 (see Methods). Isogenic strain J104x, which lacks HSP104, was used as a negative control. The effects of the HSP104 mutations, some of which are described in detail below, are summarized in Table 1. For all strains that propagated [PSI+], cytoductions were done to verify that any altered effects were caused by the HSP104 mutation and not by the generation of an atypical form of [PSI+]. Strains with mutations that abolished the [PSI+] phenotype were used as both recipients and donors in reciprocal cytoduction crosses to confirm that [PSI+] was unable to propagate rather than its presence masked by an unknown mechanism.
Table 1.
Relative effects of D184 substitutions on [PSI+] and Hsp104 activity
| Residue 184 | [PSI+] phenotype
|
Hsp104 activity
|
||||
|---|---|---|---|---|---|---|
| −ade growth
|
Stability
|
|||||
| G0 | G3 | G0 | G3 | G0 | G5 | |
| D (Asp) | 5 | 0 | 5 | 0 | 5 | 0 |
| S (Ser) | 5 | 4 | 5 | 5 | 5 | 4 |
| A (Ala) | 5 | 4 | 5 | 5 | ND | ND |
| Q (Gln) | 5 | 4 | 5 | 4 | 3 | 2 |
| L (Leu) | 5 | 4 | 5 | 3 | ND | ND |
| N (Asn) | 5 | 3 | 5 | 3 | 3 | 2 |
| F (Phe) | 5 | 3 | 5 | 2 | ND | ND |
| Y (Tyr) | 2 | 2 | 2 | 0 | 4 | 2 |
| W (Trp) | 0 | 0 | 0 | 0 | 2 | 0 |
| K (Lys) | 0 | 0 | 0 | 0 | 0 | 0 |
| Null | 0 | 0 | 0 | 0 | 0 | 0 |
G0, G3, and G5 indicate that assay conditions included 0, 3, or 5 mM guanidine, respectively. Scores range from that of wild type (D) without guanidine (5 = fastest −ade growth and completely stable) to that of the hsp104 null mutant (0 = Ade− and [psi−]). ND, not determined.
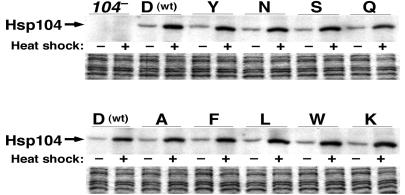
Acquired thermotolerance was used as a measure of Hsp104 activity and the sensitivity of Hsp104 to inactivation by guanidine (see Methods). Basal and heat-induced abundance of Hsp104 in the mutant strains was similar to that of wild-type cells (Fig. 2), ruling out altered expression of the mutant proteins as causing any effects. We showed previously that Hsp104 abundance is elevated when cells are grown in the presence of guanidine, indicating that guanidine does not cause Hsp104 degradation (31).
Figure 2.
Abundance of Hsp104 mutant proteins is normal. Half of the cultures of log-phase [psi−] cells grown at 27°C in YPAD (−heat shock) were shifted to 39°C for 30 min (+heat shock). Cells were collected, and Western analysis was done by using anti-Hsp104. Strains are designated as described for Fig. 1. (Lower) Coomassie-stained loading controls.
All the mutations were weakly dominant. Hsp104 acts as a hexamer, and thus this phenotype is likely caused by interactions between wild-type and mutant monomers or to the presence of dominantly active mutant oligomers. Either way, the dominance suggests that the mutations did not eliminate the ability of Hsp104 to oligomerize.
HSP104 Mutations Conferring Resistance to Guanidine-Curing of [PSI+].
The color of colonies on YPD/G3 plates correlated with resistance to curing of [PSI+]. Strains J184S, J184A, and J184Q accumulated the least pigment (Fig. 1C and data not shown). When these strains were assayed for resistance to guanidine-curing as described above for J184N, 100% of the cells were found to be [PSI+]. When assayed similarly, 77 and 28% of cells from colonies of strains J184L and J184F, respectively, were [PSI+]. Additionally, all HSP104 mutant strains capable of propagating [PSI+] were able to grow better than wild-type cells on −ade/G3 (Fig. 1D and data not shown). Thus, several substitutions of the same amino acid in Hsp104 confer resistance to the antiprion effect of guanidine.
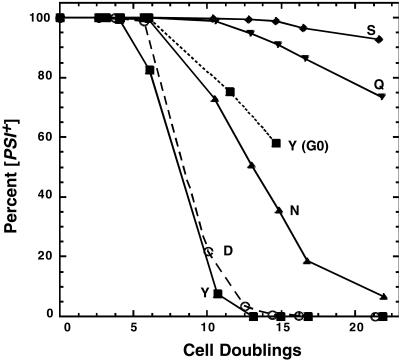
For some mutants we analyzed resistance of [PSI+]-curing more precisely by monitoring the appearance of [psi−] cells in liquid cultures after adding guanidine (Fig. 3). In wild-type cultures, [psi−] cells began to appear after 4–5 cell divisions, and after 15 generations less than 1% of the population remained [PSI+]. In contrast, more than 99% of J184S cells remained [PSI+] after 15 generations. Thus, a point mutation in HSP104 conferred essentially complete resistance to curing of [PSI+] by guanidine.
Figure 3.
Rates of curing of [PSI+] after exposure to guanidine. [PSI+] cells from −ade plates at 25°C were transferred to liquid YPAD with 3 mM guanidine at 30°C. The proportion of [PSI+] cells remaining in growing cultures then was monitored. The letters represent strains as described for Fig. 1. Loss of [PSI+] from J184Y grown without guanidine (G0) also is shown. [PSI+] was completely stable in all other strains grown without guanidine.
In addition to its prion-curing effect, guanidine is toxic to yeast and inhibits growth at concentrations above 3–5 mM. The low number of [psi−] cells in J184S cultures after prolonged exposure to guanidine thus may be caused by factors unrelated to Hsp104. To test whether the D184S substitution was altering guanidine toxicity, growth of [PSI+] and [psi−] variants of strains J104x, J184D, and J184S was tested on YPD plates containing from 0.5 to 15 mM guanidine. Growth of all three strains was inhibited similarly by guanidine at concentrations of 5 mM and above (data not shown). These results show that the observed effects of D184S were not caused by altered sensitivity to the growth-inhibitory effects of guanidine, and that guanidine toxicity is caused by a mechanism unrelated to its effects on Hsp104 or [PSI+].
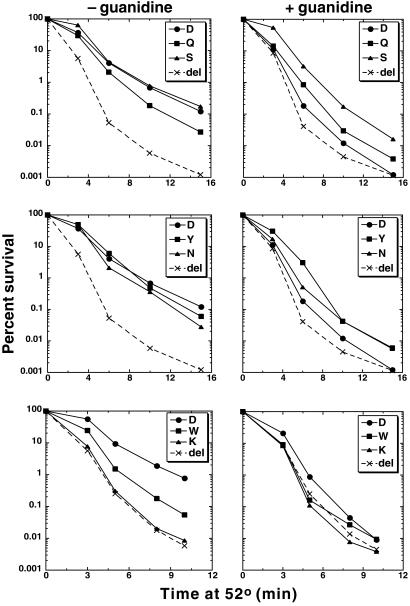
Except for J184S, the mutants capable of propagating [PSI+] had reduced thermotolerance, but all of them retained detectable Hsp104 activity when exposed to guanidine (Fig. 4). Thermotolerance of J184S was similar to that of wild type, which was 100-fold higher than that of the strain lacking HSP104. Thermotolerance of J184N and J184Q was reduced ≈4-fold, but [PSI+] was completely stable in these strains. Moreover, although guanidine reduced thermotolerance of J184S by 10-fold and that of J184Q to a level only slightly higher than the inactivated wild-type protein, [PSI+] was very stable in these strains when grown with guanidine. Together, these results show that Hsp104 activity can be reduced significantly without affecting [PSI+] propagation. Alternatively, these mutations were altering an Hsp104 activity that is more important for thermotolerance than for maintaining [PSI+].
Figure 4.
Thermotolerance of HSP104 mutant strains. After shifting log-phase cultures grown at 27–39°C for 30 min, they were shifted to 52°C, and survival was monitored as a function of time. The data on the right are from portions of the same cultures used for that shown on the left, to which guanidine was added to a final concentration of 5 mM immediately before exposure to 52°C.
HSP104 Mutations That Adversely Affect [PSI+].
Because [PSI+] propagation requires an intermediate level of Hsp104, the weakened [PSI+] of the J184Y strain could be caused by either reduced or elevated Hsp104 activity. Basal (data not shown) and acquired thermotolerance (Fig. 4) of J184Y was slightly less than that of wild type, indicating that Hsp104 activity of the D184Y protein is reduced. However, J184Y cells were more thermotolerant than J184N cells in which [PSI+] was completely stable. These results suggest that the J184Y effect on [PSI+] was not simply caused by the reduced Hsp104 function. Consistent with this explanation, increasing Hsp104 abundance by elevating the growth temperature had no effect on [PSI+] stability in J184D cells (data not shown) but significantly destabilized [PSI+] in J184Y (Table 2). Together, these results suggest that although the D184Y protein is modestly defective in its thermotolerance function, it may be overactive in or have gained an activity that interferes with [PSI+] propagation.
Table 2.
Percentage of [psi−] cells in J184Y colonies grown at different temperatures
| Incubation temperature, °C | Colony size
|
|
|---|---|---|
| 1 mm | 2 mm | |
| 25 | 0.4 ± 0.1 | 1.0 ± 0.3 |
| 30 | 3.1 ± 0.2 | 13 ± 4 |
| 37 | 15 ± 5 | 51 ± 6 |
Cells taken from −ade plates at 25°C were grown on YPAD medium, and cells from four colonies of each size at each temperature were suspended in water and spread onto YPD medium. The resulting colonies (600–900) were scored.
In agreement with the ability of J184Y cells to grow better than wild type on −ade/G3 plates, thermotolerance of guanidine-exposed J184Y was better than that of wild type (Fig. 4). It also was better than J184Q and similar to that of J184N. Unlike J184N and J184Q, however, J184Y was cured efficiently of [PSI+] by guanidine (Fig. 3). Thus, Hsp104 activity did not correlate directly with [PSI+] stability. Taken together, the differences between J184Y, J184N, and J184Q regarding [PSI+] phenotype and thermotolerance suggest that there may be a difference in the way that Hsp104 interacts with prion aggregates of Sup35p in [PSI+] cells and with protein aggregates resulting from thermal denaturation.
In addition to D184Y, only D184W and D184K noticeably impaired [PSI+]. Notably, these replacements introduce side chains that are the largest and the opposite in charge to the wild-type aspartate, respectively. Both abolished the ability of Hsp104 to support [PSI+] propagation. The thermotolerance of strain J184K was no better than that of J104x (Fig. 4), indicating that the D184K mutation inactivated Hsp104. Strain J184W had the lowest thermotolerance of the mutants that retained activity. Despite the large change in the amino acid side chain caused by this substitution, the D184W protein showed no resistance to inhibition by guanidine (Fig. 4), which is interesting in light of the fact that all the other substitutions conferred some level of guanidine resistance. Because the thermotolerance of J184W was only about half that of J184Q, which stably maintained [PSI+], the activity of the D184W protein might be just below a critical threshold level required for maintaining [PSI+]. To address this possibility, [PSI+] was transferred by cytoduction into J184W that expressed an extra copy of the hsp104D184W allele on a plasmid, which typically increases Hsp104 abundance 3–4-fold. All of 30 cytoductants remained [psi−]. Additionally, the thermotolerance of J184W without exposure to guanidine was greater than that of guanidine exposed J184Q, which stably maintains [PSI+]. As with J184Y, these results may indicate that the [PSI+] propagation defect of J184W was caused by more than a simple loss of overall Hsp104 activity.
Effects of HSP104 Mutations on [URE3].
To verify that the resistance to prion-curing was caused by altered interaction of guanidine with Hsp104 and not to [PSI+]-specific effects, isogenic ura2 derivatives of the J184 strains were made such that [URE3] could be monitored (JU-184 strains, see Methods). [URE3] [psi−] variants of JU-184D and JU-184S from USA plates (which select for [URE3]) were streaked onto YPAD and YPAD/G3 and then scored for [URE3]. Without guanidine, [URE3] was completely stable in strain JU-184D. Among three JU-184S colonies 33 ± 2% of cells had lost [URE3], indicating that the D184S substitution impaired [URE3] propagation. When grown on YPAD/G3, [URE3] was lost from all cells tested from three JU-184D colonies but only from 38 ± 3% of those from three JU-184S colonies. Thus, the D184S substitution reduced mitotic stability of [URE3] but also conferred considerable resistance of [URE3] to curing by guanidine.
We showed previously that [URE3] inhibits growth of our strains and that this effect is inversely related to the “strength” of the [URE3] phenotype (40). In agreement with the reduced stability of [URE3], when transferred from USA medium JU-184S [URE3] cells grew faster in liquid YPAD at 30°C than those of JU-184D (Table 3), suggesting that [URE3] was weaker in JU-184S cells. Together, the results suggest that propagation of [URE3] is more sensitive than that of [PSI+] to changes in Hsp104 function caused by alterations at residue 184. These results provide evidence for a distinction in the way Hsp104 interacts with [URE3] and [PSI+] and suggest that [URE3] and [PSI+] have differences in what they require of Hsp104 for their replication.
Table 3.
Growth rates of strains with and without [URE3]
| Strain ([psi−]) | Minutes/cell division
|
|
|---|---|---|
| [ure-o] | [URE3] | |
| JU-184D | 115 | 180 |
| JU-184S | 110 | 122 |
| JU-184Y | 114 | 130 |
The impaired [PSI+] propagation caused by the D184Y and D184W substitutions was also seen with [URE3]. JU-184Y could propagate [URE3], but [URE3] was very unstable, and growth on USA was reduced considerably compared with JU-184D. When transferred from USA plates and grown on YPAD, 98% of cells from resulting colonies of JU-184Y lost [URE3]. JU-184Y [URE3] cells also grew faster than JU-184D [URE3] cells on YPAD (Table 3), but much of this difference is likely caused by the appearance of cells having lost [URE3] shortly after transfer to nonselective conditions. For strain JU-184W, all of 39 cytoduction recipients of [URE3] remained USA−. To verify that the recipients were unable to propagate [URE3], they were pooled and used as donors in a reciprocal cross. None of over 100 resulting wild-type recipients were [URE3]. Thus, the D184W mutation in Hsp104 also is incompatible with [URE3] propagation. Residue 184 is therefore critical for the Hsp104 activity that is required for propagation of both prions. As shown before, [URE3] propagation depended on Hsp104 (19). All of 50 JU-104n cytoduction recipients of [URE3] remained [ure-o].
Discussion
We show that guanidine cures yeast prions by inactivating Hsp104. The correlation between substitutions of residue 184 and resistance of Hsp104 to inactivation by guanidine attests to the importance of this site in the interaction with guanidine. Amino acid residue 184 is conserved universally among HSP100/Clp proteins and is located at the N-terminal end of the first of two NBDs (NBD1) of Hsp104. There is no analogous residue in NBD2. It was reported earlier that low concentrations of guanidine inhibit the ATPase activity of purified Hsp104 (2), which likely explains its effect in vivo. Our data do not reveal how alterations at D184 affect the interaction of Hsp104 with guanidine, and a better understanding of how this residue participates in Hsp104 function will be aided by a high-resolution structure of Hsp104. Nevertheless, this specific inhibitory effect makes guanidine a useful tool for analyzing the consequences of depletion of Hsp104 activity on yeast prion propagation.
Guanidine cures [PSI+] by arresting replication of inheritable prion particles, or seeds (30). The nonreplicating seeds then are diluted among dividing cells, and the length of the lag before the appearance of [psi−] cells in the population provides an estimate of the starting number of seeds per cell. The fact that Hsp104 is inactivated by guanidine implies that Hsp104 plays an essential role in the regeneration of new prion seeds. Hsp104 also may act by promoting conformational changes in Sup35p that are necessary for prion formation (12, 15). In line with its cellular role of resolubilizing protein aggregates (1), Hsp104 may aid seed regeneration simply by breaking [PSI+] aggregates into smaller more numerous particles that function as prion seeds (21). Yet, stressful growth conditions that induce Hsp104-mediated thermotolerance increase the amount of functional Sup35p in [PSI+] cells, which weakens the [PSI+] phenotype, but do not cure [PSI+] or alter the number of inheritable [PSI+] seeds (30, 44). Moreover, modest increases in Hsp104 under conditions where it normally is not induced reduces [PSI+]-mediated nonsense suppression and causes [PSI+] to be lost (15, 34). These results suggest that Hsp104 can resolubilize Sup35p from aggregates in [PSI+] cells without generating new prion seeds.
Without affecting Hsp104 activity, expression of a mutant allele of HSP70 (SSA1-21) eliminates the lag in appearance of [psi−] cells after the addition of guanidine (34). This effect likely reflects a reduction in the number of [PSI+] seeds, which is associated with an increase in their size. As suggested earlier (32, 45), Hsp104 and Hsp70 may act together in prion seed generation. Hsp104 resolubilizes aggregated proteins, and Hsp70 interacts with partially folded proteins and prevents them from (re)aggregating (2). These distinct activities that combine to reactivate denatured protein also may promote prion replication by maintaining Sup35p aggregates in a form soluble enough to be transmitted efficiently. Because altered Hsp104 alone confers resistance to guanidine-curing of prions, any function of Hsp70 in prion seed regeneration would necessarily be unaffected by guanidine.
Although small increases in Hsp104 can destabilize [PSI+], we now show that Hsp104 activity can be reduced significantly without noticeably affecting [PSI+] propagation, which may mean that the level of Hsp104 activity required to generate new prion seeds from Sup35p aggregates in [PSI+] cells is simply less than that required to restore viability by solubilizing aggregates of heat-denatured protein. However, mutants with thermotolerance reduced by guanidine to similar levels are affected differentially with respect to [PSI+] stability.
A nonexclusive alternative explanation is that Hsp104 interacts differently with ordered aggregates of Sup35p in [PSI+] cells than it does with amorphous protein aggregates that result from thermal denaturation. Several results support this latter interpretation. The D184Y substitution considerably destabilized [PSI+] and [URE3] but impaired thermotolerance less than other substitutions that had no effect on [PSI+] propagation. In contrast, although guanidine significantly inhibited the ability of the mutant Hsp104 proteins to restore viability by acting on stress-denatured substrates, most still retained a substantial ability to act on [PSI+] in a way that promotes its propagation. Additionally, there were differences in [PSI+] and [URE3] stability in cells expressing the D184S and D184Y proteins, suggesting that these two prions require Hsp104 differently for their propagation. The dissociation of the cellular functions of Hsp104 in thermotolerance and the propagation of different prions may indicate that the structure near residue 184 is important for a specific activity of Hsp104 or for some aspect of substrate recognition. By any scenario, our results show that alterations causing subtle changes in the cellular function of Hsp104 can have profound effects on yeast prion propagation. Biochemical analysis will pinpoint the altered enzymatic activities of the mutant Hsp104 proteins and aid in understanding the function of Hsp104 in thermotolerance and prion propagation.
Acknowledgments
We thank Michael McCormick for proficient and enthusiastic technical assistance in the early stages of this study.
Abbreviation
- NBD
nucleotide-binding domain
References
- 1.Parsell D A, Kowal A S, Singer M A, Lindquist S. Nature (London) 1994;372:475–478. doi: 10.1038/372475a0. [DOI] [PubMed] [Google Scholar]
- 2.Glover J R, Lindquist S. Cell. 1998;94:73–82. doi: 10.1016/s0092-8674(00)81223-4. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez Y, Lindquist S L. Science. 1990;248:1112–1115. doi: 10.1126/science.2188365. [DOI] [PubMed] [Google Scholar]
- 4.Lindquist S, Kim G. Proc Natl Acad Sci USA. 1996;93:5301–5306. doi: 10.1073/pnas.93.11.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez Y, Taulien J, Borkovich K A, Lindquist S. EMBO J. 1992;11:2357–2364. doi: 10.1002/j.1460-2075.1992.tb05295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsell D A, Sanchez Y, Stitzel J D, Lindquist S. Nature (London) 1991;353:270–273. doi: 10.1038/353270a0. [DOI] [PubMed] [Google Scholar]
- 7.Parsell D A, Kowal A S, Lindquist S. J Biol Chem. 1994;269:4480–4487. [PubMed] [Google Scholar]
- 8.Schirmer E C, Glover J R, Singer M A, Lindquist S. Trends Biochem Sci. 1996;21:289–296. [PubMed] [Google Scholar]
- 9.Schirmer E C, Queitsch C, Kowal A S, Parsell D A, Lindquist S. J Biol Chem. 1998;273:15546–15552. doi: 10.1074/jbc.273.25.15546. [DOI] [PubMed] [Google Scholar]
- 10.Hattendorf D A, Lindquist S L. EMBO J. 2002;21:12–21. doi: 10.1093/emboj/21.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wickner R B. Science. 1994;264:566–569. doi: 10.1126/science.7909170. [DOI] [PubMed] [Google Scholar]
- 12.Patino M M, Liu J-J, Glover J R, Lindquist S. Science. 1996;273:622–626. doi: 10.1126/science.273.5275.622. [DOI] [PubMed] [Google Scholar]
- 13.Kushnirov V V, Ter-Avanesyan M D. Cell. 1998;94:13–16. doi: 10.1016/s0092-8674(00)81216-7. [DOI] [PubMed] [Google Scholar]
- 14.Edskes H K, Gray V T, Wickner R B. Proc Natl Acad Sci USA. 1999;96:1498–1503. doi: 10.1073/pnas.96.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chernoff Y O, Lindquist S L, Ono B, Inge-Vechtomov S G, Liebman S W. Science. 1995;268:880–884. doi: 10.1126/science.7754373. [DOI] [PubMed] [Google Scholar]
- 16.Derkatch I L, Bradley M E, Masse S V, Zadorsky S P, Polozkov G V, Inge-Vechtomov S G, Liebman S W. EMBO J. 2000;19:1942–1952. doi: 10.1093/emboj/19.9.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kushnirov V V, Kryndushkin D S, Boguta M, Smirnov V N, Ter-Avanesyan M D. Curr Biol. 2000;10:1443–1446. doi: 10.1016/s0960-9822(00)00802-2. [DOI] [PubMed] [Google Scholar]
- 18.Sondheimer N, Lindquist S. Mol Cell. 2000;5:163–172. doi: 10.1016/s1097-2765(00)80412-8. [DOI] [PubMed] [Google Scholar]
- 19.Moriyama H, Edskes H K, Wickner R B. Mol Cell Biol. 2000;20:8916–8922. doi: 10.1128/mcb.20.23.8916-8922.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DebBurman S K, Raymond G J, Caughey B, Lindquist S. Proc Natl Acad Sci USA. 1997;94:13938–13943. doi: 10.1073/pnas.94.25.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paushkin S V, Kushnirov V V, Smirnov V N, Ter-Avanesyan M D. EMBO J. 1996;15:3127–3134. [PMC free article] [PubMed] [Google Scholar]
- 22.Zhouravleva G, Frolova L, Le Goff X, Le Guellec R, Inge-Vechtomov S, Kisselev L, Philippe M. EMBO J. 1995;14:4065–4072. doi: 10.1002/j.1460-2075.1995.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stansfield I, Jones K M, Kushnirov V V, Dagkesamanskaya A R, Poznyakovski A I, Paushkin S V, Nierras C R, Cox B S, Ter-Avanesyan M D, Tuite M F. EMBO J. 1995;14:4365–4373. doi: 10.1002/j.1460-2075.1995.tb00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derkatch I L, Bradley M E, Hong J Y, Liebman S W. Cell. 2001;106:171–182. doi: 10.1016/s0092-8674(01)00427-5. [DOI] [PubMed] [Google Scholar]
- 25.Tuite M F, Mundy C R, Cox B S. Genetics. 1981;98:691–711. doi: 10.1093/genetics/98.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derkatch I L, Bradley M E, Zhou P, Chernoff Y O, Liebman S W. Genetics. 1997;147:509–519. doi: 10.1093/genetics/147.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kushnirov V V, Kochneva-Pervukhova N V, Chechenova M B, Frolova N S, Ter-Avanesyan M D. EMBO J. 2000;19:324–331. doi: 10.1093/emboj/19.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santoso A, Chien P, Osherovich L Z, Weissman J S. Cell. 2000;100:277–288. doi: 10.1016/s0092-8674(00)81565-2. [DOI] [PubMed] [Google Scholar]
- 29.McCready S J, Cox B S, McLaughlin C S. Mol Gen Genet. 1977;150:265–270. doi: 10.1007/BF00268125. [DOI] [PubMed] [Google Scholar]
- 30.Eaglestone S S, Ruddock L W, Cox B S, Tuite M F. Proc Natl Acad Sci USA. 2000;97:240–244. doi: 10.1073/pnas.97.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jung G, Masison D C. Curr Microbiol. 2001;43:7–10. doi: 10.1007/s002840010251. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira P C, Ness F, Edwards S R, Cox B S, Tuite M F. Mol Microbiol. 2001;40:1357–1369. doi: 10.1046/j.1365-2958.2001.02478.x. [DOI] [PubMed] [Google Scholar]
- 33.Wegrzyn R D, Bapat K, Newnam G P, Zink A D, Chernoff Y O. Mol Cell Biol. 2001;21:4656–4669. doi: 10.1128/MCB.21.14.4656-4669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung G, Jones G, Wegrzyn R D, Masison D C. Genetics. 2000;156:559–570. doi: 10.1093/genetics/156.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alani E, Cao L, Kleckner N. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sikorski R S, Boeke J D. Methods Enzymol. 1991;194:302–318. doi: 10.1016/0076-6879(91)94023-6. [DOI] [PubMed] [Google Scholar]
- 37.Guthrie C, Fink G R. Methods in Enzymology. Vol. 194. San Diego: Academic; 1991. [Google Scholar]
- 38.Cox B S. Heredity. 1965;20:505–521. [Google Scholar]
- 39.Lacroute F. J Bacteriol. 1971;106:519–522. doi: 10.1128/jb.106.2.519-522.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwimmer C, Masison D C. Mol Cell Biol. 2002;22:3590–3598. doi: 10.1128/MCB.22.11.3590-3598.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Masison D C, Maddelein M-L, Wickner R B. Proc Natl Acad Sci USA. 1997;94:12503–12508. doi: 10.1073/pnas.94.23.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sikorski R S, Hieter P. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schatz P J, Solomon F, Botstein D. Genetics. 1988;120:681–695. doi: 10.1093/genetics/120.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eaglestone S S, Cox B S, Tuite M F. EMBO J. 1999;18:1974–1981. doi: 10.1093/emboj/18.7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newnam G P, Wegrzyn R D, Lindquist S L, Chernoff Y O. Mol Cell Biol. 1999;19:1325–1333. doi: 10.1128/mcb.19.2.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]