Abstract
HIV/AIDS infection is the fourth leading cause of death worldwide, and one in every 100 adults aged 15–49 years is HIV-infected. Forty percent of AIDS patients suffer from neurological symptoms, but the selective profile of damage caused by HIV in the brain is not well understood. Here, we report 3D maps revealing how AIDS affects the human cerebral cortex, identifying the most vulnerable regions and where deficits link with cognitive decline and immune-system suppression. With high-resolution brain MRI scans, we created composite maps of cortical gray-matter thickness in 26 AIDS patients and 14 healthy controls to establish the selective pattern of brain deficits in AIDS. In AIDS, primary sensory, motor, and premotor cortices were 15% thinner. Thinner frontopolar and language cortex correlated with immune system deterioration measured through blood levels of CD4+ T lymphocytes. Prefrontal and parietal tissue loss correlated with cognitive/motor deficits. T cell depletion and cognitive impairment are, therefore, associated with specific 3D brain-deficit patterns visualized with MRI. These quantitative MRI-based maps reveal that HIV selectively damages the cortex. They provide an approach to gauge the impact of AIDS on the living brain and show that the brain is still vulnerable to infection even when patients are receiving antiretroviral therapy.
Keywords: brain, MRI, disease, T cell, immunity
In 2004, an estimated 40 million people worldwide were living with HIV/AIDS (www.unaids.org/wad2004/report_pdf.html). At least 40% of these patients suffer from cognitive impairments, ranging from minor cognitive/motor disorder to HIV-associated dementia, often with a progressive trajectory leading to death (1). For 10–30% of patients, neurologic impairment is the first sign that they are infected with HIV. Cognitive impairments commonly occur in the absence of opportunistic infections, which take advantage of the progressively declining immune system and, therefore, are thought to result from the effect of HIV on the brain. Shortly after infection, HIV enters the CNS, but it does not replicate in neural or glial cells. Virus-encoded proteins are directly toxic to glutamatergic neurons (2, 3), but at autopsy, most of the HIV material in the brain is found in infected monocytes. These monocytes migrate across the blood–brain barrier and later release HIV into the CNS (4). Neurons are primarily damaged indirectly, as infected macrophages, lymphocytes, and microglia release lymphokines and other neurotoxic substances (e.g., tumor necrosis factor, oxidative radicals, proteases, quinolinic acid, etc.) (5).
Progress in understanding the advancing profile of AIDS-related brain damage has been slowed by the lack of detailed 3D maps of deficits that consistently occur in the brain. Noninvasive measures of disease progression in living subjects are urgently needed. We set out to identify brain regions that are selectively vulnerable to HIV infection by computing 3D maps of cortical gray-matter thickness in 26 AIDS patients and 14 matched healthy controls and combining them across subjects. Cortical thickness was computed from 3D brain MRI scans as the 3D distance between the inner and outer surfaces of the cortical gray matter. This measure of cortical integrity is sensitive to subtle disease-related changes and correlates with cognitive decline in Alzheimer's disease and schizophrenia (6). We also used 3D maps to localize brain changes that are specifically linked with T cell depletion and cognitive deterioration.
Methods
Subjects. Forty subjects were scanned with MRI, of whom 26 were AIDS patients [i.e., Centers for Disease Control and Prevention (CDC) stage C; mean age, 47.2 yr ±9.8 SD; 25 men, 1 woman] and 14 were HIV-seronegative controls (age, 37.6 yr ±12.2; 8 men, 6 women) with similar HIV-related risk factors to the AIDS patients (maps were similar with and without adjustment for individual age differences). None of the patients had recent traumatic brain injury, although traumatic brain injury may be more prevalent in AIDS patients than in the general population (7), and in general, alcoholics and drug users are at elevated risk. All patients met CDC criteria for AIDS (8), and none had HIV-associated dementia (patients' mean CD4+ T cell count was 325.0 ± 233.5 per μl; patients' mean log10 viral load was 2.57 ± 1.28 RNA copies per ml of blood plasma). Viral load was measured with an ultrasensitive assay (Roche, Branchburg, NJ) with a lower limit of detectability of 50 copies per ml. Health care providers in Allegheny County, PA, served as a sentinel network for recruitment; AIDS patients seen in the sentinel offices and clinics were approached for participation by their treating physician. All AIDS patients were eligible to participate, excluding only those with a history of CNS opportunistic infections, lymphoma, or stroke.
Neurobehavioral Assessment. Each subject underwent a detailed neurobehavioral assessment within 4 weeks before their MRI scan, involving a neurological examination, psychosocial interview, and neuropsychological testing. The neurological examination was conducted by a research nurse supervised by a behavioral neurologist (O.L.L.) and covered the entire nervous system. The motor component of the Unified Rating of Parkinsonism Scale (9) measured the severity of extrapyramidal signs. Each participant underwent a psychosocial interview, including (i) a semistructured psychiatric interview, modified from the Structured Clinical Interview for DSM-III-R (10) administered by a trained interviewer; (ii) the Brief Symptom Inventory (11) and the Neuropsychiatric Inventory (12) to assess subclinical psychiatric symptoms; and (iii) Heaton's Patient's Assessment of Own Function questionnaire (13) and the Modified Instrumental Activities of Daily Living scale (14) to evaluate specific symptoms of cognitive decline, and their impact on daily living. Detailed neurocognitive test data on the subjects is summarized in Table 1, which is published as supporting information on the PNAS web site. We also computed a composite variable of psychomotor speed, which is very similar to the standardized neuropsychological test score called “NPZ” elsewhere (see Table 1). To compute it, first the individual scores on the Grooved Pegboard, Digit-Symbol Substitution, and Trailmaking tests reported in Table 1 were z-transformed based on the mean and standard deviation of the control subjects and then combined by using the arithmetic mean (adjusting the sign such that better performance was always positive). A regression equation was then created predicting normal performance based on age, education, and sex; predicted scores were then calculated for each subject (i.e., controls and AIDS subjects). Finally, the standardized neuropsychological test score was calculated by taking the difference between actual and predicted speed scores. On this variable, the AIDS patients were significantly impaired (P < 0.022). Subjects also were designated as Impaired or Unimpaired (Table 1), based on a comprehensive review of the cognitive data by a neuropsychologist (J.T.B.) blinded to information on whether each subject was HIV-positive or HIV-negative. This binary outcome variable (Impairment), although not specific to any one cognitive domain, is sensitive to AIDS-related cognitive impairments (15). In the maps that correlate impairment with cortical thickness, this binary measure of impairment was correlated with the cortical thickness.
MRI Scanning and Cortical Thickness Measurement. 3D volumetric SPGR (spoiled gradient echo) MRI scans of the brain were acquired identically for all 40 subjects (256 × 256 × 124 matrix; 24-cm field of view; 1.5-mm slices, zero gap; flip angle, 40°, echo time = 5 ms, repetition time = 25 ms). Maps of cortical thickness were created exactly as in ref. 6. All 40 individual brain volumes were rigidly reoriented into the standardized coordinate system of the ICBM-53 average brain, correcting for head tilt and alignment differences between subjects but leaving scale differences intact. Automated tissue segmentation was performed on each data set to classify voxels based on signal intensity as most representative of gray matter, white matter, cerebrospinal fluid, or an extracerebral background class. A 3D cortical surface model was extracted with automatic software by continuously deforming a mesh-like surface to fit a threshold intensity value in the brain image that best differentiates cortical cerebrospinal fluid from underlying cortical gray matter. Cortical thickness was defined as the 3D distance measured from the cortical white/gray-matter boundary in the tissue classified brain volume to the cortical surface (gray-matter/cerebrospinal fluid boundary) in each subject. Gray-matter thickness was then compared across subjects and averaged at each cortical surface location to produce spatially detailed maps of local thickness differences within and between groups. Cortical pattern matching was used to spatially relate thickness information from homologous cortical areas across subjects. This technique explicitly matches cortical gyral patterns, increasing the power to detect group differences. A set of 72 sulcal landmarks per brain constrained the mapping of one cortex onto another. This procedure eliminates much of the confounding gyral pattern variability when averaging thickness data across individual brain volumes.
Statistical Maps of Cortical Thickness. Color-coded statistical maps visualized local gray-matter thickness differences between AIDS patients and controls. Regressions at each cortical point assessed whether cortical gray-matter thickness at that point depended on (i) diagnosis, (ii) cognitive impairment, (iii) CD4+ T cell counts, and (iv) viral load. The P value describing the significance of this linkage was plotted at each cortical point by using a color code to produce a significance map. The spatial maps (uncorrected) visualize the spatial patterns of cortical thinning. Permutation methods assessed their overall significance, correcting for multiple comparisons. We also measured the fractal dimension (complexity) of the human cerebral cortex in 3D (as in ref. 6), and compared surface complexity for AIDS patients vs. controls, by using t tests.
Results
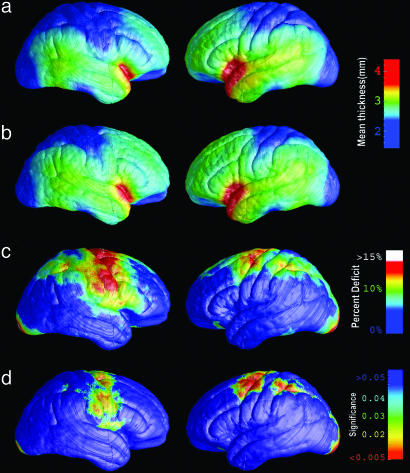
Fig. 1a shows the average profile of cortical thickness in AIDS patients. Compared with matched healthy controls (Fig. 1b), AIDS patients exhibited a severe, selective gray-matter thinning (10–15% locally) in a broad anatomic region that includes the primary sensory and motor cortices, in both brain hemispheres (Fig. 1c). These highly significant deficits extend anteriorly into the frontal eye fields and premotor cortices (left hemisphere, P < 0.0036; right hemisphere, P < 0.028, corrected for multiple comparisons). Severely atrophic regions in the parietal association cortex lie immediately adjacent to comparatively intact cortex, such as the perisylvian language areas. This profile suggests that an anatomically selective pattern of cortical deficits occurs in AIDS.
Fig. 1.
Cortical thinning on the lateral brain surface in HIV/AIDS. (a) Average profile of cortical thickness in AIDS patients. Right hemisphere is on the left. (b) Mean cortical thickness for matched healthy controls. (c) Average percentage thinning of the cortex in AIDS relative to healthy controls. (d) Color-coded map that shows the significance of the group difference, at each cortical point (reds indicate significant cortical thinning). The band of thinner cortex encompasses the primary sensorimotor, premotor, and parietal cortices.
Fig. 2 shows the pattern of AIDS-related deficits mapped across the medial brain surface. Consistent with Fig. 1, severe deficits were identified in the medial frontal and premotor cortex, sparing the immediately adjacent limbic tissue. Fifteen percent thinning was observed in the primary visual cortices of the occipital poles. This overall pattern suggests that primary cortices are more vulnerable to thinning than higher-order association cortices. No thinning was detected in temporal or prefrontal cortices.
Fig. 2.
Cortical thinning on the medial brain surface in HIV/AIDS. The same set of maps is shown as in Fig. 1 but visualizing the medial surfaces of the brain hemispheres. Shown are the average profile of cortical thickness in AIDS patients (a), mean cortical thickness for matched healthy controls (b), the average percentage thinning of the cortex in AIDS relative to healthy controls (c), and the significance of the group difference at each cortical point (d) (red indicates significant cortical thinning). Primary sensorimotor and frontal cortices are thinner on the medial wall, as are the primary visual cortices.
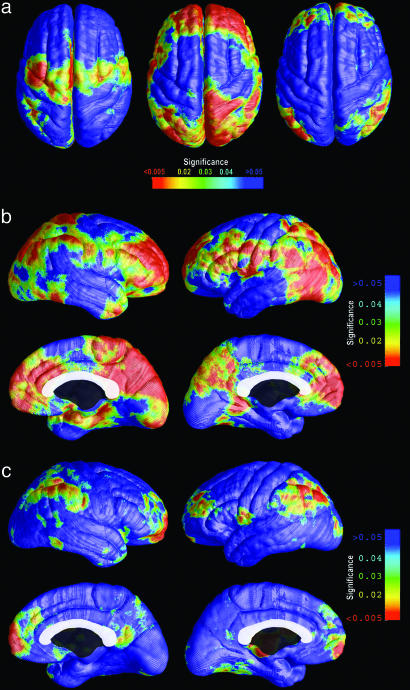
Given the trajectory of progressive cognitive decline typical of HIV infection, we also correlated these cortical deficits with the level of immune compromise (CD4+ cell count) and the degree of neuropsychological impairment. In Fig. 3a, separate maps show brain regions where cortical thinning is linked with AIDS, with cognitive impairment, and with CD4+ T cell counts. These maps identify regions where structural brain deterioration affects brain function and gauge how strongly it correlates with immune system integrity. Prefrontal and parietal cortical thinning was linked with neuropsychological impairment, in both brain hemispheres (left hemisphere, P < 0.006; right hemisphere, P < 0.007; see Fig. 3b). At this stage of HIV infection, cortical thinning in these association areas better accounted for cognitive symptoms than did cortical thinning in the primary cortical regions most devastated by AIDS.
Fig. 3.
Cortical thinning is associated with cognitive impairment and immune suppression. (a) In red, the brain regions where cortical thickness variations correlate with AIDS diagnosis (Left), with cognitive impairment (Center), and with T lymphocyte counts (Right). (b) Parietal and prefrontal cortical thinning is linked with cognitive impairment. (c) Frontopolar and perisylvian language regions where thinning is significantly associated with lower CD4+ T cell counts. The correlational analyses (b and c) are based on data from the patients only: among patients, those with most impaired cognition and immune function also have the thinnest cortex. No correlations were found in the opposite direction (see Fig. 7, which is published as supporting information on the PNAS web site).
Cortical thinning was associated with the degree of immune suppression, as indexed by blood levels of CD4+ T lymphocytes (Fig. 3 a and c). HIV selectively infects and destroys two key cell types in the immune system, helper T cells and monocytes. Progressive T cell depletion undermines the immune response to HIV and many opportunistic infections, and the transition from asymptomatic HIV-positive status to AIDS is conventionally defined when CD4+ T lymphocyte counts fall below 200 cells per μl of blood or when opportunistic infections occur (8). In these AIDS patients, cortical gray-matter thinning was still highly correlated with immune suppression (i.e., lower CD4+ T cell counts). Thinning in the frontal poles and perisylvian language areas showed the highest correlation with CD4+ cell counts (left hemisphere, P < 0.031; right hemisphere, P < 0.021). Brain structure was linked with immunity, but no associations were found between viral load and cortical thinning in any brain region (maps not shown).
Medication Effects. Thirteen of the AIDS subjects were receiving highly active antiretroviral therapy (HAART), which combines protease inhibitors and reverse transcriptase inhibitors. Chronic use of HAART may lead to systemic changes, both positive (reduced viral load, bolstered CD4+ T cell counts) and negative (altered amyloid levels, lipodystrophy, etc.). We, therefore, regressed the thickness maps against a binary variable that distinguished the 13 patients receiving HAART from the 13 who were not, and no significant thickness differences were found between the two therapy groups (P > 0.05, permutation test). The sample was too small to determine whether there were differences in cortical thinning as a function of the CNS penetrability of the medications. Although a direct HAART effect on brain structure cannot be ruled out by this lack of effect in a small sample, it suggests that the use of HAART is unlikely to mediate the severe pattern of cortical thinning seen here. Longitudinal data in larger samples are needed to fully address this question. Further supporting the idea that cortical thinning is associated with the disease process, cortical thinning correlates not only with illness but also with lower CD4+ T cell counts within patients (Fig. 3), suggesting that it tracks a widely accepted biological measure of disease progression.
Gender. Because men were overrepresented in the sample (six women were controls but there was only one female patient), we covaried for gender but did not find a main effect, so we did not adjust for gender in the maps, because there was no effect to regress out. Although there is no evidence of gender differences in how AIDS affects the brain, future samples that include more women will help identify any gender effects.
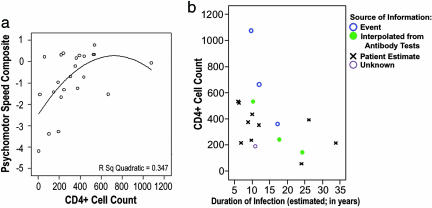
Duration of Infection. Although there is no absolutely reliable index of the duration of infection, an estimate was inferred from patients' estimates (in six patients, this information was based on successive antibody tests or inferred from a specific life event). In maps correlating duration of infection with cortical thickness, even though there appeared to be a diffuse, weak effect, in the hypothesized direction in the left and right parietal cortices, it was not significant after multiple comparisons correction (P ≈ 0.15, permutation test; maps not shown). The estimated duration of infection did correlate with CD4+ T cell counts (r2 = 0.157; P = 0.037), which is a more reliable marker for disease progression and correlates better with cortical thinning (Fig. 3). Because of the 16% shared variance between duration of infection and T cell counts (see Fig. 4b), there was insufficient power to resolve independent effects of each. Although we suspect that cortical thinning is a result of chronic infection, longitudinal data are needed to establish whether cortical thinning depends on the duration of HIV infection or the duration of AIDS.
Fig. 4.
Impairment, declining immunity, and illness duration. (a) In a quadratic regression, CD4+ T cell counts were predictive of psychomotor speed (the standardized neuropsychological test score, described in Methods). (b) T cell counts were also correlated with the duration of infection, estimated from successive antibody tests or other data. The source of information is shown in b.
Cognition and Immune Decline. As expected, psychomotor performance was poorer in subjects with lower CD4+ T cell counts and consistently higher when the immune system was more intact (in subjects with T cell counts>250 per μl). Based on a quadratic model (r2 = 0.347; P < 0.014; Fig. 4a), a prediction based on CD4+ T cell counts accounted for 35% of the variance in psychomotor speed (after adjusting for expected performance based on age, education, and sex).
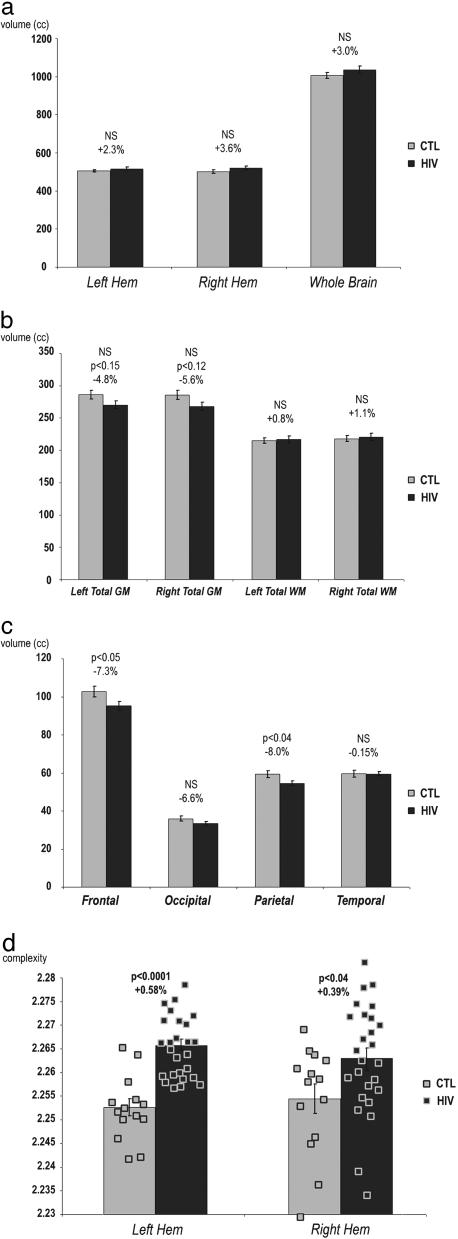
We also assessed global and lobar tissue volumes to determine whether the cortical thinning was regionally specific or whether it simply reflected global brain atrophy, i.e., a generalized, nonspecific gray- or white-matter change (Fig. 5). Supporting the locality of the gray-matter deficits, we detected no overall reduction in cerebral volume, volumes of the left or right hemispheres, or in total gray or white matter in either hemisphere (Fig. 5 a and b). Perhaps surprisingly, no global reduction was detected for total cerebral gray matter (averaging –4.8% and –5.6% for left and right hemispheres; P > 0.05). Frontal and parietal lobes had significant gray-matter loss (frontal, –7.3%, P < 0.05; parietal, –8.0%, P < 0.04), and there were nonsignificant decreases in gray-matter volumes of –6.6% in the occipital lobes and –0.2% in the temporal lobes, consistent with the cortical thinning patterns (Figs. 1 and 2).
Fig. 5.
Regional brain tissue volume reductions in HIV/AIDS relative to control subjects (CTL). Total cerebral volumes (a) and overall gray- and white-matter volumes (b) were not significantly decreased in AIDS. Frontal and parietal gray-matter volumes were significantly reduced in AIDS (c), consistent with the cortical thinning in Figs. 1 and 2. AIDS patients had higher cortical surface complexity than healthy controls in both brain hemispheres (d). Gray matter was assessed in greater detail using cortical thickness measures, computed using the sequence of steps shown in Fig. 6.
Finally, to see whether we could identify the AIDS effect with a global cortical measure, we measured the cortical surface complexity for all 40 subjects and found that it was higher in AIDS patients than in healthy controls (Fig. 5d; left hemisphere, P < 0.0001; right hemisphere, P < 0.04). This increase in surface complexity is likely a surrogate marker of the cortical atrophy seen in patients, rather than reflecting fundamental alterations in the gyral patterning of the cortex.
Discussion
The maps provide an approach to gauge the impact of AIDS on the living brain and show that the brain is still vulnerable to infection even when patients are receiving antiretroviral therapy. The findings have three implications. First, in AIDS patients without other brain infections, there is severe cortical thinning in primary sensorimotor, premotor, and visual areas. This unusual neurodegenerative pattern is the opposite of that seen in common noninfectious dementias such as Alzheimer's disease where the medial temporal, limbic, and association cortices are affected first and the primary sensorimotor and visual cortices only later (6). Second, cognitive impairment in AIDS was powerfully predicted by the level of atrophy in the prefrontal and parietal cortices. Association cortices showed more subtle atrophy than did primary cortices, but these changes may affect higher cognitive function more severely than primary cortical deficits of the same magnitude. Third, cortical thinning in the language areas and frontal poles of both brain hemispheres was a sensitive predictor of T cell counts, which itself is a sensitive index of immune system integrity, as it progressively deteriorates in AIDS.
Mechanisms. Cortical thinning on MRI is, therefore, a sensitive index of declining neurological and immune function in AIDS. Earlier studies associated HIV infection with progressive striatal, hippocampal, and white-matter volume loss beginning in the medically asymptomatic stage and accelerating later (16, 17). Supporting the atrophic patterns seen here, Oster et al. (18) and Fisher et al. (19) detected an overall neuronal loss of 27% and 25%, respectively (≈6 × 109 neurons) in the neocortex of AIDS patients. Larger neurons were most vulnerable, but neurodegeneration occurred in all lobes. Autopsy studies of AIDS patients with minor cognitive/motor disorder reveal widespread loss of synapses (20) and reduced dendritic complexity without overt neuronal loss. Other studies of minor cognitive/motor disorder reveal some cell loss in selected neuronal subpopulations, specifically in MAP-2-immunoreactive and calbindin pyramidal cells (21).
Even mild dendritic injury may lead to behavioral alterations in HIV-associated minor cognitive/motor disorder (22), and this deterioration precedes frank loss of pyramidal neurons and interneurons, which is more typically associated with HIV-associated dementia (23). Abnormal white-matter signal on in vivo MR images correlates with dendritic damage in the neocortex and with high levels of HIV viral coat protein (HIV-gp41 immunoreactivity) (17). Irreversible neuronal loss occurs primarily in frontal and occipital cortices (21) but not in archicortical regions, such as the hippocampus (23). Neuronal loss may occur only in patients with more severe cognitive decline (e.g., in HIV-associated dementia) or who have CNS opportunistic infections, and these latter subjects were excluded from our study.
The cortical alterations, mapped here, may be partly responsible for the mild cognitive alterations in AIDS, and several cellular mechanisms may contribute to them. Two major factors cause damage to neurons and their dendritic arbors. Virotoxins, such as the HIV-encoded proteins Tat and gp120, are directly toxic to neurons. These viral proteins overactivate NMDA-type glutamate receptors (2), and the excess extracellular glutamate causes excitotoxic injury and cell death (21). MAP-2 immunoreactive pyramidal cells are especially vulnerable (24). Also, cytokines produced by HIV-infected macrophages may be toxic to neurons and myelin. Axonal damage may lead to dendritic simplification and reductions in somal size, as have been observed in AIDS for subsets of neurons in the frontal cortex.
Cognition. The presence of dementia in HIV-infected subjects is linked with the overall degree of cerebral atrophy (computed as a ratio of ventricular to total brain volume). In nondemented subjects, the degree of cognitive impairment links with volume loss in the striatum, white matter, and posterior cortex (25). The frontal cortical thinning observed here was strongly linked with cognitive impairment and may underlie the commonly observed HIV-related decline in frontal lobe functions such as attention, executive function, and working memory. These changes are strongly associated with HIV-related brain disease (26) and independently predict diminished survival (27).
Immunity. Immunological measures of the stage of HIV infection (CD4+ cell counts) also were associated with specific regions of cortical atrophy. CD4+ cell counts predict vulnerability to opportunistic infections and imminent death. In prior longitudinal studies, rapid decreases in CD4+ lymphocyte counts were associated with rapidly increasing ventricular and sulcal cerebrospinal fluid volumes, which tend to reflect global cerebral atrophy (16). In our study, cortical atrophy was not associated with viral load, perhaps because of the waxing and waning nature of viral load in AIDS patients undergoing treatment. By contrast, cortical atrophy is likely to be cumulative, and like T cell depletion, it is likely to advance inexorably with longer duration of illness. Alternatively, atrophy and viral load may be more strongly associated in subcortical regions than in the cortex. The caudate, for example, exhibits significant atrophy in HIV, as well as the highest viral load (16).
Psychomotor slowing in AIDS is correlated with CD4+ T cell decline, even though HIV damages neurons via a different cellular pathway (e.g., via cytokines and envelope proteins) from that involved in its attack on the immune system. We were not able to statistically partial out the effects of CD4+ counts from impairment because they were so highly correlated and, ultimately, they have a common cause (HIV). If therapy only improved immune function but did not resist cortical atrophy, one might expect CD4+ counts and psychomotor measures to become decoupled over time, but this appears not to be the case. Once patients are affected beyond a threshold level of immune decline, both measures track disease, and both correlate with cortical thinning.
Most patients in this study were treated with antiretroviral combination therapy, which combines nucleoside and nonnucleoside analog reverse transcriptase inhibitors [such as 3′-azido-3′-deoxythymidine (AZT)] and protease inhibitors. HIV induces neuronal damage and immune system decline through separate mechanisms, and HIV-infected patients do not necessarily have immune failure before nervous system signs and neuropathologic changes appear. HAART has emerged as the treatment of choice since 1996, because it combats immunosuppression and reduces disease progression and mortality. However, HAART may not prevent HIV-induced neuronal damage even when viral load is low (28). Progressive CNS damage is now more common as patients live longer and is somewhat resistant even to therapeutic agents that cross the blood–brain barrier (29). The incidence of HIV-related neuropathology at autopsy also has increased since HAART therapy became more widely available (28).
With 40 million patients worldwide now living with AIDS, of whom an estimated 5 million were first infected in 2004, detailed biomarkers of CNS deficits, such as the cortical maps presented here, are increasingly needed to help gauge the success of neuroprotective therapies (e.g., memantine, CPI-1189, or NMDA-receptor antagonists). Cortical maps may assist research into other disease processes, e.g., in revealing the sequence and extent of brain changes in the dementias, epilepsy, psychosis, and developmental disorders. Here, they reveal how AIDS impacts the brain and may help identify early atrophic changes in neurologically asymptomatic patients with HIV, who might benefit most from neuroprotective agents.
Supplementary Material
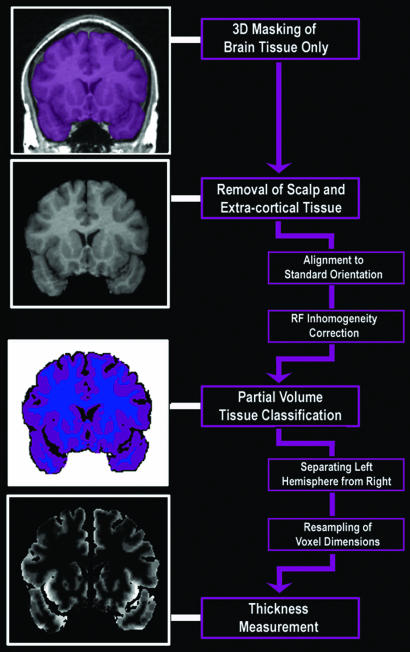
Fig. 6.
Mapping cortical thickness by using MRI. This flow chart summarizes the sequence of steps used to compute cortical thickness from MRI scans of the brain.
Acknowledgments
This work was supported in part by grants from the National Institute on Aging (to J.T.B. and P.M.T.), the National Institute for Biomedical Imaging and Bioengineering (to P.M.T.), and the National Center for Research Resources (to P.M.T.).
Author contributions: P.M.T. and J.T.B. designed research; P.M.T., R.A.D., K.M.H., A.W.T., O.L.L., H.J.A., and J.T.B. performed research; P.M.T. and A.W.T. contributed new reagents/analytic tools; P.M.T., R.A.D., K.M.H., and J.T.B. analyzed data; and P.M.T., A.W.T., O.L.L., H.J.A., and J.T.B. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviation: HAART, highly active antiretroviral therapy.
References
- 1.Levy, R. M., Bredesen, D. E. & Rosenblum, M. L. (1986) J. Neurosurg. 62, 475–495. [DOI] [PubMed] [Google Scholar]
- 2.Sardar, A. M., Hutson, P. H. & Reynolds, G. P. (1999) NeuroReport 10, 3513–3515. [DOI] [PubMed] [Google Scholar]
- 3.Nath, A., Haughey, N. J., Jones, M., Anderson, C., Bell, J. E. & Geiger, J. D. (2000) Ann. Neurol. 47, 186–194. [PubMed] [Google Scholar]
- 4.Koenig, S., Gendelman, H. E., Orenstein, J. M., Canto, M. C. D., Pezeshkpour G. H., Yungbluth, M., Janotta, F., Aksamit, A., Martin M. A. & Fauci, A. S. (1986) Science 233, 1089–1093. [DOI] [PubMed] [Google Scholar]
- 5.Heyes, M. P., Ellis, R. J., Ryan, L., Childers, M. E., Grant, I., Wolfson, T., Archibald, S., Jernigan, T. L. & HIV Neurobehavioral Research Center Group (2001) Brain 124, 1033–1042. [DOI] [PubMed] [Google Scholar]
- 6.Thompson, P. M., Hayashi, K. M., Sowell, E. R., Gogtay, N., Giedd, J. N., Rapoport, J. L., de Zubicaray, G. I., Janke, A. L., Rose, S. E., Semple, J., et al. (2004) NeuroImage 23, Suppl. 1, S2–S18. [DOI] [PubMed] [Google Scholar]
- 7.Jaffe, M. P., O'Neill, J., Vandergoot, D., Gordon, W. A. & Small, B. (2000) Brain Inj. 14, 35–44. [DOI] [PubMed] [Google Scholar]
- 8.Center for Disease Control and Prevention (1992) Morbid. Mortal. Wkly. Rep. Recomm. Rep. 41(RR-17), 1–19. [Google Scholar]
- 9.Fahn, S., Elton, R. L. & Members of the UPDRS Development Committee (1987) in Recent Developments in Parkinson's Disease, eds. Fahn, S., Marsden, C. D. & Calne, D. B. (Macmillan, New York), Vol. 2, pp. 153–163. [Google Scholar]
- 10.Spitzer, R. L., Williams, J. B., Gibbon, M. & First, M. B. (1989) Instruction Manual for the Structured Clinical Interview for DSM-III-R (Biometrics Research, New York State Psychiatric Institute, New York).
- 11.Derogatis, L. (1992) The Brief Symptom Inventory (BSI): Administration, Scoring, and Procedures Manual (Clinical Psychometric Research, Baltimore Research, Baltimore).
- 12.Cummings, J. L., Mega, M., Gray, K., Rosenberg-Thompson, S., Carusi, D. A. & Gornbein, J. (1994) Neurology 44, 2308–2314. [DOI] [PubMed] [Google Scholar]
- 13.Heaton, R. K. (1981) Wisconsin Card Sorting Test Manual (Psychological Assessment Resources, Odessa, FL).
- 14.Lawton, M. P. & Brody, E. M. (1969) Gerontologist 9, 179–186. [PubMed] [Google Scholar]
- 15.Becker, J. T., Lopez, O. L., Dew, M. A. & Aizenstein, H. J. (2004) AIDS 18, Suppl. 1, S11–S18. [PubMed] [Google Scholar]
- 16.Stout, J. C., Ellis, R. J., Jernigan, T. L., Archibald, S. L., Abramson, I., Wolfson, T., McCutchan, J. A., Wallace, M. R., Atkinson, J. H. & Grant, I. (1998) Arch. Neurol. 55, 161–168. [DOI] [PubMed] [Google Scholar]
- 17.Archibald, S. L., Masliah, E., Fennema-Notestine, C., Marcotte, T. D., Ellis, R. J., McCutchan, J. A., Heaton, R. K., Grant, I., Mallory, M., Miller, A., et al. (2004) Arch. Neurol. 61, 369–376. [DOI] [PubMed] [Google Scholar]
- 18.Oster, S., Christoffersen, P., Gundersen, H. J., Nielsen, J. O., Pedersen, C. & Pakkenberg, B. (1995) APMIS 103, 525–529. [DOI] [PubMed] [Google Scholar]
- 19.Fischer, C. P., Jorgen, G., Gundersen, H. & Pakkenberg, B. (1999) Brain Res. 15, 119–126. [DOI] [PubMed] [Google Scholar]
- 20.Wiley, C. A., Masliah, E., Morey, M., Lemere. C., DeTeresa, R., Grafe, M., Hansen, L. & Terry, R. (1991) Ann. Neurol. 29, 651–657. [DOI] [PubMed] [Google Scholar]
- 21.Everall, I., Luthert, P. & Lantos, P. (1993) J. Neuropathol. Exp. Neurol. 52, 561–566. [DOI] [PubMed] [Google Scholar]
- 22.Masliah, E., Heaton, R. K., Marcotte, T. D., Ellis, R. J., Wiley, C. A., Mallory, M., Achim, C. L., McCutchan, A., Nelson, J. A., Atkinson J. H., Grant, I., et al. (1997) Ann. Neurol. 42, 963–972. [DOI] [PubMed] [Google Scholar]
- 23.Sá, M. J., Madeira, M. D., Ruela, C., Volk, B., Mota-Miranda, A., Lecour, H., Goncalves, V. & Paula-Barbosa, M. M. (2000) Acta Neuropathol. 99, 643–653. [DOI] [PubMed] [Google Scholar]
- 24.Masliah, E., Achim, C. L., Ge, N., DeTeresa, R., Terry, R. D. & Wiley, C. A. (1992) Ann. Neurol. 32, 321–329. [DOI] [PubMed] [Google Scholar]
- 25.Patel, S. H., Kolson, D. L., Glosser, G., Matozzo, I., Ge, Y., Babb, J. S., Mannon, L. J. & Grossman, R. I. (2002) Am. J. Neuroradiol. 23, 543–549. [PMC free article] [PubMed] [Google Scholar]
- 26.Cherner, M., Masliah, E., Ellis, R. J., Marcotte, T. D., Moore, D. J., Grant, I. & Heaton, R. K. (2002) Neurology 59, 1563–1567. [DOI] [PubMed] [Google Scholar]
- 27.Ellis, R. J., Deutsch, R., Heaton, R. K., Marcotte, T. D., McCutchan, J. A., Nelson, J. A., Abramson, I., Thal, L. J., Atkinson, J. H., Wallace, M. R. & Grant, I. (1997) Arch. Neurol. 54, 416–424. [DOI] [PubMed] [Google Scholar]
- 28.Neuenburg, J. K., Brodt, H. R., Herndier, B. G., Bickel, M., Bacchetti, P., Price, R. W., Grant, R. M. & Schlote, W. (2002) J. Acquired Immune Defic. Syndr. 31, 171–177. [DOI] [PubMed] [Google Scholar]
- 29.Cysique, L. A., Maruff, P. & Brew, B. J. (2004) Arch. Neurol. 61, 1699–1704. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.